User login
In the 11 studies that have examined the incidence of leiomyosarcoma (LMS) since the 2014 communications by the US Food and Drug Administration (FDA),1 Dr. Matthew Siedhoff and colleagues found that the cumulative LMS incidence in 318,006 women was 0.0017%, or approximately 1 in 600. This is according to data Dr. Seidhoff presented November 16, 2016, at the 45th annual AAGL Global Congress on MIGS.2 This reported risk is smaller than the 1 in 350 cited by the FDA in its 2014 notice.1 Dr. Seidhoff concluded that, particularly in women aged younger than 50 years, minimally invasive hysterectomy remains a safe option for the informed patient to consider.
Regardless of the incidence of unsuspected LMS found in the studies published since 2014, what have been the practice changes among gynecologic surgeons as a result of the FDA’s 2014 actions? Kerac N. Falk, MD, explored this question with his colleagues at the Icahn School of Medicine in New York, New York. He presented these findings to AAGL congress attendees on November 16 in Orlando, Florida.3 Notably, of 197 responders to a survey sent to members of the Society of Gynecologic Oncologists, 12.5% reported decreasing their use of power morcellation. A full 38.8% reported discontinuing altogether the use of a power morcellator.
One in 5 (20%) of gyn oncologists previously using power morcellation reported switching to laparotomy. Importantly, the drive for the switch was “driven by media, patient request, and the FDA rather than physician choice,” Falk said.
What if physicians, and patients, have another choice?
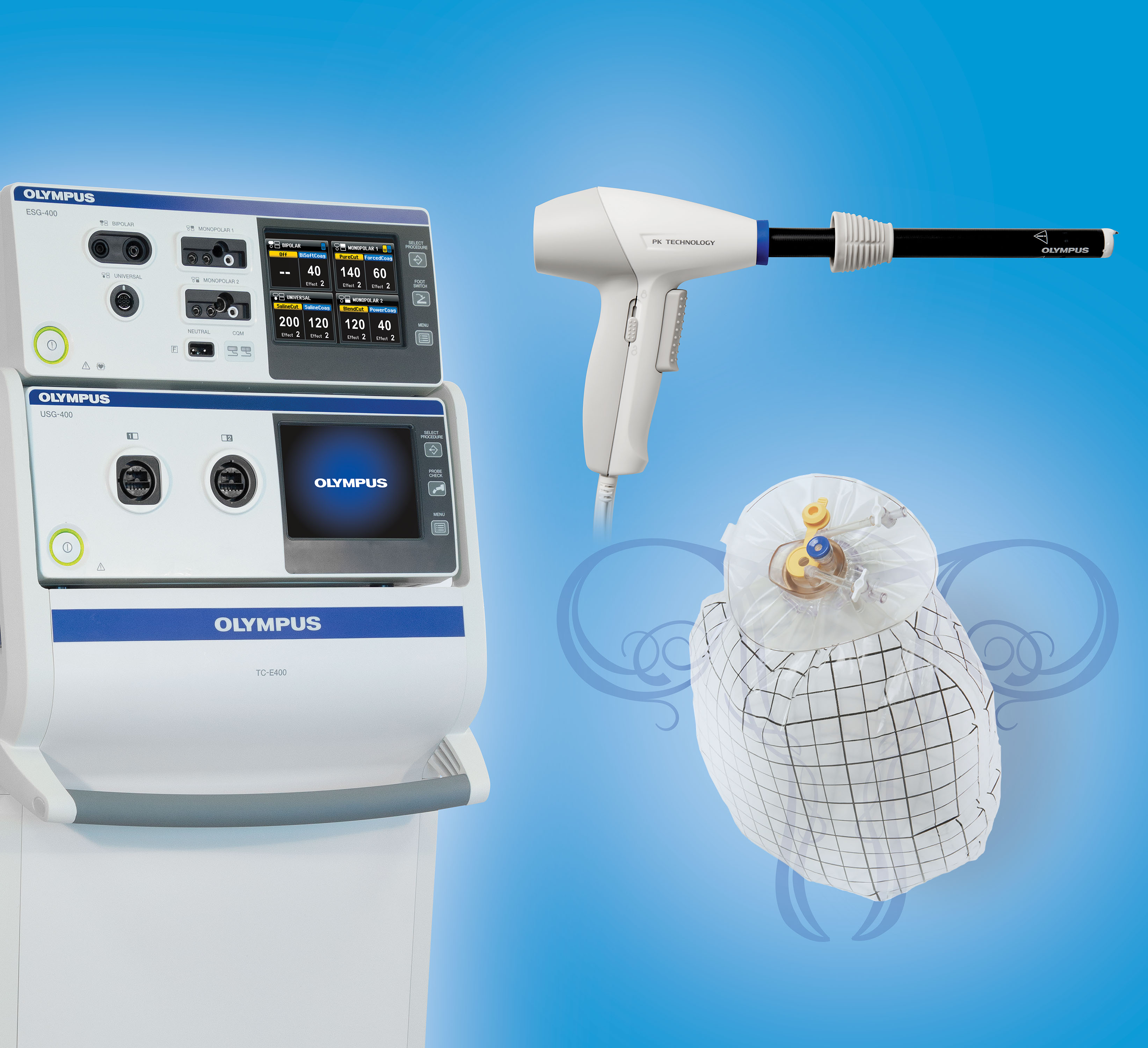
Here at the AAGL meeting, Olympus announced the FDA clearance, marketing, and initial training on its contained tissue extraction system—the PneumoLiner containment device and its accompanying laparoscopic PK Morcellator (FIGURE).
