User login
A protein found in the cerebrospinal fluid and peripheral blood cells of patients with a common type of amyotrophic lateral sclerosis may serve as a “pharmacodynamic marker,” providing a mechanism to assess RNA-based therapies that are now in clinical trials.
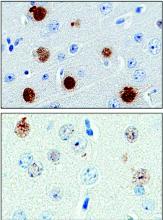
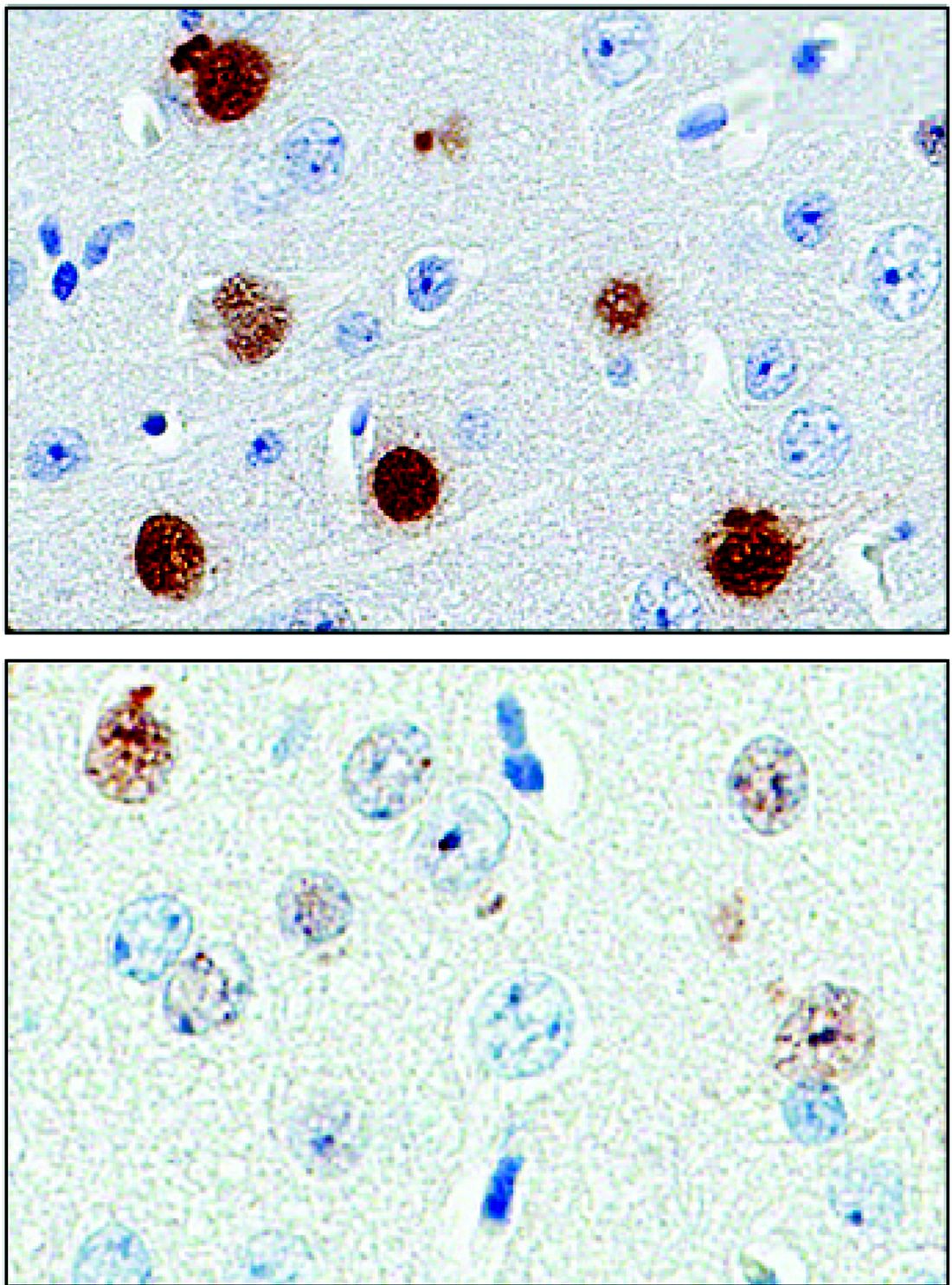
The protein, poly(GP), is expressed in patients who have a mutation in the gene chromosome 9 open reading frame 72 (C9ORF72), which causes one type of amyotrophic lateral sclerosis (ALS). This means that detecting poly(GP) also, eventually, may help to identify “presymptomatic individuals who are expected to benefit from early therapeutic interventions,” wrote the authors of a newly published paper (Sci Transl Med. 2017;9[383]:eaai7866).
