User login
Although the definition of Parkinson disease (PD) is based on the presence of motor features, these are just the “tip of the iceberg.” Nonmotor manifestations are nearly ubiquitous in PD, with behavior problems often being the most malignant. Almost all patients with PD have nonmotor and neuropsychiatric features, including sleep disturbances, compulsive and impulsive behaviors, autonomic dysfunction, and psychosis.
The neuropsychiatric and behavioral features of PD can be classified as intrinsic features, which occur as part of PD, and iatrogenic features, which are complications that arise from treatments used to manage the motor symptoms of PD.
DEMENTIA IN PD
An intrinsic nonmotor feature of PD is dementia, which occurs at a rate four to six times greater in patients with PD than in age-matched controls without PD.1 The prevalence of dementia in PD varies among studies and depends on the demographics of the population being studied. The cross-sectional prevalence of dementia is 40% in patients with PD.2 Seventy-eight percent of a population-based, representative cohort of patients with PD developed dementia during an 8-year study period.3
Dementia is a burden to the caregiver, the patient, and society. Cognitive and behavioral symptoms in patients with PD are the greatest contributors to caregiver distress.4 Dementia and associated behavioral symptoms (ie, hallucinations) hasten nursing home placement, contributing to the financial burden of caring for patients with PD.5 The risk of mortality is increased when dementia develops.6
PSYCHOTIC SYMPTOMS IN PD: AN EFFECT OF EXCESS DOPAMINE STIMULATION
Most of the complications observed in PD can be explained by the dopamine effect of medications and by dopamine deficiencies. An excess of dopamine stimulation caused by administration of prodopaminergic agents manifests as dyskinesias, hallucinations, or delusions. Withdrawal of levodopa will reverse these complications but leads to dopamine deficiency and thus a worsening of PD symptoms. Most patients with PD will tolerate mild dyskinesias or hallucinations if their PD symptoms are well controlled.
The hallucinations in PD tend to be visual as opposed to auditory (as in schizophrenia). They are usually benign and involve figures of people, furry animals, or complex scenes. About 10% to 40% of hallucinations in PD are secondary auditory hallucinations, which tend to be nondistinct, non-paranoid, and often incomprehensible (ie, voices in a crowd).
In the same way, the delusions experienced in patients with PD are distinct from those in schizophrenia. The delusions in PD are usually paranoid in nature and involve stereotyped themes (ie, spousal infidelity, feelings of abandonment) rather than the grandiose delusions that are common in schizophrenia.
The reported prevalence of psychotic symptoms in PD, including hallucinations and delusions, ranges from 20% to 50%.8,9 Auditory hallucinations are a feature in about 10%, and they usually occur with visual hallucinations. Less common are delusions and hallucinations with loss of insight, which are more likely with increasing severity of dementia.
Once a PD patient experiences hallucinations, they are likely to continue. In a 6-year longitudinal study, the prevalence of hallucinations increased from 33% at baseline to 55% at 72 months.10 Persistent psychosis was found in 69% of participants in the Psychosis and Clozapine in PD Study (PSYCLOPS) with 26 months of follow-up.11
High caregiver burden
Psychotic symptoms in PD are associated with high caregiver stress and increased rates of nursing home placement. Goetz et al12 showed that PD patients with psychosis had a much greater risk of nursing home placement than those without psychosis. The prognosis for PD patients in extended-care facilities is worse for those with psychotic symptoms.13
Management of psychotic symptoms
The first step in managing psychosis in PD is to rule out other causes of changes in mental status, such as infection, electrolyte imbalance, or introduction of new medications.
Adjusting anti-PD medications to a tolerable yet effective dose may help to reduce the incidence and severity of psychotic complications. If necessary, selective discontinuation of anti-PD medications may be tried in the following sequence: anticholinergics, amantadine, monoamine oxidase B inhibitors, dopamine agonists, catechol-O-methyltransferase inhibitors, and levodopa/carbidopa.
If motor symptoms prevent dosage minimization or discontinuation of some medications, then the addition of an atypical antipsychotic medication should be considered. Before the advent of atypical antipsychotics, the management of psychosis and hallucinations in PD was unsatisfactory, reflected by a mortality of 100% within 2 years among psychotic PD patients placed in nursing homes compared with 32% among age-matched community dwellers.13 The introduction of atypical antipsychotics has improved survival among PD patients with psychosis. In one study, mortality over 5 years was 44% among PD patients taking long-term clozapine for the treatment of psychosis.14 Recurrence of psychosis is rapid (within 8 weeks) even when PD patients are slowly weaned from atypical antipsychotics.15
Receptor affinities differ among antipsychotics. Because dopamine has been implicated as the principal neurotransmitter in the development of PD psychosis, atypical antipsychotics, with milder dopamine-blocking action, have played a central role in the treatment of PD psychosis. The dopamine D2 receptor is the main target for conventional antipsychotic drugs to exert their clinical effects. Atypical antipsychotics have different affinities for the D2 receptors.16 Occupancy of D2 receptors with atypical antipsychotics is 40% to 70% (risperidone and olanzapine have higher affinity for the D2 receptor than clozapine and quetiapine), and affinity for 5-HT2A receptors can be as high as 70%. This affinity for 5-HT2A receptors relative to D2 receptors may be important for therapeutic efficacy of the atypical antipsychotics. Antagonism of muscarinic, histaminergic, noradrenergic, and other serotonergic receptors also differs among the atypical antipsychotics.
Clozapine remains the gold standard atypical antipsychotic agent, based on results from three relatively small (N = 6 to 60) double-blind, placebo-controlled studies in PD patients with dopaminergic drug-induced psychosis.17–19 Quetiapine improved psychotic symptoms associated with PD in several open-label studies, but has not demonstrated the same success in double-blind clinical trials.20,21
Loss of cholinergic neurons and implications for treatment. In autopsy studies, the loss of cholinergic neurons is more profound in PD than in Alzheimer disease, which suggests that procholinergic drugs may improve symptoms of PD dementia, a major risk factor for hallucinations. In open-label studies, acetylcholinesterase inhibitors have reduced the frequency of hallucinations in patients who have dementia with Lewy bodies (DLB) and in patients with PD dementia. Double-blind trials of patients with DLB and PD dementia concentrated on the effect of cholinesterase inhibitors on dementia and not hallucinations. One concern with the use of a procholinergic drug in patients with PD has been worsening of parkinsonism, but studies of acetylcholinesterase inhibitors have shown no worsening of parkinsonism and only transient worsening of tremor.
Ondansetron, a 5-HT3 receptor antagonist used as an antinausea medication, produced moderate improvements in hallucinations and delusions in an open-label trial for the treatment of psychosis in advanced PD.22 For PD patients with psychosis and comorbid depression, antidepressant therapy and electroconvulsive therapy may be effective options.23,24
MOOD DISTURBANCES IN PD
Depression and apathy occur more frequently in patients with PD than in those who do not have PD.