Both tools, FDA cleared in April and October 2016, respectively, provide low-risk, appropriate women with “an improved safety device,” said Jubilee Brown, MD, AAGL representative on power morcellation to the FDA and Associate Director of Gynecologic Oncology at the Levine Cancer Institute of the Carolinas HealthCare System in Charlotte, North Carolina.
“We have found at our institution that we have had to do more opens and minilaps on patients without the option for power morcellation, and this new device offers a way for us not to have to do that. Minimally invasive surgery stays truly minimally invasive surgery,” said Dr. Brown.
“Although I have a handful of patients who have chosen open surgery over minimally invasive surgery when MIS was appropriate, by and large most patients prefer the minimally invasive approach to surgery—especially when they learn that they have reduced risk for complications, blood loss, and pain; will be back to normal life faster; and will have less cosmetic incisions,” said Dr. Brown. “We all have tried workarounds, but for low-risk, appropriate patients under FDA guidelines, this new device by Olympus brings us back to minimally invasive options, and that is a good thing.”
How does the Olympus containment system work?
The PneumoLiner is the first containment device to receive FDA market clearance that is designed for use with certain laparoscopic morcellators to isolate uterine tissue that is not suspected to contain cancer.4
The containment bag and morcellator are not indicated for use in women with tissue that is known or suspected to contain malignancy; and should not be used for removal of uterine tissue containing suspected fibroids in patients who are peri- or post-menopausal, or candidates for en bloc tissue removal vaginally or via mini-laparotomy.4
When insufflated, the PneumoLiner bag allows for space and surgeon visibility throughout the power morcellation procedure while maintaining a barrier to the escape of fluids, cells, and tissue fragments. The bag creates a barrier between the targeted tissue and nontargeted abdominal contents, minimizing the risk of inadvertent damage to adjacent structures, says Olympus.4 See this video, which demonstrates the procedure.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Filtration, immersion, and stress testing were conducted to demonstrate the PneumoLiner as impermeable to human cells, that it maintains its integrity following morcellation, and that its mechanical strength withholds forces in excess of those demonstrated during actual use.5 To support FDA 510 clearance of the PK Morcellator, Olympus performed additional testing with both devices used together, demonstrating compatibility.4
Surgeon training on new device use is rigid, comprehensive, and not to be overlooked
“This is one of the rare times in my career when, right out of the gate, we have a very rigid and comprehensive way of educating and training physicians on the proper utilization of a new technology (avoiding the cart-before-the-horse phenomenon, in which a clinician is given the tools without the proper education),” said AAGL immediate past president Arnold Advincula, MD, “and it is important to underscore that.” Dr. Advincula is the Levine Family Professor, Vice-Chair of Women’s Health, and Chief of Gynecology at Sloane Women’s Hospital, Columbia University Medical Center/New York-Presbyterian Hospital, New York, New York.
Training plans
Instead of its sales force, Olympus is having surgeons train other surgeons. Clinical Education Specialists, a small, select group of individuals within the company, also will be able to train. The required training follows a rigid protocol that was validated and submitted to the FDA as part of the approval process, said Jerilyn Hitchings, Director of Procedure Marketing at Olympus. After training up to 40 surgeons in the past 3 weeks, Olympus expects to train another 100 at AAGL.