Tania Gendron, PhD, of the department of neuroscience at the Mayo Clinic, Jacksonville, Fla., and her colleagues noted that poly(GP) was found in the cerebrospinal fluid (CSF) of both symptomatic and asymptomatic patients who carried the C9ORF72 mutation. The mutation can cause ALS, and is also associated with frontotemporal dementia (FTD) in a pattern with incomplete overlap with ALS.
However, “A limitation in moving such treatments from bench to bedside is a lack of pharmacodynamics markers for use in clinical trials,” Dr. Gendron and her collaborators wrote. The discovery that poly(GP) tracks well with C9ORF72 means that it has the potential to serve as the kind of biomarker that’s been missing in drug development for this family of neurodegenerative diseases, they said.
“To prepare for upcoming clinical trials for c9ALS, the present study used patient CSF and several preclinical models to investigate the hypothesis that poly(GP) proteins could serve as an urgently needed pharmacodynamics marker for developing and testing therapies for treating c9ALS,” Dr. Gendron and her colleagues wrote.
They looked at CSF samples from 83 patients with c9ALS and 27 patients who were asymptomatic C9ORF72 repeat expansion carriers, as well as 24 carriers who had a neurologic disease besides ALS or FTD (total n = 134). They also examined CSF samples from 120 study participants who lacked the mutation, 48 of whom were healthy controls; the remainder had ALS (n = 57) or another neurological disease.
The investigators, who were blinded to individuals’ disease status in each study arm, found that CSF poly(GP) levels were significantly higher in patients who had the C9ORF72 mutation (P less than .0001 in unadjusted and adjusted analyses). Poly(GP) was present in both asymptomatic and symptomatic carriers of the mutation, and not significantly different between these groups when data were adjusted for multiple comparisons, age, and gender.
When Dr. Gendron and her colleagues looked at poly(GP) levels over time for patients whose longitudinal data were available, they found that levels for an individual study participant were “relatively constant,” without any significant change over the median 12.9 months that these levels were tracked (P = .84).
However, “poly(GP) is not a prognostic marker,” wrote Dr. Gendron and her colleagues. They found no consistent association between levels of the protein and disease severity of progression, age at onset, or the development of FTD. Women were more likely to have lower levels, but the significance of that finding is not known, they said. There was a trend, which lost significance after statistical adjustment, for patients with cognitive impairment to have higher poly(GP) levels (adjusted P = .12).
Treatments under investigation for ALS and FTD include the use of an antisense oligonucleotide (ASO) to bind to the repeated RNA sequences and negate their ill effects. The investigators wrote that in vitro investigations using patient-derived cell models showed that poly(GP) levels dropped when cells were exposed to an ASO for 10 days. “The data indicate that poly(GP) production mirrors expression of repeat-containing C9ORF72 transcripts in lymphoblastoid cell lines,” they wrote.
The authors reported multiple governmental and private foundation sources of support for the research. Dr. Gendron and several of her coauthors are investigators in clinical trials for an ASO to target C9ORF72. Several authors reported paid and unpaid relationships and stock positions with pharmaceutical companies, including ones developing treatments for ALS and FTD.
koakes@frontlinemedcom.com
On Twitter @karioakes
A protein found in the cerebrospinal fluid and peripheral blood cells of patients with a common type of amyotrophic lateral sclerosis may serve as a “pharmacodynamic marker,” providing a mechanism to assess RNA-based therapies that are now in clinical trials.