Depression
Challenges in the management of depression in PD include recognition of depression and distinguishing depressive disorders from mood fluctuations. Whereas a depressive disorder lasts from weeks to years and can occur at any stage of illness, mood fluctuations can change many times daily and appear as nonmotor manifestations during the “off” medication state. Mood fluctuations occur mostly in patients who have developed motor fluctuations. The implication for treatment is that the treatment strategy for a depressive disorder is antidepressant therapy, whereas the strategy for mood fluctuations in PD is to increase the levodopa dose.
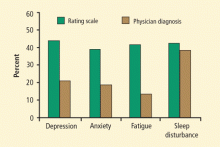
Recognition of depression in PD is confounded by the depression criteria of the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; many of these criteria can be intrinsic features of PD itself—for example, anhedonia, weight/appetite loss or gain, insomnia or hypersomnia, psychomotor retardation, and fatigue. Questions such as “are you feeling sad” or “are you feeling blue” may be superior to questions about associative symptoms when evaluating PD patients for depression.
Most of the medications used for the treatment of depression also work well for depression in patients with PD. Double-blind controlled studies have demonstrated superiority of nortriptyline, citalopram, desipramine, and pramipexole over placebo in improving mood.26–29
Apathy
The overlap between apathy and depressive symptoms can also complicate recognition of apathy, which can be described as a lack of motivation or failure to initiate goal-directed behavior. Apathy involves three domains30:
- Cognitive: expressed as a loss of interest in new experience or a lack of concern about a personal problem
- Diminished affect: flattened affect or a lack of reaction to positive or negative events
- Final: diminished goal-directed cognition, as indicated by a lack of effort or requiring others to structure activities.
Unlike depression, which is similarly representative of PD and other episodic conditions such as dystonia, apathy is more common in PD than in dystonia. In fact, the occurrence of apathy alone distinguishes PD from dystonia. Apathy in PD has no known treatment. If it is associated with depression, apathy may respond to antidepressants.
Repetitive transcranial magnetic stimulation (rTMS) manipulates activity in specific brain neural circuits through the skull to induce changes in behavior. Some studies suggest that modulation of behavior may last beyond the actual stimulation. A randomized, sham-controlled trial of rTMS over the middorsolateral frontal cortex has been conducted with the primary aim of improving apathy in PD. Unfortunately, while patients who were randomized to rTMS experienced some improvement in apathy during the study, the improvement was not significantly different from that observed in patients who received sham treatment.31
IMPULSE CONTROL AND COMPULSIVE DISORDERS IN PD
Impulse control disorders are characterized by the inability to resist an urge to act; the resulting irrational desire to pursue self-gratification may inflict suffering on friends and relatives that compromises relationships and impairs social- and work-related functioning.
Examples of impulse control disorders in PD are pathologic gambling, hypersexuality, compulsive shopping, excessive spending, and binge eating. Patients taking dopamine agonists are two to three times more likely to develop impulse control disorders than those receiving other treatments for PD. Dopamine agonists with relative selectivity for D3 receptors have been implicated in impulse control disorders in PD because D3 receptors are abundant in a region of the brain (ventral striatum) associated with behavioral and substance addictions. Higher levodopa dosages were also associated with impulse control disorders.
Factors associated with impulse control disorders in PD are young age, being single, a family history of impulse control disorders, and levodopa treatment.32 Modifications to dopamine agonist or levodopa therapy are important in the treatment of dopamine agonist–induced impulse disorders.
Compulsive disorders have been described as a class distinct from impulse control disorders and involve repetitive stereotypes and well-ordered acts to decrease inner anxiety and avoid harm. Punding is the engagement of stereotyped behaviors that are repeated compulsively—for example, repetitive manipulation of technical equipment; continual handling, sorting, and examining of objects; grooming; and hoarding. The punder has poor insight into the disruptive and senseless nature of his or her acts. Punding has consistently been related to dopaminergic therapy. Its prevalence in PD patients on dopaminergic therapy ranges from 1.4%33 to 14%.34 An improvement in behavior is observed with a reduction in dosage or discontinuation of levodopa.
Pathologic gambling, or the inability to control gambling, can result in lying to obtain money for gambling, thereby complicating relationships. It can affect up to 8% of patients with PD.35
SUMMARY
Dementia, psychotic symptoms, mood disturbances, and impulse control disorders are important nonmotor manifestations of PD that present management challenges. Some of these manifestations are intrinsic to PD, and some are complications of therapies used to treat the motor manifestations of PD.
Dementia and psychotic symptoms extract a considerable toll on the patient, caregivers, and society. Psychotic symptoms generally manifest as hallucinations (mostly visual) and other sensory disturbances. Initial management involves adjustment of anti-PD medications. The use of atypical antipsychotic drugs has been shown to improve survival among patients with PD. Clozapine is the preferred agent.
Mood disturbances such as depression and apathy may be difficult to diagnose. Depression may be treated similarly to depression unassociated with PD.
Dopamine agonists and levodopa have been associated with impulse control disorders in PD. Compulsive disorders, which are distinct from impulse control disorders, may improve with reduction or discontinuation of levodopa therapy.
- Aarsland D, Andersen K, Larsen JP, Lolk A, Nielsen H, Kragh-Sørensen P. Risk of dementia in Parkinson’s disease: a community-based, prospective study. Neurology 2001; 56:730–736.
- Cummings JL. Intellectual impairment in Parkinson’s disease: clinical, pathologic, and biochemical correlates. J Geriatr Psychiatry Neurol 1988; 1:24–36.
- Aarsland D, Andersen K, Larsen JP, Lolk A, Kragh-Sørensen P. Prevalence and characteristics of dementia in Parkinson disease: an 8-year prospective study. Arch Neurol 2003; 60:387–392.
- Aarsland D, Larsen JP, Karlsen K, Lim NG, Tandberg E. Mental symptoms in Parkinson’s disease are important contributors to caregiver distress. Int J Geriatr Psychiatry 1999; 14:866–874.
- Aarsland D, Larsen JP, Tandberg E, Laake K. Predictors of nursing home placement in Parkinson’s disease: a population-based, prospective study. J Am Geriatr Soc 2000; 48:938–942.
- Hughes TA, Ross HF, Mindham RH, Spokes EG. Mortality in Parkinson’s disease and its association with dementia and depression. Acta Neurol Scand 2004; 110:118–123.
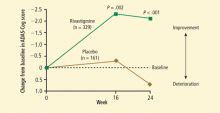
- Emre M, Aarsland D, Albanese A, et al. Rivastigmine for dementia associated with Parkinson’s disease. N Engl J Med 2004; 351:2509–2518.
- Fénelon G, Mahieux F, Huon R, Ziégler M. Hallucinations in Parkinson’s disease: prevalence, phenomenology and risk factors. Brain 2000; 123:733–745.
- Zahodne LB, Fernandez HH. Pathophysiology and treatment of psychosis in Parkinson’s disease: a review. Drugs Aging 2008; 25:665–682.
- Goetz CG, Wuu J, Curgian LM, Leurgans S. Hallucinations and sleep disorders in PD: six-year prospective longitudinal study. Neurology 2005; 64:81–86.