“We have strict criteria for training, including that they are advanced laparoscopists,” fulfilling a minimum number of surgeries within a 90-day period, said Hitchings.
The 1-hour training requires surgeons to perform a 4-step protocol and demonstrate unaided proficiency at training conclusion. Olympus will not sell its product to a facility until an advanced surgeon has been trained there, according to Hitchings. And many physicians are approaching their institutions inquiring about training now, she reports.
Applications for training will be submitted to the Olympus Professional Education Team for approval. “We want this to go well. We know that there are many physicians who are hoping that this becomes a good reality, and we want to make sure that it is done properly. The training process adheres to that agenda.”
Having the technology is step 1
OBG Management Board of Editors member Dr. Advincula struck an optimistic tone in his June 2016 Guest Editorial for the journal,6 indicating his belief that the “tissue morcellation pendulum,” which has swung toward non−minimally invasive approaches since 2014, can change direction. At the Olympus containment system official introduction event at AAGL, he expressed the same optimism:
“We now have technology that has the promise of being able to deliver to women a safe way to undergo tissue extraction, to undergo a minimally invasive surgical procedure. …When you combine innovation and education together, you ultimately are going to advance MIS worldwide. With all the things going on around the world today, I think it is important that women be able to have a choice, an autonomous choice with her physician. Having the technology that allows physicians to extract tissue safely, to give women the option of undergoing a minimally invasive surgery, is critically important. I look forward to seeing how we can continue to advance surgery. Partnership with clinicians and with industry, such as Olympus, is going to be key to the success of how we advance women’s health care in general.”
What do AAGL attendees have to say about it?
“It’s a good thing for the minimally invasive market,” said John B. Gebhart, MD, MS, vaginal hysterectomy representative at the star-studded Operating with the Stars event on Thursday, November 17, and Professor of Obstetrics and Gynecology at the Mayo Clinic in Rochester, Minnesota.
May Thomassee, MD, who practices in Lafayette, Louisiana, said that, for the past 3 years, she has been performing extracorporeal morcellation at her institution. “I think the new containment bag and morcellator devices that are attempting to be safer are a very good thing; however, my concern of introducing this new technology, such as bags, is that it may increase the cost within our health care system. We have had great success, and patients feel that they have options when we offer them—after informed consent and appropriate preoperative workup—an abdominal, vaginal, or extracorporeal morcellation approach.”
As a developer and proponent of the Extracorporeal Tissue Extraction (ExCITE) technique, Mireille Truong, MD, who demonstrated the ExCITE surgical approach with Dr. Advincula as part of the Research and Science Plenary here at AAGL, said, “I think the PneumoLiner is a great example of creativity and innovation. It’s good that we now have an FDA-approved device to offer and counsel patients as one of many options.” She added that, overall, the morcellation controversy has been “a positive experience. We have learned better as a community about how to approach new technology.”
- US Food and Drug Administration. Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication. April 17, 2014. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm393576.htm. Updated November 24, 2014. Accessed November 17, 2016.
- Siedhoff MT, Doll KM, Rutstein SE, et al. Laparoscopic hysterectomy with morcellation versus abdominal hysterectomy for presumed uterine leiomyomata: an updated decision analysis. J Minim Invasiv Gynecol. 2016;23(7):S4-S5.
- Mandelberger AH, Mathews S, Chuang L. Practice changes in power morcellation among gynecologic-oncologists since 2014. J Minim Invasiv Gynecol. 2016;23(7):S3.
Olympus introduces first-of-its-kind contained tissue extraction system, restoring healthcare option for gynecologists and women [press release]. November 16, 2016. Olympus website. http://www.olympusamerica.com/corporate/corp_presscenter_headline.asp?pressNo=2079. Accessed November 16, 2016.
- FDA allows marketing of first-of-kind tissue containment system for use with certain laparoscopic power morcellators in select patients. FDA website. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm494650.htm. Updated April 7, 2016. Accessed November 16, 2016.
- Advincula AP. Tissue extraction: can the pendulum change direction? OBG Manag. 2016;28(6):8, 10, 12.