The protein, poly(GP), is expressed in patients who have a mutation in the gene chromosome 9 open reading frame 72 (C9ORF72), which causes one type of amyotrophic lateral sclerosis (ALS). This means that detecting poly(GP) also, eventually, may help to identify “presymptomatic individuals who are expected to benefit from early therapeutic interventions,” wrote the authors of a newly published paper (Sci Transl Med. 2017;9[383]:eaai7866).
Tania Gendron, PhD, of the department of neuroscience at the Mayo Clinic, Jacksonville, Fla., and her colleagues noted that poly(GP) was found in the cerebrospinal fluid (CSF) of both symptomatic and asymptomatic patients who carried the C9ORF72 mutation. The mutation can cause ALS, and is also associated with frontotemporal dementia (FTD) in a pattern with incomplete overlap with ALS.
However, “A limitation in moving such treatments from bench to bedside is a lack of pharmacodynamics markers for use in clinical trials,” Dr. Gendron and her collaborators wrote. The discovery that poly(GP) tracks well with C9ORF72 means that it has the potential to serve as the kind of biomarker that’s been missing in drug development for this family of neurodegenerative diseases, they said.
“To prepare for upcoming clinical trials for c9ALS, the present study used patient CSF and several preclinical models to investigate the hypothesis that poly(GP) proteins could serve as an urgently needed pharmacodynamics marker for developing and testing therapies for treating c9ALS,” Dr. Gendron and her colleagues wrote.
They looked at CSF samples from 83 patients with c9ALS and 27 patients who were asymptomatic C9ORF72 repeat expansion carriers, as well as 24 carriers who had a neurologic disease besides ALS or FTD (total n = 134). They also examined CSF samples from 120 study participants who lacked the mutation, 48 of whom were healthy controls; the remainder had ALS (n = 57) or another neurological disease.
The investigators, who were blinded to individuals’ disease status in each study arm, found that CSF poly(GP) levels were significantly higher in patients who had the C9ORF72 mutation (P less than .0001 in unadjusted and adjusted analyses). Poly(GP) was present in both asymptomatic and symptomatic carriers of the mutation, and not significantly different between these groups when data were adjusted for multiple comparisons, age, and gender.
When Dr. Gendron and her colleagues looked at poly(GP) levels over time for patients whose longitudinal data were available, they found that levels for an individual study participant were “relatively constant,” without any significant change over the median 12.9 months that these levels were tracked (P = .84).
However, “poly(GP) is not a prognostic marker,” wrote Dr. Gendron and her colleagues. They found no consistent association between levels of the protein and disease severity of progression, age at onset, or the development of FTD. Women were more likely to have lower levels, but the significance of that finding is not known, they said. There was a trend, which lost significance after statistical adjustment, for patients with cognitive impairment to have higher poly(GP) levels (adjusted P = .12).
Treatments under investigation for ALS and FTD include the use of an antisense oligonucleotide (ASO) to bind to the repeated RNA sequences and negate their ill effects. The investigators wrote that in vitro investigations using patient-derived cell models showed that poly(GP) levels dropped when cells were exposed to an ASO for 10 days. “The data indicate that poly(GP) production mirrors expression of repeat-containing C9ORF72 transcripts in lymphoblastoid cell lines,” they wrote.
The authors reported multiple governmental and private foundation sources of support for the research. Dr. Gendron and several of her coauthors are investigators in clinical trials for an ASO to target C9ORF72. Several authors reported paid and unpaid relationships and stock positions with pharmaceutical companies, including ones developing treatments for ALS and FTD.
koakes@frontlinemedcom.com
On Twitter @karioakes
A protein found in the cerebrospinal fluid and peripheral blood cells of patients with a common type of amyotrophic lateral sclerosis may serve as a “pharmacodynamic marker,” providing a mechanism to assess RNA-based therapies that are now in clinical trials.
The protein, poly(GP), is expressed in patients who have a mutation in the gene chromosome 9 open reading frame 72 (C9ORF72), which causes one type of amyotrophic lateral sclerosis (ALS). This means that detecting poly(GP) also, eventually, may help to identify “presymptomatic individuals who are expected to benefit from early therapeutic interventions,” wrote the authors of a newly published paper (Sci Transl Med. 2017;9[383]:eaai7866).