- Factor SA, Feustel PJ, Friedman JH, et al. Longitudinal outcome of Parkinson’s disease patients with psychosis. Neurology 2003; 60:1756–1761.
- Goetz CG, Stebbins GT. Risk factors for nursing home placement in advanced Parkinson’s disease. Neurology 1993; 43:2227–2229.
- Goetz CG, Stebbins GT. Mortality and hallucinations in nursing home patients with advanced Parkinson’s disease. Neurology 1995; 45:669–671.
- Fernandez HH, Donnelly EM, Friedman JH. Long-term outcome of clozapine use for psychosis in parkinsonian patients. Mov Disord 2004; 19:831–833.
- Fernandez HH, Trieschmann ME, Okun MS. Rebound psychosis: effect of discontinuation of antipsychotics in Parkinson’s disease. Mov Disord 2005; 20:104–115.
- Goldstein JM. Atypical antipsychotic drugs: beyond acute psychosis, new directions. Emerging Drugs 1999; 4:127–151.
- Pollak P, Tison F, Rascol O, et al; on behalf of the French Clozapine Parkinson Study Group. Clozapine in drug induced psychosis in Parkinson’s disease: a randomised, placebo controlled study with open follow up. J Neurol Neurosurg Psychiatry 2004; 75:689–695.
- The Parkinson Study Group. Low-dose clozapine for the treatment of drug-induced psychosis in Parkinson’s disease. N Engl J Med 1999; 340:757–763.
- Wolters ECh, Hurwitz TA, Mak E, et al. Clozapine in the treatment of parkinsonian patients with dopaminomimetic psychosis. Neurology 1990; 40:832–834.
- Ondo WG, Tintner R, Voung KD, Lai D, Ringholz G. Double-blind, placebo-controlled, unforced titration parallel trial of quetiapine for dopaminergic-induced hallucinations in Parkinson’s disease. Mov Disord 2005; 20:958–963.
- Fernandez HH, Okun MS, Rodriguez RL, Malaty IA, Romrell J. Quetiapine improves visual hallucinations in Parkinson disease but not through normalization of sleep architecture: results from a double-blind clinical-polysomnography study. Int J Neurosci 2009; 119:2196–2205.
- Zoldan J, Friedberg G, Livneh M, Melamed E. Psychosis in advanced Parkinson’s disease: treatment with ondansetron, a 5-HT3 receptor antagonist. Neurology 1995; 45:1305–1308.
- Voon V, Lang AE. Antidepressants in the treatment of psychosis with comorbid depression in Parkinson disease. Clin Neuropharmacol 2004; 27:90–92.
- Ozer F, Meral H, Aydin B, Hanoglu L, Aydemir T, Oral T. Electroconvulsive therapy in drug-induced psychiatric states and neuroleptic malignant syndrome. J ECT 2005; 21:125–127.
- Shulman LM, Taback RL, Rabinstein AA, Weiner WJ. Non-recognition of depression and other non-motor symptoms in Parkinson’s disease. Parkinsonism Relat Disord 2002; 8:193–197.
- Devos D, Dujardin K, Poirot I, et al. Comparison of desipramine and citalopram treatments for depression in Parkinson’s disease: a double-blind, randomized, placebo-controlled study. Mov Disord 2008; 23:850–857.
- Menza M, Dobkin RD, Marin H, et al. A controlled trial of antidepressants in patients with Parkinson disease and depression. Neurology 2009; 72:886–892.
- Barone P, Poewe W, Albrecht S, et al. Pramipexole for the treatment of depressive symptoms in patients with Parkinson’s disease: a randomised, double-blind, placebo-controlled trial. Lancet Neurol 2010; 9:573–580.
- Fernandez HH, Merello M. Pramipexole for depression and motor symptoms in Parkinson disease: can we kill two birds with one stone? Lancet Neurol 2010; 9:556–557.
- Marin RS. Apathy: a neuropsychiatric syndrome. J Neuropsychiatry Clin Neurosci 1991; 3:243–254.
- Fernandez HH, Bowers D, Triggs WJ, et al. Repetitive transcranial magnetic stimulation for the treatment of apathy in Parkinson’s disease: results from a double-blind, sham-controlled, randomized, controlled trial. Neurology 2010; 74( suppl 2):352.
- Weintraub D, Koester J, Potenza MN, et al. Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients. Arch Neurol 2010; 67:589–595.
- Miyasaki JM, Al Hassan K, Lang AE, Voon V. Punding prevalence in Parkinson’s disease. Mov Disord 2007; 22:1179–1181.
- Evans AH, Katzenschlager R, Paviour D, et al. Punding in Parkinson’s disease: its relation to the dopamine dysregulation syndrome. Mov Disord 2004; 19:397–405.
- Grosset KA, Macphee G, Pal G, et al. Problematic gambling on dopamine agonists: not such a rarity. Mov Disord 2006; 21:2206–2208.
Although the definition of Parkinson disease (PD) is based on the presence of motor features, these are just the “tip of the iceberg.” Nonmotor manifestations are nearly ubiquitous in PD, with behavior problems often being the most malignant. Almost all patients with PD have nonmotor and neuropsychiatric features, including sleep disturbances, compulsive and impulsive behaviors, autonomic dysfunction, and psychosis.
The neuropsychiatric and behavioral features of PD can be classified as intrinsic features, which occur as part of PD, and iatrogenic features, which are complications that arise from treatments used to manage the motor symptoms of PD.
DEMENTIA IN PD
An intrinsic nonmotor feature of PD is dementia, which occurs at a rate four to six times greater in patients with PD than in age-matched controls without PD.1 The prevalence of dementia in PD varies among studies and depends on the demographics of the population being studied. The cross-sectional prevalence of dementia is 40% in patients with PD.2 Seventy-eight percent of a population-based, representative cohort of patients with PD developed dementia during an 8-year study period.3
Dementia is a burden to the caregiver, the patient, and society. Cognitive and behavioral symptoms in patients with PD are the greatest contributors to caregiver distress.4 Dementia and associated behavioral symptoms (ie, hallucinations) hasten nursing home placement, contributing to the financial burden of caring for patients with PD.5 The risk of mortality is increased when dementia develops.6
PSYCHOTIC SYMPTOMS IN PD: AN EFFECT OF EXCESS DOPAMINE STIMULATION
Most of the complications observed in PD can be explained by the dopamine effect of medications and by dopamine deficiencies. An excess of dopamine stimulation caused by administration of prodopaminergic agents manifests as dyskinesias, hallucinations, or delusions. Withdrawal of levodopa will reverse these complications but leads to dopamine deficiency and thus a worsening of PD symptoms. Most patients with PD will tolerate mild dyskinesias or hallucinations if their PD symptoms are well controlled.
The hallucinations in PD tend to be visual as opposed to auditory (as in schizophrenia). They are usually benign and involve figures of people, furry animals, or complex scenes. About 10% to 40% of hallucinations in PD are secondary auditory hallucinations, which tend to be nondistinct, non-paranoid, and often incomprehensible (ie, voices in a crowd).
