User login
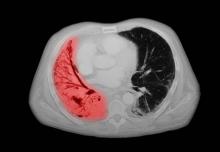
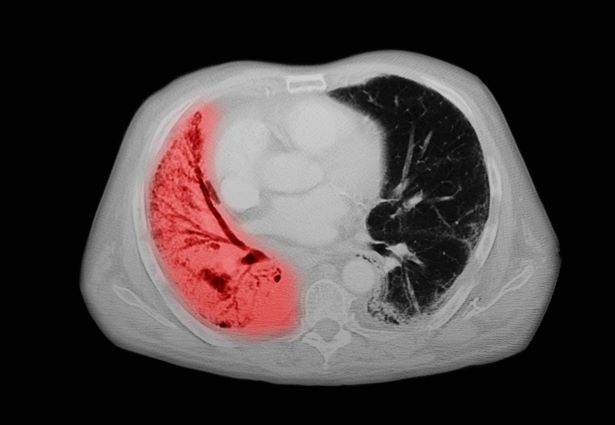
Small cell lung cancer (SCLC) is the most aggressive lung cancer subtype, has a poor prognosis, and is highly associated with smoking. It accounts for 10%-15% of all lung cancers. Rapid tumor growth may lead to obstruction of major airways, with distal collapse leading to postobstructive pneumonitis, infection, and fever. SCLCs usually grow rapidly and metastasize to mediastinal lymph nodes relatively early in the course of the disease. At presentation, patients may have very large intrathoracic tumors, and distinguishing the primary tumor from lymph node metastases may be difficult.
CT of all common sites of metastasis should be performed to stage the disease. CT scanning of the thorax (lungs and mediastinum) and commonly involved abdominal viscera is the minimum requirement in standard staging workup of SCLC. Intravenous contrast agents should be used whenever possible. In the United States, CT scans of the chest and upper abdomen to include the liver and adrenal glands are standard. The liver is a common site of metastasis.
Most cases of SCLC will present in advanced stages, be inoperable, and have a dismal prognosis. Only about 5% of patients present at an early stage (Ia, Ib, or IIa). The National Comprehensive Cancer Network (NCCN) guidelines recommend that these patients be managed with aggressive chemoradiation therapy; in some, lobectomy associated with mediastinal lymph node dissection may be performed. The NCCN notes that advanced disease is managed primarily with chemotherapy, mainly for palliation and symptom control. Older patients, such as the current patient, who have a good performance status (ECOG 0 or 1) and intact organ function should receive standard carboplatin-based chemotherapy. However, even those who have poor prognostic factors (eg, poor performance status, medically significant concomitant conditions) may still be considered for chemotherapy if appropriate precautions are taken to avoid excessive toxicity and further decline in performance status.
Unlike non-SCLC, which has seen waves of new drug approvals from the US Food and Drug Administration (FDA), the prognosis for SCLC has not changed substantially in the past two decades and remains poor. However, the FDA approved atezolizumab in combination with carboplatin and etoposide for first-line treatment of adult patients with extensive-stage SCLC. Further, the approval of durvalumab in combination with chemotherapy; and the approval of lurbinectedin, a novel chemotherapy agent approved for second-line treatment of SCLC, have added to the therapeutic options for patients with SCLC.
Karl J. D'Silva, MD, Clinical Assistant Professor, Department of Medicine, Tufts University School of Medicine, Boston; Medical Director, Department of Oncology and Hematology, Lahey Hospital and Medical Center, Peabody, Massachusetts
Karl J. D'Silva, MD, has disclosed no relevant financial relationships
Small cell lung cancer (SCLC) is the most aggressive lung cancer subtype, has a poor prognosis, and is highly associated with smoking. It accounts for 10%-15% of all lung cancers. Rapid tumor growth may lead to obstruction of major airways, with distal collapse leading to postobstructive pneumonitis, infection, and fever. SCLCs usually grow rapidly and metastasize to mediastinal lymph nodes relatively early in the course of the disease. At presentation, patients may have very large intrathoracic tumors, and distinguishing the primary tumor from lymph node metastases may be difficult.
CT of all common sites of metastasis should be performed to stage the disease. CT scanning of the thorax (lungs and mediastinum) and commonly involved abdominal viscera is the minimum requirement in standard staging workup of SCLC. Intravenous contrast agents should be used whenever possible. In the United States, CT scans of the chest and upper abdomen to include the liver and adrenal glands are standard. The liver is a common site of metastasis.
