User login
BARCELONA – Neuropsychopharmacologists want to change the way you describe psychoactive drugs.
A team of representatives from four international neuropsychopharmacology groups is finishing a first draft of its proposed new system for drug classification and nomenclature that – in its current form – describes each of approximately 120 psychoactive drugs by five different parameters, or axes, including neurobiologic activity, efficacy, and approved indications worldwide. The goal is a new, Web-based framework for classifying and identifying drugs that is more consistent, informative, and transparent than the existing nomenclature.
"We expect drug nomenclature to reflect current scientific knowledge, give useful pharmacologic information to clinicians, and give patients a rationale for using a drug," but the existing nomenclature does not accomplish those goals, said Dr. Joseph Zohar, director of psychiatry at Sheba Medical Center in Tel Hashomer, Israel, and a member of the nomenclature group. "Existing nomenclature dates from the 1960s; no wonder it does not reflect current understanding. Drugs called antidepressants are often prescribed to patients with anxiety disorders. Patients ask, why give me an antidepressant if I have anxiety? Antipsychotics are prescribed to patients with depression, and they ask why they are getting an antipsychotic? The nomenclature is confusing," Dr. Zohar said at a session at the annual congress of the European College of Neuropsychopharmacology devoted to presenting the new nomenclature system to congress participants.
"We want to rationalize classification and have it better reflect science. We want to focus on mechanisms; knowing the entire profile of medications will help make more informed choices," he said.
"It’s getting people to think more about what drugs do. If people understood drugs better, they would use them better," said Dr. David J. Nutt, professor of neuropsychopharmacology at Imperial College London and another member of the nomenclature group. "The shorthands we currently use [for classifying psychoactive drugs] may be useful, but we need to determine what’s not useful" and get rid of it.
Leadership from the European College of Neuropsychopharmacology (ECNP) arranged for a panel with two representatives each from the American College of Neuropsychopharmacology, the International College of Neuropsychopharmacology, the Asian College of Neuropsychopharmacology, and the ECNP. A draft version of the nomenclature document should appear for comment on the ECNP website by early next year, and the current plan is to publish a finalized version before the end of next year. But the panel members stressed that since the document will primarily be Web-based, it will be open to frequent revision.
"This is the first step in a long process. We’re trying to wake up a 50-year-old nomenclature into a living document that will be used over the next decades and may someday merge with DSM [Diagnostic and Statistical Manual of Mental Disorders]," said Dr. Stephen M. Stahl, a panel member and psychiatrist affiliated with the University of California, San Diego.
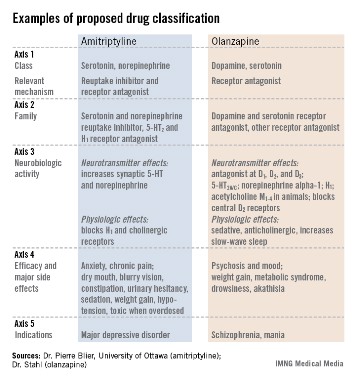
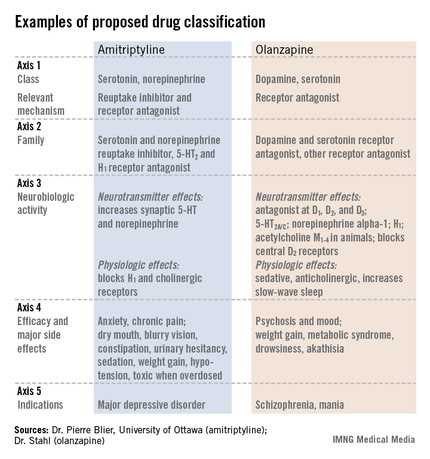
The format the panel has in place currently describes every psychoactive drug in terms of five axes: class and relevant mechanisms; family; neurobiologic activity, neurotransmitter effects, and physiologic effects; efficacy and major side effects; and approved and licenses indications in the major countries and regions that perform drug licensing. Some of the collaborators on the panel published an explanation of the five axes template online in September (Euro. Neuropsychopharmacol. 2013 [doi:10.1016/j.euroneuro.2013.08.004]).
All this information for each drug "should tell you everything you need to know to make a rational decision about what to do with the drug," Dr. Nutt said.