In the same way, the delusions experienced in patients with PD are distinct from those in schizophrenia. The delusions in PD are usually paranoid in nature and involve stereotyped themes (ie, spousal infidelity, feelings of abandonment) rather than the grandiose delusions that are common in schizophrenia.
The reported prevalence of psychotic symptoms in PD, including hallucinations and delusions, ranges from 20% to 50%.8,9 Auditory hallucinations are a feature in about 10%, and they usually occur with visual hallucinations. Less common are delusions and hallucinations with loss of insight, which are more likely with increasing severity of dementia.
Once a PD patient experiences hallucinations, they are likely to continue. In a 6-year longitudinal study, the prevalence of hallucinations increased from 33% at baseline to 55% at 72 months.10 Persistent psychosis was found in 69% of participants in the Psychosis and Clozapine in PD Study (PSYCLOPS) with 26 months of follow-up.11
High caregiver burden
Psychotic symptoms in PD are associated with high caregiver stress and increased rates of nursing home placement. Goetz et al12 showed that PD patients with psychosis had a much greater risk of nursing home placement than those without psychosis. The prognosis for PD patients in extended-care facilities is worse for those with psychotic symptoms.13
Management of psychotic symptoms
The first step in managing psychosis in PD is to rule out other causes of changes in mental status, such as infection, electrolyte imbalance, or introduction of new medications.
Adjusting anti-PD medications to a tolerable yet effective dose may help to reduce the incidence and severity of psychotic complications. If necessary, selective discontinuation of anti-PD medications may be tried in the following sequence: anticholinergics, amantadine, monoamine oxidase B inhibitors, dopamine agonists, catechol-O-methyltransferase inhibitors, and levodopa/carbidopa.
If motor symptoms prevent dosage minimization or discontinuation of some medications, then the addition of an atypical antipsychotic medication should be considered. Before the advent of atypical antipsychotics, the management of psychosis and hallucinations in PD was unsatisfactory, reflected by a mortality of 100% within 2 years among psychotic PD patients placed in nursing homes compared with 32% among age-matched community dwellers.13 The introduction of atypical antipsychotics has improved survival among PD patients with psychosis. In one study, mortality over 5 years was 44% among PD patients taking long-term clozapine for the treatment of psychosis.14 Recurrence of psychosis is rapid (within 8 weeks) even when PD patients are slowly weaned from atypical antipsychotics.15
Receptor affinities differ among antipsychotics. Because dopamine has been implicated as the principal neurotransmitter in the development of PD psychosis, atypical antipsychotics, with milder dopamine-blocking action, have played a central role in the treatment of PD psychosis. The dopamine D2 receptor is the main target for conventional antipsychotic drugs to exert their clinical effects. Atypical antipsychotics have different affinities for the D2 receptors.16 Occupancy of D2 receptors with atypical antipsychotics is 40% to 70% (risperidone and olanzapine have higher affinity for the D2 receptor than clozapine and quetiapine), and affinity for 5-HT2A receptors can be as high as 70%. This affinity for 5-HT2A receptors relative to D2 receptors may be important for therapeutic efficacy of the atypical antipsychotics. Antagonism of muscarinic, histaminergic, noradrenergic, and other serotonergic receptors also differs among the atypical antipsychotics.
Clozapine remains the gold standard atypical antipsychotic agent, based on results from three relatively small (N = 6 to 60) double-blind, placebo-controlled studies in PD patients with dopaminergic drug-induced psychosis.17–19 Quetiapine improved psychotic symptoms associated with PD in several open-label studies, but has not demonstrated the same success in double-blind clinical trials.20,21
Loss of cholinergic neurons and implications for treatment. In autopsy studies, the loss of cholinergic neurons is more profound in PD than in Alzheimer disease, which suggests that procholinergic drugs may improve symptoms of PD dementia, a major risk factor for hallucinations. In open-label studies, acetylcholinesterase inhibitors have reduced the frequency of hallucinations in patients who have dementia with Lewy bodies (DLB) and in patients with PD dementia. Double-blind trials of patients with DLB and PD dementia concentrated on the effect of cholinesterase inhibitors on dementia and not hallucinations. One concern with the use of a procholinergic drug in patients with PD has been worsening of parkinsonism, but studies of acetylcholinesterase inhibitors have shown no worsening of parkinsonism and only transient worsening of tremor.
Ondansetron, a 5-HT3 receptor antagonist used as an antinausea medication, produced moderate improvements in hallucinations and delusions in an open-label trial for the treatment of psychosis in advanced PD.22 For PD patients with psychosis and comorbid depression, antidepressant therapy and electroconvulsive therapy may be effective options.23,24
MOOD DISTURBANCES IN PD
Depression and apathy occur more frequently in patients with PD than in those who do not have PD.
Depression
Challenges in the management of depression in PD include recognition of depression and distinguishing depressive disorders from mood fluctuations. Whereas a depressive disorder lasts from weeks to years and can occur at any stage of illness, mood fluctuations can change many times daily and appear as nonmotor manifestations during the “off” medication state. Mood fluctuations occur mostly in patients who have developed motor fluctuations. The implication for treatment is that the treatment strategy for a depressive disorder is antidepressant therapy, whereas the strategy for mood fluctuations in PD is to increase the levodopa dose.
Recognition of depression in PD is confounded by the depression criteria of the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; many of these criteria can be intrinsic features of PD itself—for example, anhedonia, weight/appetite loss or gain, insomnia or hypersomnia, psychomotor retardation, and fatigue. Questions such as “are you feeling sad” or “are you feeling blue” may be superior to questions about associative symptoms when evaluating PD patients for depression.
Most of the medications used for the treatment of depression also work well for depression in patients with PD. Double-blind controlled studies have demonstrated superiority of nortriptyline, citalopram, desipramine, and pramipexole over placebo in improving mood.26–29
Apathy
The overlap between apathy and depressive symptoms can also complicate recognition of apathy, which can be described as a lack of motivation or failure to initiate goal-directed behavior. Apathy involves three domains30:
- Cognitive: expressed as a loss of interest in new experience or a lack of concern about a personal problem
- Diminished affect: flattened affect or a lack of reaction to positive or negative events
- Final: diminished goal-directed cognition, as indicated by a lack of effort or requiring others to structure activities.
Unlike depression, which is similarly representative of PD and other episodic conditions such as dystonia, apathy is more common in PD than in dystonia. In fact, the occurrence of apathy alone distinguishes PD from dystonia. Apathy in PD has no known treatment. If it is associated with depression, apathy may respond to antidepressants.
Repetitive transcranial magnetic stimulation (rTMS) manipulates activity in specific brain neural circuits through the skull to induce changes in behavior. Some studies suggest that modulation of behavior may last beyond the actual stimulation. A randomized, sham-controlled trial of rTMS over the middorsolateral frontal cortex has been conducted with the primary aim of improving apathy in PD. Unfortunately, while patients who were randomized to rTMS experienced some improvement in apathy during the study, the improvement was not significantly different from that observed in patients who received sham treatment.31
IMPULSE CONTROL AND COMPULSIVE DISORDERS IN PD
Impulse control disorders are characterized by the inability to resist an urge to act; the resulting irrational desire to pursue self-gratification may inflict suffering on friends and relatives that compromises relationships and impairs social- and work-related functioning.