In the 11 studies that have examined the incidence of leiomyosarcoma (LMS) since the 2014 communications by the US Food and Drug Administration (FDA),1 Dr. Matthew Siedhoff and colleagues found that the cumulative LMS incidence in 318,006 women was 0.0017%, or approximately 1 in 600. This is according to data Dr. Seidhoff presented November 16, 2016, at the 45th annual AAGL Global Congress on MIGS.2 This reported risk is smaller than the 1 in 350 cited by the FDA in its 2014 notice.1 Dr. Seidhoff concluded that, particularly in women aged younger than 50 years, minimally invasive hysterectomy remains a safe option for the informed patient to consider.
Regardless of the incidence of unsuspected LMS found in the studies published since 2014, what have been the practice changes among gynecologic surgeons as a result of the FDA’s 2014 actions? Kerac N. Falk, MD, explored this question with his colleagues at the Icahn School of Medicine in New York, New York. He presented these findings to AAGL congress attendees on November 16 in Orlando, Florida.3 Notably, of 197 responders to a survey sent to members of the Society of Gynecologic Oncologists, 12.5% reported decreasing their use of power morcellation. A full 38.8% reported discontinuing altogether the use of a power morcellator.
One in 5 (20%) of gyn oncologists previously using power morcellation reported switching to laparotomy. Importantly, the drive for the switch was “driven by media, patient request, and the FDA rather than physician choice,” Falk said.
What if physicians, and patients, have another choice?
Here at the AAGL meeting, Olympus announced the FDA clearance, marketing, and initial training on its contained tissue extraction system—the PneumoLiner containment device and its accompanying laparoscopic PK Morcellator (FIGURE).

Both tools, FDA cleared in April and October 2016, respectively, provide low-risk, appropriate women with “an improved safety device,” said Jubilee Brown, MD, AAGL representative on power morcellation to the FDA and Associate Director of Gynecologic Oncology at the Levine Cancer Institute of the Carolinas HealthCare System in Charlotte, North Carolina.
“We have found at our institution that we have had to do more opens and minilaps on patients without the option for power morcellation, and this new device offers a way for us not to have to do that. Minimally invasive surgery stays truly minimally invasive surgery,” said Dr. Brown.
“Although I have a handful of patients who have chosen open surgery over minimally invasive surgery when MIS was appropriate, by and large most patients prefer the minimally invasive approach to surgery—especially when they learn that they have reduced risk for complications, blood loss, and pain; will be back to normal life faster; and will have less cosmetic incisions,” said Dr. Brown. “We all have tried workarounds, but for low-risk, appropriate patients under FDA guidelines, this new device by Olympus brings us back to minimally invasive options, and that is a good thing.”
How does the Olympus containment system work?
The PneumoLiner is the first containment device to receive FDA market clearance that is designed for use with certain laparoscopic morcellators to isolate uterine tissue that is not suspected to contain cancer.4
The containment bag and morcellator are not indicated for use in women with tissue that is known or suspected to contain malignancy; and should not be used for removal of uterine tissue containing suspected fibroids in patients who are peri- or post-menopausal, or candidates for en bloc tissue removal vaginally or via mini-laparotomy.4
When insufflated, the PneumoLiner bag allows for space and surgeon visibility throughout the power morcellation procedure while maintaining a barrier to the escape of fluids, cells, and tissue fragments. The bag creates a barrier between the targeted tissue and nontargeted abdominal contents, minimizing the risk of inadvertent damage to adjacent structures, says Olympus.4 See this video, which demonstrates the procedure.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Filtration, immersion, and stress testing were conducted to demonstrate the PneumoLiner as impermeable to human cells, that it maintains its integrity following morcellation, and that its mechanical strength withholds forces in excess of those demonstrated during actual use.5 To support FDA 510 clearance of the PK Morcellator, Olympus performed additional testing with both devices used together, demonstrating compatibility.4
Surgeon training on new device use is rigid, comprehensive, and not to be overlooked
“This is one of the rare times in my career when, right out of the gate, we have a very rigid and comprehensive way of educating and training physicians on the proper utilization of a new technology (avoiding the cart-before-the-horse phenomenon, in which a clinician is given the tools without the proper education),” said AAGL immediate past president Arnold Advincula, MD, “and it is important to underscore that.” Dr. Advincula is the Levine Family Professor, Vice-Chair of Women’s Health, and Chief of Gynecology at Sloane Women’s Hospital, Columbia University Medical Center/New York-Presbyterian Hospital, New York, New York.
Training plans
Instead of its sales force, Olympus is having surgeons train other surgeons. Clinical Education Specialists, a small, select group of individuals within the company, also will be able to train. The required training follows a rigid protocol that was validated and submitted to the FDA as part of the approval process, said Jerilyn Hitchings, Director of Procedure Marketing at Olympus. After training up to 40 surgeons in the past 3 weeks, Olympus expects to train another 100 at AAGL.