Most cases of SCLC will present in advanced stages, be inoperable, and have a dismal prognosis. Only about 5% of patients present at an early stage (Ia, Ib, or IIa). The National Comprehensive Cancer Network (NCCN) guidelines recommend that these patients be managed with aggressive chemoradiation therapy; in some, lobectomy associated with mediastinal lymph node dissection may be performed. The NCCN notes that advanced disease is managed primarily with chemotherapy, mainly for palliation and symptom control. Older patients, such as the current patient, who have a good performance status (ECOG 0 or 1) and intact organ function should receive standard carboplatin-based chemotherapy. However, even those who have poor prognostic factors (eg, poor performance status, medically significant concomitant conditions) may still be considered for chemotherapy if appropriate precautions are taken to avoid excessive toxicity and further decline in performance status.
Unlike non-SCLC, which has seen waves of new drug approvals from the US Food and Drug Administration (FDA), the prognosis for SCLC has not changed substantially in the past two decades and remains poor. However, the FDA approved atezolizumab in combination with carboplatin and etoposide for first-line treatment of adult patients with extensive-stage SCLC. Further, the approval of durvalumab in combination with chemotherapy; and the approval of lurbinectedin, a novel chemotherapy agent approved for second-line treatment of SCLC, have added to the therapeutic options for patients with SCLC.
Karl J. D'Silva, MD, Clinical Assistant Professor, Department of Medicine, Tufts University School of Medicine, Boston; Medical Director, Department of Oncology and Hematology, Lahey Hospital and Medical Center, Peabody, Massachusetts
Karl J. D'Silva, MD, has disclosed no relevant financial relationships
Small cell lung cancer (SCLC) is the most aggressive lung cancer subtype, has a poor prognosis, and is highly associated with smoking. It accounts for 10%-15% of all lung cancers. Rapid tumor growth may lead to obstruction of major airways, with distal collapse leading to postobstructive pneumonitis, infection, and fever. SCLCs usually grow rapidly and metastasize to mediastinal lymph nodes relatively early in the course of the disease. At presentation, patients may have very large intrathoracic tumors, and distinguishing the primary tumor from lymph node metastases may be difficult.
CT of all common sites of metastasis should be performed to stage the disease. CT scanning of the thorax (lungs and mediastinum) and commonly involved abdominal viscera is the minimum requirement in standard staging workup of SCLC. Intravenous contrast agents should be used whenever possible. In the United States, CT scans of the chest and upper abdomen to include the liver and adrenal glands are standard. The liver is a common site of metastasis.
Most cases of SCLC will present in advanced stages, be inoperable, and have a dismal prognosis. Only about 5% of patients present at an early stage (Ia, Ib, or IIa). The National Comprehensive Cancer Network (NCCN) guidelines recommend that these patients be managed with aggressive chemoradiation therapy; in some, lobectomy associated with mediastinal lymph node dissection may be performed. The NCCN notes that advanced disease is managed primarily with chemotherapy, mainly for palliation and symptom control. Older patients, such as the current patient, who have a good performance status (ECOG 0 or 1) and intact organ function should receive standard carboplatin-based chemotherapy. However, even those who have poor prognostic factors (eg, poor performance status, medically significant concomitant conditions) may still be considered for chemotherapy if appropriate precautions are taken to avoid excessive toxicity and further decline in performance status.
Unlike non-SCLC, which has seen waves of new drug approvals from the US Food and Drug Administration (FDA), the prognosis for SCLC has not changed substantially in the past two decades and remains poor. However, the FDA approved atezolizumab in combination with carboplatin and etoposide for first-line treatment of adult patients with extensive-stage SCLC. Further, the approval of durvalumab in combination with chemotherapy; and the approval of lurbinectedin, a novel chemotherapy agent approved for second-line treatment of SCLC, have added to the therapeutic options for patients with SCLC.
Karl J. D'Silva, MD, Clinical Assistant Professor, Department of Medicine, Tufts University School of Medicine, Boston; Medical Director, Department of Oncology and Hematology, Lahey Hospital and Medical Center, Peabody, Massachusetts
Karl J. D'Silva, MD, has disclosed no relevant financial relationships
A 72-year-old woman presents with shortness of breath, a productive cough, chest pain, some fatigue, anorexia, a recent 18-pound weight loss, and a history of hypertension. She is currently a smoker and has a 45–pack-year smoking history. On physical examination she has some dullness to percussion with some decreased breath sounds. She has a distended abdomen and complains of itchy skin. The chest x-ray shows a left hilar mass and a 5.4-cm left upper-lobe mass. A CT scan reveals a hilar mass with a mediastinal extension.