"We need to be very humble about these medications and what they do. You have multiple things going on simultaneously," said Dr. Stahl, also founder of the Neuroscience Education Institute in San Diego. "The second-generation antipsychotics are the most complicated drugs in medicine; one patient’s side effect is another’s therapeutic effect.
"We propose that it is no longer appropriate to name all drugs that treat psychosis as ‘antipsychotics.’ We propose a multiaxial classification system based on pharmacologic mechanisms of action. A mechanism-based nomenclature may clarify the differing mechanisms of individual agents, especially actions in psychosis versus mood disorders. This approach has the potential to better inform those who work with drugs that treat depression, and prevent confusion with other drugs that treat both psychosis and multiple additional disorders such as depression and mania. This approach is also a strategy for naming drugs yet to be discovered that target novel mechanisms of action."
"What we don’t want is industry or a regulator to define a new glycine-reuptake blocker as an antipsychotic," Dr. Nutt said.
Several panel members stressed that the five-axis format will allow a diverse list of useful information.
"We don’t want to be constrained by the approved indications," Dr. Nutt said. "This is an opportunity to document off-label indications and to link to the evidence for off-label uses," said Dr. Guy Goodwin, chairman of psychiatry at the University of Oxford (England) and another collaborator on the panel.
The audience of about 150 congress attendees at the session seemed persuaded by the speakers. At the session’s end, in an electronic vote, 67% said they fully agreed, and another 26% said they partly agreed, that the existing nomenclature needs revision. Asked whether the proposed nomenclature was a step in the right direction, 48% said they fully agreed, and another 39% said they partly agreed. Another question asked what feature should drive a drug’s top-line definition. Sixty percent of the audience said pharmacologic action, and an additional 29% voted in favor of clinical indication.
Dr. Zohar said he has been an adviser or consultant to or received research support from eight drug companies. Dr. Nutt said he has been an adviser or consultant to eight drug companies. Dr. Stahl said he has been an adviser or consultant to more than 30 drug companies. Dr. Goodwin said he had been an adviser or consultant to 12 drug companies.
On Twitter @mitchelzoler
BARCELONA – Neuropsychopharmacologists want to change the way you describe psychoactive drugs.
A team of representatives from four international neuropsychopharmacology groups is finishing a first draft of its proposed new system for drug classification and nomenclature that – in its current form – describes each of approximately 120 psychoactive drugs by five different parameters, or axes, including neurobiologic activity, efficacy, and approved indications worldwide. The goal is a new, Web-based framework for classifying and identifying drugs that is more consistent, informative, and transparent than the existing nomenclature.

"We expect drug nomenclature to reflect current scientific knowledge, give useful pharmacologic information to clinicians, and give patients a rationale for using a drug," but the existing nomenclature does not accomplish those goals, said Dr. Joseph Zohar, director of psychiatry at Sheba Medical Center in Tel Hashomer, Israel, and a member of the nomenclature group. "Existing nomenclature dates from the 1960s; no wonder it does not reflect current understanding. Drugs called antidepressants are often prescribed to patients with anxiety disorders. Patients ask, why give me an antidepressant if I have anxiety? Antipsychotics are prescribed to patients with depression, and they ask why they are getting an antipsychotic? The nomenclature is confusing," Dr. Zohar said at a session at the annual congress of the European College of Neuropsychopharmacology devoted to presenting the new nomenclature system to congress participants.
"We want to rationalize classification and have it better reflect science. We want to focus on mechanisms; knowing the entire profile of medications will help make more informed choices," he said.
"It’s getting people to think more about what drugs do. If people understood drugs better, they would use them better," said Dr. David J. Nutt, professor of neuropsychopharmacology at Imperial College London and another member of the nomenclature group. "The shorthands we currently use [for classifying psychoactive drugs] may be useful, but we need to determine what’s not useful" and get rid of it.
Leadership from the European College of Neuropsychopharmacology (ECNP) arranged for a panel with two representatives each from the American College of Neuropsychopharmacology, the International College of Neuropsychopharmacology, the Asian College of Neuropsychopharmacology, and the ECNP. A draft version of the nomenclature document should appear for comment on the ECNP website by early next year, and the current plan is to publish a finalized version before the end of next year. But the panel members stressed that since the document will primarily be Web-based, it will be open to frequent revision.