Examples of impulse control disorders in PD are pathologic gambling, hypersexuality, compulsive shopping, excessive spending, and binge eating. Patients taking dopamine agonists are two to three times more likely to develop impulse control disorders than those receiving other treatments for PD. Dopamine agonists with relative selectivity for D3 receptors have been implicated in impulse control disorders in PD because D3 receptors are abundant in a region of the brain (ventral striatum) associated with behavioral and substance addictions. Higher levodopa dosages were also associated with impulse control disorders.
Factors associated with impulse control disorders in PD are young age, being single, a family history of impulse control disorders, and levodopa treatment.32 Modifications to dopamine agonist or levodopa therapy are important in the treatment of dopamine agonist–induced impulse disorders.
Compulsive disorders have been described as a class distinct from impulse control disorders and involve repetitive stereotypes and well-ordered acts to decrease inner anxiety and avoid harm. Punding is the engagement of stereotyped behaviors that are repeated compulsively—for example, repetitive manipulation of technical equipment; continual handling, sorting, and examining of objects; grooming; and hoarding. The punder has poor insight into the disruptive and senseless nature of his or her acts. Punding has consistently been related to dopaminergic therapy. Its prevalence in PD patients on dopaminergic therapy ranges from 1.4%33 to 14%.34 An improvement in behavior is observed with a reduction in dosage or discontinuation of levodopa.
Pathologic gambling, or the inability to control gambling, can result in lying to obtain money for gambling, thereby complicating relationships. It can affect up to 8% of patients with PD.35
SUMMARY
Dementia, psychotic symptoms, mood disturbances, and impulse control disorders are important nonmotor manifestations of PD that present management challenges. Some of these manifestations are intrinsic to PD, and some are complications of therapies used to treat the motor manifestations of PD.
Dementia and psychotic symptoms extract a considerable toll on the patient, caregivers, and society. Psychotic symptoms generally manifest as hallucinations (mostly visual) and other sensory disturbances. Initial management involves adjustment of anti-PD medications. The use of atypical antipsychotic drugs has been shown to improve survival among patients with PD. Clozapine is the preferred agent.
Mood disturbances such as depression and apathy may be difficult to diagnose. Depression may be treated similarly to depression unassociated with PD.
Dopamine agonists and levodopa have been associated with impulse control disorders in PD. Compulsive disorders, which are distinct from impulse control disorders, may improve with reduction or discontinuation of levodopa therapy.
Although the definition of Parkinson disease (PD) is based on the presence of motor features, these are just the “tip of the iceberg.” Nonmotor manifestations are nearly ubiquitous in PD, with behavior problems often being the most malignant. Almost all patients with PD have nonmotor and neuropsychiatric features, including sleep disturbances, compulsive and impulsive behaviors, autonomic dysfunction, and psychosis.
The neuropsychiatric and behavioral features of PD can be classified as intrinsic features, which occur as part of PD, and iatrogenic features, which are complications that arise from treatments used to manage the motor symptoms of PD.
DEMENTIA IN PD
An intrinsic nonmotor feature of PD is dementia, which occurs at a rate four to six times greater in patients with PD than in age-matched controls without PD.1 The prevalence of dementia in PD varies among studies and depends on the demographics of the population being studied. The cross-sectional prevalence of dementia is 40% in patients with PD.2 Seventy-eight percent of a population-based, representative cohort of patients with PD developed dementia during an 8-year study period.3
Dementia is a burden to the caregiver, the patient, and society. Cognitive and behavioral symptoms in patients with PD are the greatest contributors to caregiver distress.4 Dementia and associated behavioral symptoms (ie, hallucinations) hasten nursing home placement, contributing to the financial burden of caring for patients with PD.5 The risk of mortality is increased when dementia develops.6
PSYCHOTIC SYMPTOMS IN PD: AN EFFECT OF EXCESS DOPAMINE STIMULATION
Most of the complications observed in PD can be explained by the dopamine effect of medications and by dopamine deficiencies. An excess of dopamine stimulation caused by administration of prodopaminergic agents manifests as dyskinesias, hallucinations, or delusions. Withdrawal of levodopa will reverse these complications but leads to dopamine deficiency and thus a worsening of PD symptoms. Most patients with PD will tolerate mild dyskinesias or hallucinations if their PD symptoms are well controlled.
The hallucinations in PD tend to be visual as opposed to auditory (as in schizophrenia). They are usually benign and involve figures of people, furry animals, or complex scenes. About 10% to 40% of hallucinations in PD are secondary auditory hallucinations, which tend to be nondistinct, non-paranoid, and often incomprehensible (ie, voices in a crowd).
In the same way, the delusions experienced in patients with PD are distinct from those in schizophrenia. The delusions in PD are usually paranoid in nature and involve stereotyped themes (ie, spousal infidelity, feelings of abandonment) rather than the grandiose delusions that are common in schizophrenia.
The reported prevalence of psychotic symptoms in PD, including hallucinations and delusions, ranges from 20% to 50%.8,9 Auditory hallucinations are a feature in about 10%, and they usually occur with visual hallucinations. Less common are delusions and hallucinations with loss of insight, which are more likely with increasing severity of dementia.
Once a PD patient experiences hallucinations, they are likely to continue. In a 6-year longitudinal study, the prevalence of hallucinations increased from 33% at baseline to 55% at 72 months.10 Persistent psychosis was found in 69% of participants in the Psychosis and Clozapine in PD Study (PSYCLOPS) with 26 months of follow-up.11
High caregiver burden
Psychotic symptoms in PD are associated with high caregiver stress and increased rates of nursing home placement. Goetz et al12 showed that PD patients with psychosis had a much greater risk of nursing home placement than those without psychosis. The prognosis for PD patients in extended-care facilities is worse for those with psychotic symptoms.13
Management of psychotic symptoms
The first step in managing psychosis in PD is to rule out other causes of changes in mental status, such as infection, electrolyte imbalance, or introduction of new medications.
Adjusting anti-PD medications to a tolerable yet effective dose may help to reduce the incidence and severity of psychotic complications. If necessary, selective discontinuation of anti-PD medications may be tried in the following sequence: anticholinergics, amantadine, monoamine oxidase B inhibitors, dopamine agonists, catechol-O-methyltransferase inhibitors, and levodopa/carbidopa.