“We have strict criteria for training, including that they are advanced laparoscopists,” fulfilling a minimum number of surgeries within a 90-day period, said Hitchings.
The 1-hour training requires surgeons to perform a 4-step protocol and demonstrate unaided proficiency at training conclusion. Olympus will not sell its product to a facility until an advanced surgeon has been trained there, according to Hitchings. And many physicians are approaching their institutions inquiring about training now, she reports.
Applications for training will be submitted to the Olympus Professional Education Team for approval. “We want this to go well. We know that there are many physicians who are hoping that this becomes a good reality, and we want to make sure that it is done properly. The training process adheres to that agenda.”
Having the technology is step 1
OBG Management Board of Editors member Dr. Advincula struck an optimistic tone in his June 2016 Guest Editorial for the journal,6 indicating his belief that the “tissue morcellation pendulum,” which has swung toward non−minimally invasive approaches since 2014, can change direction. At the Olympus containment system official introduction event at AAGL, he expressed the same optimism:
“We now have technology that has the promise of being able to deliver to women a safe way to undergo tissue extraction, to undergo a minimally invasive surgical procedure. …When you combine innovation and education together, you ultimately are going to advance MIS worldwide. With all the things going on around the world today, I think it is important that women be able to have a choice, an autonomous choice with her physician. Having the technology that allows physicians to extract tissue safely, to give women the option of undergoing a minimally invasive surgery, is critically important. I look forward to seeing how we can continue to advance surgery. Partnership with clinicians and with industry, such as Olympus, is going to be key to the success of how we advance women’s health care in general.”
What do AAGL attendees have to say about it?
“It’s a good thing for the minimally invasive market,” said John B. Gebhart, MD, MS, vaginal hysterectomy representative at the star-studded Operating with the Stars event on Thursday, November 17, and Professor of Obstetrics and Gynecology at the Mayo Clinic in Rochester, Minnesota.
May Thomassee, MD, who practices in Lafayette, Louisiana, said that, for the past 3 years, she has been performing extracorporeal morcellation at her institution. “I think the new containment bag and morcellator devices that are attempting to be safer are a very good thing; however, my concern of introducing this new technology, such as bags, is that it may increase the cost within our health care system. We have had great success, and patients feel that they have options when we offer them—after informed consent and appropriate preoperative workup—an abdominal, vaginal, or extracorporeal morcellation approach.”
As a developer and proponent of the Extracorporeal Tissue Extraction (ExCITE) technique, Mireille Truong, MD, who demonstrated the ExCITE surgical approach with Dr. Advincula as part of the Research and Science Plenary here at AAGL, said, “I think the PneumoLiner is a great example of creativity and innovation. It’s good that we now have an FDA-approved device to offer and counsel patients as one of many options.” She added that, overall, the morcellation controversy has been “a positive experience. We have learned better as a community about how to approach new technology.”
In the 11 studies that have examined the incidence of leiomyosarcoma (LMS) since the 2014 communications by the US Food and Drug Administration (FDA),1 Dr. Matthew Siedhoff and colleagues found that the cumulative LMS incidence in 318,006 women was 0.0017%, or approximately 1 in 600. This is according to data Dr. Seidhoff presented November 16, 2016, at the 45th annual AAGL Global Congress on MIGS.2 This reported risk is smaller than the 1 in 350 cited by the FDA in its 2014 notice.1 Dr. Seidhoff concluded that, particularly in women aged younger than 50 years, minimally invasive hysterectomy remains a safe option for the informed patient to consider.
Regardless of the incidence of unsuspected LMS found in the studies published since 2014, what have been the practice changes among gynecologic surgeons as a result of the FDA’s 2014 actions? Kerac N. Falk, MD, explored this question with his colleagues at the Icahn School of Medicine in New York, New York. He presented these findings to AAGL congress attendees on November 16 in Orlando, Florida.3 Notably, of 197 responders to a survey sent to members of the Society of Gynecologic Oncologists, 12.5% reported decreasing their use of power morcellation. A full 38.8% reported discontinuing altogether the use of a power morcellator.
One in 5 (20%) of gyn oncologists previously using power morcellation reported switching to laparotomy. Importantly, the drive for the switch was “driven by media, patient request, and the FDA rather than physician choice,” Falk said.