"This is the first step in a long process. We’re trying to wake up a 50-year-old nomenclature into a living document that will be used over the next decades and may someday merge with DSM [Diagnostic and Statistical Manual of Mental Disorders]," said Dr. Stephen M. Stahl, a panel member and psychiatrist affiliated with the University of California, San Diego.
The format the panel has in place currently describes every psychoactive drug in terms of five axes: class and relevant mechanisms; family; neurobiologic activity, neurotransmitter effects, and physiologic effects; efficacy and major side effects; and approved and licenses indications in the major countries and regions that perform drug licensing. Some of the collaborators on the panel published an explanation of the five axes template online in September (Euro. Neuropsychopharmacol. 2013 [doi:10.1016/j.euroneuro.2013.08.004]).
All this information for each drug "should tell you everything you need to know to make a rational decision about what to do with the drug," Dr. Nutt said.
"We need to be very humble about these medications and what they do. You have multiple things going on simultaneously," said Dr. Stahl, also founder of the Neuroscience Education Institute in San Diego. "The second-generation antipsychotics are the most complicated drugs in medicine; one patient’s side effect is another’s therapeutic effect.
"We propose that it is no longer appropriate to name all drugs that treat psychosis as ‘antipsychotics.’ We propose a multiaxial classification system based on pharmacologic mechanisms of action. A mechanism-based nomenclature may clarify the differing mechanisms of individual agents, especially actions in psychosis versus mood disorders. This approach has the potential to better inform those who work with drugs that treat depression, and prevent confusion with other drugs that treat both psychosis and multiple additional disorders such as depression and mania. This approach is also a strategy for naming drugs yet to be discovered that target novel mechanisms of action."
"What we don’t want is industry or a regulator to define a new glycine-reuptake blocker as an antipsychotic," Dr. Nutt said.
Several panel members stressed that the five-axis format will allow a diverse list of useful information.
"We don’t want to be constrained by the approved indications," Dr. Nutt said. "This is an opportunity to document off-label indications and to link to the evidence for off-label uses," said Dr. Guy Goodwin, chairman of psychiatry at the University of Oxford (England) and another collaborator on the panel.
The audience of about 150 congress attendees at the session seemed persuaded by the speakers. At the session’s end, in an electronic vote, 67% said they fully agreed, and another 26% said they partly agreed, that the existing nomenclature needs revision. Asked whether the proposed nomenclature was a step in the right direction, 48% said they fully agreed, and another 39% said they partly agreed. Another question asked what feature should drive a drug’s top-line definition. Sixty percent of the audience said pharmacologic action, and an additional 29% voted in favor of clinical indication.
Dr. Zohar said he has been an adviser or consultant to or received research support from eight drug companies. Dr. Nutt said he has been an adviser or consultant to eight drug companies. Dr. Stahl said he has been an adviser or consultant to more than 30 drug companies. Dr. Goodwin said he had been an adviser or consultant to 12 drug companies.
On Twitter @mitchelzoler
BARCELONA – Neuropsychopharmacologists want to change the way you describe psychoactive drugs.
A team of representatives from four international neuropsychopharmacology groups is finishing a first draft of its proposed new system for drug classification and nomenclature that – in its current form – describes each of approximately 120 psychoactive drugs by five different parameters, or axes, including neurobiologic activity, efficacy, and approved indications worldwide. The goal is a new, Web-based framework for classifying and identifying drugs that is more consistent, informative, and transparent than the existing nomenclature.

"We expect drug nomenclature to reflect current scientific knowledge, give useful pharmacologic information to clinicians, and give patients a rationale for using a drug," but the existing nomenclature does not accomplish those goals, said Dr. Joseph Zohar, director of psychiatry at Sheba Medical Center in Tel Hashomer, Israel, and a member of the nomenclature group. "Existing nomenclature dates from the 1960s; no wonder it does not reflect current understanding. Drugs called antidepressants are often prescribed to patients with anxiety disorders. Patients ask, why give me an antidepressant if I have anxiety? Antipsychotics are prescribed to patients with depression, and they ask why they are getting an antipsychotic? The nomenclature is confusing," Dr. Zohar said at a session at the annual congress of the European College of Neuropsychopharmacology devoted to presenting the new nomenclature system to congress participants.
