User login
An investigational smartphone app performed similarly to a transcutaneous bilirubinometer to screen for newborn jaundice in a pilot study of 100 babies. Measurements from the BiliCam app yielded a rank order correlation of 0.85 with the gold standard blood test to measure total serum bilirubin levels. Transcutaneous bilirubinometer readings had a rank order correlation of 0.92 with total serum bilirubin levels, according to data presented by investigators from the University of Washington, Seattle, at the Association for Computing Machinery’s International Joint Conference on Pervasive and Ubiquitous Computing (UbiComp2014).
The BiliCam had a mean error of 2 mg/dL and transcutaneous bilirubinometer readings had a mean error of 1.5 mg/dL, compared with total serum bilirubin levels, Dr. James W. Stout said in an interview.
The BiliCam “compared favorably” with transcutaneous bilirubinometry, according to the investigators, Dr. Stout and Dr. James A. Taylor, both professors of pediatrics at the university.
“Where we’re at now is very much a work in progress. It will almost certainly perform better as the data set grows” to 400-500 and eventually 1,000 babies, Dr. Stout said. “The way it performs now is more than anything a promising signal about how we think it can perform.”
Getting the BiliCam into the hands of clinicians in general within a year is an “optimistic” goal, and “I feel pretty confident we can have this out in the world in 2 years,” he said. Food and Drug Administration approval would be needed before parents could use the BiliCam at home, he added.
If it pans out, the BiliCam could be more accessible and less expensive than transcutaneous bilirubinometers, which cost $7,000-$8,000, he said. The expensive devices usually aren’t found in general pediatric offices and certainly aren’t used by many parents at home.
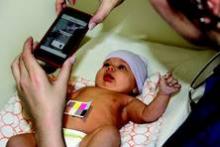
In the study, users downloaded the app to an iPhone 4, placed a business card–size color-calibration card on the baby’s belly, and triggered the app to take photos with the card in view. The app sent the data to the Cloud for analysis by algorithms, and a report on the newborn’s bilirubin levels was sent almost instantly to the user’s phone. All babies were tested between 2-5 days of age using the BiliCam, a transcutaneous bilirubinometer, and serum testing.
Although an estimated 84% of newborns develop jaundice, only approximately 20 U.S. babies per year develop kernicterus from severe jaundice, Dr. Stout said. Visual assessment, even by experienced clinicians, has been shown to be only moderately accurate, at best, for estimating the severity of jaundice, leading many parents to return to clinics with “yellow babies” who are not really at risk but may undergo blood draws, he said. “We want to reduce the churn.”
More importantly, the investigators hope that the BiliCam eventually will prove useful in resource-poor nations where kernicterus causes many newborn deaths, he added. Before that can happen, the BiliCam must be tested on more racially diverse populations so that the algorithms can extract the relevant wavelengths to incorporate into the app. “We don’t know yet that we can do that in really dark babies,” he said.
Other testing should begin within the year to adapt the BiliCam to other types of phones beyond iPhones. The investigator are exploring infrastructures for national and international trials.
Grants from the Coulter Foundation and the National Science Foundation funded the research. The investigators and the university have filed a patent on BiliCam, which is expected to generate future revenue from fees for the app or for processing photos, or from coverage by insurers or some other commercial model.
On Twitter @sherryboschert
An investigational smartphone app performed similarly to a transcutaneous bilirubinometer to screen for newborn jaundice in a pilot study of 100 babies. Measurements from the BiliCam app yielded a rank order correlation of 0.85 with the gold standard blood test to measure total serum bilirubin levels. Transcutaneous bilirubinometer readings had a rank order correlation of 0.92 with total serum bilirubin levels, according to data presented by investigators from the University of Washington, Seattle, at the Association for Computing Machinery’s International Joint Conference on Pervasive and Ubiquitous Computing (UbiComp2014).
The BiliCam had a mean error of 2 mg/dL and transcutaneous bilirubinometer readings had a mean error of 1.5 mg/dL, compared with total serum bilirubin levels, Dr. James W. Stout said in an interview.
The BiliCam “compared favorably” with transcutaneous bilirubinometry, according to the investigators, Dr. Stout and Dr. James A. Taylor, both professors of pediatrics at the university.
“Where we’re at now is very much a work in progress. It will almost certainly perform better as the data set grows” to 400-500 and eventually 1,000 babies, Dr. Stout said. “The way it performs now is more than anything a promising signal about how we think it can perform.”
Getting the BiliCam into the hands of clinicians in general within a year is an “optimistic” goal, and “I feel pretty confident we can have this out in the world in 2 years,” he said. Food and Drug Administration approval would be needed before parents could use the BiliCam at home, he added.