If motor symptoms prevent dosage minimization or discontinuation of some medications, then the addition of an atypical antipsychotic medication should be considered. Before the advent of atypical antipsychotics, the management of psychosis and hallucinations in PD was unsatisfactory, reflected by a mortality of 100% within 2 years among psychotic PD patients placed in nursing homes compared with 32% among age-matched community dwellers.13 The introduction of atypical antipsychotics has improved survival among PD patients with psychosis. In one study, mortality over 5 years was 44% among PD patients taking long-term clozapine for the treatment of psychosis.14 Recurrence of psychosis is rapid (within 8 weeks) even when PD patients are slowly weaned from atypical antipsychotics.15
Receptor affinities differ among antipsychotics. Because dopamine has been implicated as the principal neurotransmitter in the development of PD psychosis, atypical antipsychotics, with milder dopamine-blocking action, have played a central role in the treatment of PD psychosis. The dopamine D2 receptor is the main target for conventional antipsychotic drugs to exert their clinical effects. Atypical antipsychotics have different affinities for the D2 receptors.16 Occupancy of D2 receptors with atypical antipsychotics is 40% to 70% (risperidone and olanzapine have higher affinity for the D2 receptor than clozapine and quetiapine), and affinity for 5-HT2A receptors can be as high as 70%. This affinity for 5-HT2A receptors relative to D2 receptors may be important for therapeutic efficacy of the atypical antipsychotics. Antagonism of muscarinic, histaminergic, noradrenergic, and other serotonergic receptors also differs among the atypical antipsychotics.
Clozapine remains the gold standard atypical antipsychotic agent, based on results from three relatively small (N = 6 to 60) double-blind, placebo-controlled studies in PD patients with dopaminergic drug-induced psychosis.17–19 Quetiapine improved psychotic symptoms associated with PD in several open-label studies, but has not demonstrated the same success in double-blind clinical trials.20,21
Loss of cholinergic neurons and implications for treatment. In autopsy studies, the loss of cholinergic neurons is more profound in PD than in Alzheimer disease, which suggests that procholinergic drugs may improve symptoms of PD dementia, a major risk factor for hallucinations. In open-label studies, acetylcholinesterase inhibitors have reduced the frequency of hallucinations in patients who have dementia with Lewy bodies (DLB) and in patients with PD dementia. Double-blind trials of patients with DLB and PD dementia concentrated on the effect of cholinesterase inhibitors on dementia and not hallucinations. One concern with the use of a procholinergic drug in patients with PD has been worsening of parkinsonism, but studies of acetylcholinesterase inhibitors have shown no worsening of parkinsonism and only transient worsening of tremor.
Ondansetron, a 5-HT3 receptor antagonist used as an antinausea medication, produced moderate improvements in hallucinations and delusions in an open-label trial for the treatment of psychosis in advanced PD.22 For PD patients with psychosis and comorbid depression, antidepressant therapy and electroconvulsive therapy may be effective options.23,24
MOOD DISTURBANCES IN PD
Depression and apathy occur more frequently in patients with PD than in those who do not have PD.
Depression
Challenges in the management of depression in PD include recognition of depression and distinguishing depressive disorders from mood fluctuations. Whereas a depressive disorder lasts from weeks to years and can occur at any stage of illness, mood fluctuations can change many times daily and appear as nonmotor manifestations during the “off” medication state. Mood fluctuations occur mostly in patients who have developed motor fluctuations. The implication for treatment is that the treatment strategy for a depressive disorder is antidepressant therapy, whereas the strategy for mood fluctuations in PD is to increase the levodopa dose.
Recognition of depression in PD is confounded by the depression criteria of the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; many of these criteria can be intrinsic features of PD itself—for example, anhedonia, weight/appetite loss or gain, insomnia or hypersomnia, psychomotor retardation, and fatigue. Questions such as “are you feeling sad” or “are you feeling blue” may be superior to questions about associative symptoms when evaluating PD patients for depression.
Most of the medications used for the treatment of depression also work well for depression in patients with PD. Double-blind controlled studies have demonstrated superiority of nortriptyline, citalopram, desipramine, and pramipexole over placebo in improving mood.26–29
Apathy
The overlap between apathy and depressive symptoms can also complicate recognition of apathy, which can be described as a lack of motivation or failure to initiate goal-directed behavior. Apathy involves three domains30:
- Cognitive: expressed as a loss of interest in new experience or a lack of concern about a personal problem
- Diminished affect: flattened affect or a lack of reaction to positive or negative events
- Final: diminished goal-directed cognition, as indicated by a lack of effort or requiring others to structure activities.
Unlike depression, which is similarly representative of PD and other episodic conditions such as dystonia, apathy is more common in PD than in dystonia. In fact, the occurrence of apathy alone distinguishes PD from dystonia. Apathy in PD has no known treatment. If it is associated with depression, apathy may respond to antidepressants.
Repetitive transcranial magnetic stimulation (rTMS) manipulates activity in specific brain neural circuits through the skull to induce changes in behavior. Some studies suggest that modulation of behavior may last beyond the actual stimulation. A randomized, sham-controlled trial of rTMS over the middorsolateral frontal cortex has been conducted with the primary aim of improving apathy in PD. Unfortunately, while patients who were randomized to rTMS experienced some improvement in apathy during the study, the improvement was not significantly different from that observed in patients who received sham treatment.31
IMPULSE CONTROL AND COMPULSIVE DISORDERS IN PD
Impulse control disorders are characterized by the inability to resist an urge to act; the resulting irrational desire to pursue self-gratification may inflict suffering on friends and relatives that compromises relationships and impairs social- and work-related functioning.
Examples of impulse control disorders in PD are pathologic gambling, hypersexuality, compulsive shopping, excessive spending, and binge eating. Patients taking dopamine agonists are two to three times more likely to develop impulse control disorders than those receiving other treatments for PD. Dopamine agonists with relative selectivity for D3 receptors have been implicated in impulse control disorders in PD because D3 receptors are abundant in a region of the brain (ventral striatum) associated with behavioral and substance addictions. Higher levodopa dosages were also associated with impulse control disorders.
Factors associated with impulse control disorders in PD are young age, being single, a family history of impulse control disorders, and levodopa treatment.32 Modifications to dopamine agonist or levodopa therapy are important in the treatment of dopamine agonist–induced impulse disorders.
Compulsive disorders have been described as a class distinct from impulse control disorders and involve repetitive stereotypes and well-ordered acts to decrease inner anxiety and avoid harm. Punding is the engagement of stereotyped behaviors that are repeated compulsively—for example, repetitive manipulation of technical equipment; continual handling, sorting, and examining of objects; grooming; and hoarding. The punder has poor insight into the disruptive and senseless nature of his or her acts. Punding has consistently been related to dopaminergic therapy. Its prevalence in PD patients on dopaminergic therapy ranges from 1.4%33 to 14%.34 An improvement in behavior is observed with a reduction in dosage or discontinuation of levodopa.
Pathologic gambling, or the inability to control gambling, can result in lying to obtain money for gambling, thereby complicating relationships. It can affect up to 8% of patients with PD.35
SUMMARY
Dementia, psychotic symptoms, mood disturbances, and impulse control disorders are important nonmotor manifestations of PD that present management challenges. Some of these manifestations are intrinsic to PD, and some are complications of therapies used to treat the motor manifestations of PD.
Dementia and psychotic symptoms extract a considerable toll on the patient, caregivers, and society. Psychotic symptoms generally manifest as hallucinations (mostly visual) and other sensory disturbances. Initial management involves adjustment of anti-PD medications. The use of atypical antipsychotic drugs has been shown to improve survival among patients with PD. Clozapine is the preferred agent.