What if physicians, and patients, have another choice?
Here at the AAGL meeting, Olympus announced the FDA clearance, marketing, and initial training on its contained tissue extraction system—the PneumoLiner containment device and its accompanying laparoscopic PK Morcellator (FIGURE).

Both tools, FDA cleared in April and October 2016, respectively, provide low-risk, appropriate women with “an improved safety device,” said Jubilee Brown, MD, AAGL representative on power morcellation to the FDA and Associate Director of Gynecologic Oncology at the Levine Cancer Institute of the Carolinas HealthCare System in Charlotte, North Carolina.
“We have found at our institution that we have had to do more opens and minilaps on patients without the option for power morcellation, and this new device offers a way for us not to have to do that. Minimally invasive surgery stays truly minimally invasive surgery,” said Dr. Brown.
“Although I have a handful of patients who have chosen open surgery over minimally invasive surgery when MIS was appropriate, by and large most patients prefer the minimally invasive approach to surgery—especially when they learn that they have reduced risk for complications, blood loss, and pain; will be back to normal life faster; and will have less cosmetic incisions,” said Dr. Brown. “We all have tried workarounds, but for low-risk, appropriate patients under FDA guidelines, this new device by Olympus brings us back to minimally invasive options, and that is a good thing.”
How does the Olympus containment system work?
The PneumoLiner is the first containment device to receive FDA market clearance that is designed for use with certain laparoscopic morcellators to isolate uterine tissue that is not suspected to contain cancer.4
The containment bag and morcellator are not indicated for use in women with tissue that is known or suspected to contain malignancy; and should not be used for removal of uterine tissue containing suspected fibroids in patients who are peri- or post-menopausal, or candidates for en bloc tissue removal vaginally or via mini-laparotomy.4
When insufflated, the PneumoLiner bag allows for space and surgeon visibility throughout the power morcellation procedure while maintaining a barrier to the escape of fluids, cells, and tissue fragments. The bag creates a barrier between the targeted tissue and nontargeted abdominal contents, minimizing the risk of inadvertent damage to adjacent structures, says Olympus.4 See this video, which demonstrates the procedure.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Filtration, immersion, and stress testing were conducted to demonstrate the PneumoLiner as impermeable to human cells, that it maintains its integrity following morcellation, and that its mechanical strength withholds forces in excess of those demonstrated during actual use.5 To support FDA 510 clearance of the PK Morcellator, Olympus performed additional testing with both devices used together, demonstrating compatibility.4
Surgeon training on new device use is rigid, comprehensive, and not to be overlooked
“This is one of the rare times in my career when, right out of the gate, we have a very rigid and comprehensive way of educating and training physicians on the proper utilization of a new technology (avoiding the cart-before-the-horse phenomenon, in which a clinician is given the tools without the proper education),” said AAGL immediate past president Arnold Advincula, MD, “and it is important to underscore that.” Dr. Advincula is the Levine Family Professor, Vice-Chair of Women’s Health, and Chief of Gynecology at Sloane Women’s Hospital, Columbia University Medical Center/New York-Presbyterian Hospital, New York, New York.
Training plans
Instead of its sales force, Olympus is having surgeons train other surgeons. Clinical Education Specialists, a small, select group of individuals within the company, also will be able to train. The required training follows a rigid protocol that was validated and submitted to the FDA as part of the approval process, said Jerilyn Hitchings, Director of Procedure Marketing at Olympus. After training up to 40 surgeons in the past 3 weeks, Olympus expects to train another 100 at AAGL.
“We have strict criteria for training, including that they are advanced laparoscopists,” fulfilling a minimum number of surgeries within a 90-day period, said Hitchings.
The 1-hour training requires surgeons to perform a 4-step protocol and demonstrate unaided proficiency at training conclusion. Olympus will not sell its product to a facility until an advanced surgeon has been trained there, according to Hitchings. And many physicians are approaching their institutions inquiring about training now, she reports.
Applications for training will be submitted to the Olympus Professional Education Team for approval. “We want this to go well. We know that there are many physicians who are hoping that this becomes a good reality, and we want to make sure that it is done properly. The training process adheres to that agenda.”