If it pans out, the BiliCam could be more accessible and less expensive than transcutaneous bilirubinometers, which cost $7,000-$8,000, he said. The expensive devices usually aren’t found in general pediatric offices and certainly aren’t used by many parents at home.
In the study, users downloaded the app to an iPhone 4, placed a business card–size color-calibration card on the baby’s belly, and triggered the app to take photos with the card in view. The app sent the data to the Cloud for analysis by algorithms, and a report on the newborn’s bilirubin levels was sent almost instantly to the user’s phone. All babies were tested between 2-5 days of age using the BiliCam, a transcutaneous bilirubinometer, and serum testing.
Although an estimated 84% of newborns develop jaundice, only approximately 20 U.S. babies per year develop kernicterus from severe jaundice, Dr. Stout said. Visual assessment, even by experienced clinicians, has been shown to be only moderately accurate, at best, for estimating the severity of jaundice, leading many parents to return to clinics with “yellow babies” who are not really at risk but may undergo blood draws, he said. “We want to reduce the churn.”
More importantly, the investigators hope that the BiliCam eventually will prove useful in resource-poor nations where kernicterus causes many newborn deaths, he added. Before that can happen, the BiliCam must be tested on more racially diverse populations so that the algorithms can extract the relevant wavelengths to incorporate into the app. “We don’t know yet that we can do that in really dark babies,” he said.
Other testing should begin within the year to adapt the BiliCam to other types of phones beyond iPhones. The investigator are exploring infrastructures for national and international trials.
Grants from the Coulter Foundation and the National Science Foundation funded the research. The investigators and the university have filed a patent on BiliCam, which is expected to generate future revenue from fees for the app or for processing photos, or from coverage by insurers or some other commercial model.
On Twitter @sherryboschert
An investigational smartphone app performed similarly to a transcutaneous bilirubinometer to screen for newborn jaundice in a pilot study of 100 babies. Measurements from the BiliCam app yielded a rank order correlation of 0.85 with the gold standard blood test to measure total serum bilirubin levels. Transcutaneous bilirubinometer readings had a rank order correlation of 0.92 with total serum bilirubin levels, according to data presented by investigators from the University of Washington, Seattle, at the Association for Computing Machinery’s International Joint Conference on Pervasive and Ubiquitous Computing (UbiComp2014).
The BiliCam had a mean error of 2 mg/dL and transcutaneous bilirubinometer readings had a mean error of 1.5 mg/dL, compared with total serum bilirubin levels, Dr. James W. Stout said in an interview.
The BiliCam “compared favorably” with transcutaneous bilirubinometry, according to the investigators, Dr. Stout and Dr. James A. Taylor, both professors of pediatrics at the university.
“Where we’re at now is very much a work in progress. It will almost certainly perform better as the data set grows” to 400-500 and eventually 1,000 babies, Dr. Stout said. “The way it performs now is more than anything a promising signal about how we think it can perform.”
Getting the BiliCam into the hands of clinicians in general within a year is an “optimistic” goal, and “I feel pretty confident we can have this out in the world in 2 years,” he said. Food and Drug Administration approval would be needed before parents could use the BiliCam at home, he added.
If it pans out, the BiliCam could be more accessible and less expensive than transcutaneous bilirubinometers, which cost $7,000-$8,000, he said. The expensive devices usually aren’t found in general pediatric offices and certainly aren’t used by many parents at home.
In the study, users downloaded the app to an iPhone 4, placed a business card–size color-calibration card on the baby’s belly, and triggered the app to take photos with the card in view. The app sent the data to the Cloud for analysis by algorithms, and a report on the newborn’s bilirubin levels was sent almost instantly to the user’s phone. All babies were tested between 2-5 days of age using the BiliCam, a transcutaneous bilirubinometer, and serum testing.
Although an estimated 84% of newborns develop jaundice, only approximately 20 U.S. babies per year develop kernicterus from severe jaundice, Dr. Stout said. Visual assessment, even by experienced clinicians, has been shown to be only moderately accurate, at best, for estimating the severity of jaundice, leading many parents to return to clinics with “yellow babies” who are not really at risk but may undergo blood draws, he said. “We want to reduce the churn.”
More importantly, the investigators hope that the BiliCam eventually will prove useful in resource-poor nations where kernicterus causes many newborn deaths, he added. Before that can happen, the BiliCam must be tested on more racially diverse populations so that the algorithms can extract the relevant wavelengths to incorporate into the app. “We don’t know yet that we can do that in really dark babies,” he said.
Other testing should begin within the year to adapt the BiliCam to other types of phones beyond iPhones. The investigator are exploring infrastructures for national and international trials.
Grants from the Coulter Foundation and the National Science Foundation funded the research. The investigators and the university have filed a patent on BiliCam, which is expected to generate future revenue from fees for the app or for processing photos, or from coverage by insurers or some other commercial model.
On Twitter @sherryboschert