Mood disturbances such as depression and apathy may be difficult to diagnose. Depression may be treated similarly to depression unassociated with PD.
Dopamine agonists and levodopa have been associated with impulse control disorders in PD. Compulsive disorders, which are distinct from impulse control disorders, may improve with reduction or discontinuation of levodopa therapy.
- Aarsland D, Andersen K, Larsen JP, Lolk A, Nielsen H, Kragh-Sørensen P. Risk of dementia in Parkinson’s disease: a community-based, prospective study. Neurology 2001; 56:730–736.
- Cummings JL. Intellectual impairment in Parkinson’s disease: clinical, pathologic, and biochemical correlates. J Geriatr Psychiatry Neurol 1988; 1:24–36.
- Aarsland D, Andersen K, Larsen JP, Lolk A, Kragh-Sørensen P. Prevalence and characteristics of dementia in Parkinson disease: an 8-year prospective study. Arch Neurol 2003; 60:387–392.
- Aarsland D, Larsen JP, Karlsen K, Lim NG, Tandberg E. Mental symptoms in Parkinson’s disease are important contributors to caregiver distress. Int J Geriatr Psychiatry 1999; 14:866–874.
- Aarsland D, Larsen JP, Tandberg E, Laake K. Predictors of nursing home placement in Parkinson’s disease: a population-based, prospective study. J Am Geriatr Soc 2000; 48:938–942.
- Hughes TA, Ross HF, Mindham RH, Spokes EG. Mortality in Parkinson’s disease and its association with dementia and depression. Acta Neurol Scand 2004; 110:118–123.
- Emre M, Aarsland D, Albanese A, et al. Rivastigmine for dementia associated with Parkinson’s disease. N Engl J Med 2004; 351:2509–2518.
- Fénelon G, Mahieux F, Huon R, Ziégler M. Hallucinations in Parkinson’s disease: prevalence, phenomenology and risk factors. Brain 2000; 123:733–745.
- Zahodne LB, Fernandez HH. Pathophysiology and treatment of psychosis in Parkinson’s disease: a review. Drugs Aging 2008; 25:665–682.
- Goetz CG, Wuu J, Curgian LM, Leurgans S. Hallucinations and sleep disorders in PD: six-year prospective longitudinal study. Neurology 2005; 64:81–86.
- Factor SA, Feustel PJ, Friedman JH, et al. Longitudinal outcome of Parkinson’s disease patients with psychosis. Neurology 2003; 60:1756–1761.
- Goetz CG, Stebbins GT. Risk factors for nursing home placement in advanced Parkinson’s disease. Neurology 1993; 43:2227–2229.
- Goetz CG, Stebbins GT. Mortality and hallucinations in nursing home patients with advanced Parkinson’s disease. Neurology 1995; 45:669–671.
- Fernandez HH, Donnelly EM, Friedman JH. Long-term outcome of clozapine use for psychosis in parkinsonian patients. Mov Disord 2004; 19:831–833.
- Fernandez HH, Trieschmann ME, Okun MS. Rebound psychosis: effect of discontinuation of antipsychotics in Parkinson’s disease. Mov Disord 2005; 20:104–115.
- Goldstein JM. Atypical antipsychotic drugs: beyond acute psychosis, new directions. Emerging Drugs 1999; 4:127–151.
- Pollak P, Tison F, Rascol O, et al; on behalf of the French Clozapine Parkinson Study Group. Clozapine in drug induced psychosis in Parkinson’s disease: a randomised, placebo controlled study with open follow up. J Neurol Neurosurg Psychiatry 2004; 75:689–695.
- The Parkinson Study Group. Low-dose clozapine for the treatment of drug-induced psychosis in Parkinson’s disease. N Engl J Med 1999; 340:757–763.
- Wolters ECh, Hurwitz TA, Mak E, et al. Clozapine in the treatment of parkinsonian patients with dopaminomimetic psychosis. Neurology 1990; 40:832–834.
- Ondo WG, Tintner R, Voung KD, Lai D, Ringholz G. Double-blind, placebo-controlled, unforced titration parallel trial of quetiapine for dopaminergic-induced hallucinations in Parkinson’s disease. Mov Disord 2005; 20:958–963.
- Fernandez HH, Okun MS, Rodriguez RL, Malaty IA, Romrell J. Quetiapine improves visual hallucinations in Parkinson disease but not through normalization of sleep architecture: results from a double-blind clinical-polysomnography study. Int J Neurosci 2009; 119:2196–2205.
- Zoldan J, Friedberg G, Livneh M, Melamed E. Psychosis in advanced Parkinson’s disease: treatment with ondansetron, a 5-HT3 receptor antagonist. Neurology 1995; 45:1305–1308.
- Voon V, Lang AE. Antidepressants in the treatment of psychosis with comorbid depression in Parkinson disease. Clin Neuropharmacol 2004; 27:90–92.
- Ozer F, Meral H, Aydin B, Hanoglu L, Aydemir T, Oral T. Electroconvulsive therapy in drug-induced psychiatric states and neuroleptic malignant syndrome. J ECT 2005; 21:125–127.
- Shulman LM, Taback RL, Rabinstein AA, Weiner WJ. Non-recognition of depression and other non-motor symptoms in Parkinson’s disease. Parkinsonism Relat Disord 2002; 8:193–197.
- Devos D, Dujardin K, Poirot I, et al. Comparison of desipramine and citalopram treatments for depression in Parkinson’s disease: a double-blind, randomized, placebo-controlled study. Mov Disord 2008; 23:850–857.
- Menza M, Dobkin RD, Marin H, et al. A controlled trial of antidepressants in patients with Parkinson disease and depression. Neurology 2009; 72:886–892.
- Barone P, Poewe W, Albrecht S, et al. Pramipexole for the treatment of depressive symptoms in patients with Parkinson’s disease: a randomised, double-blind, placebo-controlled trial. Lancet Neurol 2010; 9:573–580.
- Fernandez HH, Merello M. Pramipexole for depression and motor symptoms in Parkinson disease: can we kill two birds with one stone? Lancet Neurol 2010; 9:556–557.
- Marin RS. Apathy: a neuropsychiatric syndrome. J Neuropsychiatry Clin Neurosci 1991; 3:243–254.
- Fernandez HH, Bowers D, Triggs WJ, et al. Repetitive transcranial magnetic stimulation for the treatment of apathy in Parkinson’s disease: results from a double-blind, sham-controlled, randomized, controlled trial. Neurology 2010; 74( suppl 2):352.
- Weintraub D, Koester J, Potenza MN, et al. Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients. Arch Neurol 2010; 67:589–595.
- Miyasaki JM, Al Hassan K, Lang AE, Voon V. Punding prevalence in Parkinson’s disease. Mov Disord 2007; 22:1179–1181.
- Evans AH, Katzenschlager R, Paviour D, et al. Punding in Parkinson’s disease: its relation to the dopamine dysregulation syndrome. Mov Disord 2004; 19:397–405.