Tania Gendron, PhD, of the department of neuroscience at the Mayo Clinic, Jacksonville, Fla., and her colleagues noted that poly(GP) was found in the cerebrospinal fluid (CSF) of both symptomatic and asymptomatic patients who carried the C9ORF72 mutation. The mutation can cause ALS, and is also associated with frontotemporal dementia (FTD) in a pattern with incomplete overlap with ALS.
However, “A limitation in moving such treatments from bench to bedside is a lack of pharmacodynamics markers for use in clinical trials,” Dr. Gendron and her collaborators wrote. The discovery that poly(GP) tracks well with C9ORF72 means that it has the potential to serve as the kind of biomarker that’s been missing in drug development for this family of neurodegenerative diseases, they said.
“To prepare for upcoming clinical trials for c9ALS, the present study used patient CSF and several preclinical models to investigate the hypothesis that poly(GP) proteins could serve as an urgently needed pharmacodynamics marker for developing and testing therapies for treating c9ALS,” Dr. Gendron and her colleagues wrote.
They looked at CSF samples from 83 patients with c9ALS and 27 patients who were asymptomatic C9ORF72 repeat expansion carriers, as well as 24 carriers who had a neurologic disease besides ALS or FTD (total n = 134). They also examined CSF samples from 120 study participants who lacked the mutation, 48 of whom were healthy controls; the remainder had ALS (n = 57) or another neurological disease.
The investigators, who were blinded to individuals’ disease status in each study arm, found that CSF poly(GP) levels were significantly higher in patients who had the C9ORF72 mutation (P less than .0001 in unadjusted and adjusted analyses). Poly(GP) was present in both asymptomatic and symptomatic carriers of the mutation, and not significantly different between these groups when data were adjusted for multiple comparisons, age, and gender.
When Dr. Gendron and her colleagues looked at poly(GP) levels over time for patients whose longitudinal data were available, they found that levels for an individual study participant were “relatively constant,” without any significant change over the median 12.9 months that these levels were tracked (P = .84).
However, “poly(GP) is not a prognostic marker,” wrote Dr. Gendron and her colleagues. They found no consistent association between levels of the protein and disease severity of progression, age at onset, or the development of FTD. Women were more likely to have lower levels, but the significance of that finding is not known, they said. There was a trend, which lost significance after statistical adjustment, for patients with cognitive impairment to have higher poly(GP) levels (adjusted P = .12).
Treatments under investigation for ALS and FTD include the use of an antisense oligonucleotide (ASO) to bind to the repeated RNA sequences and negate their ill effects. The investigators wrote that in vitro investigations using patient-derived cell models showed that poly(GP) levels dropped when cells were exposed to an ASO for 10 days. “The data indicate that poly(GP) production mirrors expression of repeat-containing C9ORF72 transcripts in lymphoblastoid cell lines,” they wrote.
The authors reported multiple governmental and private foundation sources of support for the research. Dr. Gendron and several of her coauthors are investigators in clinical trials for an ASO to target C9ORF72. Several authors reported paid and unpaid relationships and stock positions with pharmaceutical companies, including ones developing treatments for ALS and FTD.
koakes@frontlinemedcom.com
On Twitter @karioakes
Key clinical point:
Major finding: Patients with a mutation predisposing them to ALS had significantly higher levels of the protein poly(GP) (P less than .0001 in unadjusted and adjusted analyses).
Data source: Prospective blinded study of CSF samples from 83 patients with c9ALS, 27 patients who were asymptomatic C9ORF72 repeat expansion carriers, 24 carriers who had a neurologic disease besides ALS or FTD, and 120 study participants who lacked the C9ORF72 mutation.
Disclosures: The authors reported multiple governmental and private foundation sources of support for their research. Dr. Gendron and several of her coauthors are investigators in clinical trials for an ASO to target C9ORF72. Several authors reported paid and unpaid relationships and stock positions with pharmaceutical companies, including ones developing treatments for ALS and FTD.