"We want to rationalize classification and have it better reflect science. We want to focus on mechanisms; knowing the entire profile of medications will help make more informed choices," he said.
"It’s getting people to think more about what drugs do. If people understood drugs better, they would use them better," said Dr. David J. Nutt, professor of neuropsychopharmacology at Imperial College London and another member of the nomenclature group. "The shorthands we currently use [for classifying psychoactive drugs] may be useful, but we need to determine what’s not useful" and get rid of it.
Leadership from the European College of Neuropsychopharmacology (ECNP) arranged for a panel with two representatives each from the American College of Neuropsychopharmacology, the International College of Neuropsychopharmacology, the Asian College of Neuropsychopharmacology, and the ECNP. A draft version of the nomenclature document should appear for comment on the ECNP website by early next year, and the current plan is to publish a finalized version before the end of next year. But the panel members stressed that since the document will primarily be Web-based, it will be open to frequent revision.
"This is the first step in a long process. We’re trying to wake up a 50-year-old nomenclature into a living document that will be used over the next decades and may someday merge with DSM [Diagnostic and Statistical Manual of Mental Disorders]," said Dr. Stephen M. Stahl, a panel member and psychiatrist affiliated with the University of California, San Diego.
The format the panel has in place currently describes every psychoactive drug in terms of five axes: class and relevant mechanisms; family; neurobiologic activity, neurotransmitter effects, and physiologic effects; efficacy and major side effects; and approved and licenses indications in the major countries and regions that perform drug licensing. Some of the collaborators on the panel published an explanation of the five axes template online in September (Euro. Neuropsychopharmacol. 2013 [doi:10.1016/j.euroneuro.2013.08.004]).
All this information for each drug "should tell you everything you need to know to make a rational decision about what to do with the drug," Dr. Nutt said.
"We need to be very humble about these medications and what they do. You have multiple things going on simultaneously," said Dr. Stahl, also founder of the Neuroscience Education Institute in San Diego. "The second-generation antipsychotics are the most complicated drugs in medicine; one patient’s side effect is another’s therapeutic effect.
"We propose that it is no longer appropriate to name all drugs that treat psychosis as ‘antipsychotics.’ We propose a multiaxial classification system based on pharmacologic mechanisms of action. A mechanism-based nomenclature may clarify the differing mechanisms of individual agents, especially actions in psychosis versus mood disorders. This approach has the potential to better inform those who work with drugs that treat depression, and prevent confusion with other drugs that treat both psychosis and multiple additional disorders such as depression and mania. This approach is also a strategy for naming drugs yet to be discovered that target novel mechanisms of action."
"What we don’t want is industry or a regulator to define a new glycine-reuptake blocker as an antipsychotic," Dr. Nutt said.
Several panel members stressed that the five-axis format will allow a diverse list of useful information.
"We don’t want to be constrained by the approved indications," Dr. Nutt said. "This is an opportunity to document off-label indications and to link to the evidence for off-label uses," said Dr. Guy Goodwin, chairman of psychiatry at the University of Oxford (England) and another collaborator on the panel.
The audience of about 150 congress attendees at the session seemed persuaded by the speakers. At the session’s end, in an electronic vote, 67% said they fully agreed, and another 26% said they partly agreed, that the existing nomenclature needs revision. Asked whether the proposed nomenclature was a step in the right direction, 48% said they fully agreed, and another 39% said they partly agreed. Another question asked what feature should drive a drug’s top-line definition. Sixty percent of the audience said pharmacologic action, and an additional 29% voted in favor of clinical indication.
Dr. Zohar said he has been an adviser or consultant to or received research support from eight drug companies. Dr. Nutt said he has been an adviser or consultant to eight drug companies. Dr. Stahl said he has been an adviser or consultant to more than 30 drug companies. Dr. Goodwin said he had been an adviser or consultant to 12 drug companies.
On Twitter @mitchelzoler
EXPERT ANALYSIS FROM THE ECNP CONGRESS