- Grosset KA, Macphee G, Pal G, et al. Problematic gambling on dopamine agonists: not such a rarity. Mov Disord 2006; 21:2206–2208.
- Aarsland D, Andersen K, Larsen JP, Lolk A, Nielsen H, Kragh-Sørensen P. Risk of dementia in Parkinson’s disease: a community-based, prospective study. Neurology 2001; 56:730–736.
- Cummings JL. Intellectual impairment in Parkinson’s disease: clinical, pathologic, and biochemical correlates. J Geriatr Psychiatry Neurol 1988; 1:24–36.
- Aarsland D, Andersen K, Larsen JP, Lolk A, Kragh-Sørensen P. Prevalence and characteristics of dementia in Parkinson disease: an 8-year prospective study. Arch Neurol 2003; 60:387–392.
- Aarsland D, Larsen JP, Karlsen K, Lim NG, Tandberg E. Mental symptoms in Parkinson’s disease are important contributors to caregiver distress. Int J Geriatr Psychiatry 1999; 14:866–874.
- Aarsland D, Larsen JP, Tandberg E, Laake K. Predictors of nursing home placement in Parkinson’s disease: a population-based, prospective study. J Am Geriatr Soc 2000; 48:938–942.
- Hughes TA, Ross HF, Mindham RH, Spokes EG. Mortality in Parkinson’s disease and its association with dementia and depression. Acta Neurol Scand 2004; 110:118–123.
- Emre M, Aarsland D, Albanese A, et al. Rivastigmine for dementia associated with Parkinson’s disease. N Engl J Med 2004; 351:2509–2518.
- Fénelon G, Mahieux F, Huon R, Ziégler M. Hallucinations in Parkinson’s disease: prevalence, phenomenology and risk factors. Brain 2000; 123:733–745.
- Zahodne LB, Fernandez HH. Pathophysiology and treatment of psychosis in Parkinson’s disease: a review. Drugs Aging 2008; 25:665–682.
- Goetz CG, Wuu J, Curgian LM, Leurgans S. Hallucinations and sleep disorders in PD: six-year prospective longitudinal study. Neurology 2005; 64:81–86.
- Factor SA, Feustel PJ, Friedman JH, et al. Longitudinal outcome of Parkinson’s disease patients with psychosis. Neurology 2003; 60:1756–1761.
- Goetz CG, Stebbins GT. Risk factors for nursing home placement in advanced Parkinson’s disease. Neurology 1993; 43:2227–2229.
- Goetz CG, Stebbins GT. Mortality and hallucinations in nursing home patients with advanced Parkinson’s disease. Neurology 1995; 45:669–671.
- Fernandez HH, Donnelly EM, Friedman JH. Long-term outcome of clozapine use for psychosis in parkinsonian patients. Mov Disord 2004; 19:831–833.
- Fernandez HH, Trieschmann ME, Okun MS. Rebound psychosis: effect of discontinuation of antipsychotics in Parkinson’s disease. Mov Disord 2005; 20:104–115.
- Goldstein JM. Atypical antipsychotic drugs: beyond acute psychosis, new directions. Emerging Drugs 1999; 4:127–151.
- Pollak P, Tison F, Rascol O, et al; on behalf of the French Clozapine Parkinson Study Group. Clozapine in drug induced psychosis in Parkinson’s disease: a randomised, placebo controlled study with open follow up. J Neurol Neurosurg Psychiatry 2004; 75:689–695.
- The Parkinson Study Group. Low-dose clozapine for the treatment of drug-induced psychosis in Parkinson’s disease. N Engl J Med 1999; 340:757–763.
- Wolters ECh, Hurwitz TA, Mak E, et al. Clozapine in the treatment of parkinsonian patients with dopaminomimetic psychosis. Neurology 1990; 40:832–834.
- Ondo WG, Tintner R, Voung KD, Lai D, Ringholz G. Double-blind, placebo-controlled, unforced titration parallel trial of quetiapine for dopaminergic-induced hallucinations in Parkinson’s disease. Mov Disord 2005; 20:958–963.
- Fernandez HH, Okun MS, Rodriguez RL, Malaty IA, Romrell J. Quetiapine improves visual hallucinations in Parkinson disease but not through normalization of sleep architecture: results from a double-blind clinical-polysomnography study. Int J Neurosci 2009; 119:2196–2205.
- Zoldan J, Friedberg G, Livneh M, Melamed E. Psychosis in advanced Parkinson’s disease: treatment with ondansetron, a 5-HT3 receptor antagonist. Neurology 1995; 45:1305–1308.
- Voon V, Lang AE. Antidepressants in the treatment of psychosis with comorbid depression in Parkinson disease. Clin Neuropharmacol 2004; 27:90–92.
- Ozer F, Meral H, Aydin B, Hanoglu L, Aydemir T, Oral T. Electroconvulsive therapy in drug-induced psychiatric states and neuroleptic malignant syndrome. J ECT 2005; 21:125–127.
- Shulman LM, Taback RL, Rabinstein AA, Weiner WJ. Non-recognition of depression and other non-motor symptoms in Parkinson’s disease. Parkinsonism Relat Disord 2002; 8:193–197.
- Devos D, Dujardin K, Poirot I, et al. Comparison of desipramine and citalopram treatments for depression in Parkinson’s disease: a double-blind, randomized, placebo-controlled study. Mov Disord 2008; 23:850–857.
- Menza M, Dobkin RD, Marin H, et al. A controlled trial of antidepressants in patients with Parkinson disease and depression. Neurology 2009; 72:886–892.
- Barone P, Poewe W, Albrecht S, et al. Pramipexole for the treatment of depressive symptoms in patients with Parkinson’s disease: a randomised, double-blind, placebo-controlled trial. Lancet Neurol 2010; 9:573–580.
- Fernandez HH, Merello M. Pramipexole for depression and motor symptoms in Parkinson disease: can we kill two birds with one stone? Lancet Neurol 2010; 9:556–557.
- Marin RS. Apathy: a neuropsychiatric syndrome. J Neuropsychiatry Clin Neurosci 1991; 3:243–254.
- Fernandez HH, Bowers D, Triggs WJ, et al. Repetitive transcranial magnetic stimulation for the treatment of apathy in Parkinson’s disease: results from a double-blind, sham-controlled, randomized, controlled trial. Neurology 2010; 74( suppl 2):352.
- Weintraub D, Koester J, Potenza MN, et al. Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients. Arch Neurol 2010; 67:589–595.
- Miyasaki JM, Al Hassan K, Lang AE, Voon V. Punding prevalence in Parkinson’s disease. Mov Disord 2007; 22:1179–1181.
- Evans AH, Katzenschlager R, Paviour D, et al. Punding in Parkinson’s disease: its relation to the dopamine dysregulation syndrome. Mov Disord 2004; 19:397–405.
- Grosset KA, Macphee G, Pal G, et al. Problematic gambling on dopamine agonists: not such a rarity. Mov Disord 2006; 21:2206–2208.