Having the technology is step 1
OBG Management Board of Editors member Dr. Advincula struck an optimistic tone in his June 2016 Guest Editorial for the journal,6 indicating his belief that the “tissue morcellation pendulum,” which has swung toward non−minimally invasive approaches since 2014, can change direction. At the Olympus containment system official introduction event at AAGL, he expressed the same optimism:
“We now have technology that has the promise of being able to deliver to women a safe way to undergo tissue extraction, to undergo a minimally invasive surgical procedure. …When you combine innovation and education together, you ultimately are going to advance MIS worldwide. With all the things going on around the world today, I think it is important that women be able to have a choice, an autonomous choice with her physician. Having the technology that allows physicians to extract tissue safely, to give women the option of undergoing a minimally invasive surgery, is critically important. I look forward to seeing how we can continue to advance surgery. Partnership with clinicians and with industry, such as Olympus, is going to be key to the success of how we advance women’s health care in general.”
What do AAGL attendees have to say about it?
“It’s a good thing for the minimally invasive market,” said John B. Gebhart, MD, MS, vaginal hysterectomy representative at the star-studded Operating with the Stars event on Thursday, November 17, and Professor of Obstetrics and Gynecology at the Mayo Clinic in Rochester, Minnesota.
May Thomassee, MD, who practices in Lafayette, Louisiana, said that, for the past 3 years, she has been performing extracorporeal morcellation at her institution. “I think the new containment bag and morcellator devices that are attempting to be safer are a very good thing; however, my concern of introducing this new technology, such as bags, is that it may increase the cost within our health care system. We have had great success, and patients feel that they have options when we offer them—after informed consent and appropriate preoperative workup—an abdominal, vaginal, or extracorporeal morcellation approach.”
As a developer and proponent of the Extracorporeal Tissue Extraction (ExCITE) technique, Mireille Truong, MD, who demonstrated the ExCITE surgical approach with Dr. Advincula as part of the Research and Science Plenary here at AAGL, said, “I think the PneumoLiner is a great example of creativity and innovation. It’s good that we now have an FDA-approved device to offer and counsel patients as one of many options.” She added that, overall, the morcellation controversy has been “a positive experience. We have learned better as a community about how to approach new technology.”
- US Food and Drug Administration. Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication. April 17, 2014. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm393576.htm. Updated November 24, 2014. Accessed November 17, 2016.
- Siedhoff MT, Doll KM, Rutstein SE, et al. Laparoscopic hysterectomy with morcellation versus abdominal hysterectomy for presumed uterine leiomyomata: an updated decision analysis. J Minim Invasiv Gynecol. 2016;23(7):S4-S5.
- Mandelberger AH, Mathews S, Chuang L. Practice changes in power morcellation among gynecologic-oncologists since 2014. J Minim Invasiv Gynecol. 2016;23(7):S3.
Olympus introduces first-of-its-kind contained tissue extraction system, restoring healthcare option for gynecologists and women [press release]. November 16, 2016. Olympus website. http://www.olympusamerica.com/corporate/corp_presscenter_headline.asp?pressNo=2079. Accessed November 16, 2016.
- FDA allows marketing of first-of-kind tissue containment system for use with certain laparoscopic power morcellators in select patients. FDA website. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm494650.htm. Updated April 7, 2016. Accessed November 16, 2016.
- Advincula AP. Tissue extraction: can the pendulum change direction? OBG Manag. 2016;28(6):8, 10, 12.
- US Food and Drug Administration. Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication. April 17, 2014. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm393576.htm. Updated November 24, 2014. Accessed November 17, 2016.
- Siedhoff MT, Doll KM, Rutstein SE, et al. Laparoscopic hysterectomy with morcellation versus abdominal hysterectomy for presumed uterine leiomyomata: an updated decision analysis. J Minim Invasiv Gynecol. 2016;23(7):S4-S5.
- Mandelberger AH, Mathews S, Chuang L. Practice changes in power morcellation among gynecologic-oncologists since 2014. J Minim Invasiv Gynecol. 2016;23(7):S3.
Olympus introduces first-of-its-kind contained tissue extraction system, restoring healthcare option for gynecologists and women [press release]. November 16, 2016. Olympus website. http://www.olympusamerica.com/corporate/corp_presscenter_headline.asp?pressNo=2079. Accessed November 16, 2016.
- FDA allows marketing of first-of-kind tissue containment system for use with certain laparoscopic power morcellators in select patients. FDA website. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm494650.htm. Updated April 7, 2016. Accessed November 16, 2016.
- Advincula AP. Tissue extraction: can the pendulum change direction? OBG Manag. 2016;28(6):8, 10, 12.