User login
Fertility preservation and sexual health are main concerns in reproductive-age cancer survivors. Approximately 1% of cancer survivors are younger than age 20 and up to 10% are estimated to be younger than age 45.1 For many of these survivors, a cancer diagnosis may have occurred prior to their completion of childbearing.
Infertility or premature ovarian failure has been reported in 40% to 80% of cancer survivors due to chemotoxicity-induced accelerated loss of oocytes.2 Most gonadotoxic chemotherapeutic agents cause DNA double-strand breaks that cannot be adequately repaired, eventually leading to apoptotic cell death.3 Therefore, any chemotherapeutic agent that induces apoptotic death will cause irreversible depletion of ovarian reserve, since primordial follicles cannot be regenerated.
Alkylating agents, such as cyclophosphamide, have been shown to be most cytotoxic, and young cancer survivors who have received a combination of alkylating agents and abdominopelvic radiation—such as those with Hodgkin’s lymphoma—are at higher risk. Other poor prognostic factors for fertility include a hypothalamic-pituitary radiation dose greater than 30 Gy, an ovarian-uterine radiation dose greater than 5 Gy, summed alkylating agent dose score of 3 to 4 for each agent, and treatment with lomustine or cyclophosphamide.4
In general, a woman’s age (which reflects her existing ovarian reserve), type of therapeutic agents used, and duration of therapy impact the posttreatment viability of ovarian function. Despite conflicting information in published literature, medical suppression by gonadotropin-releasing hormone agonists is not effective.
Fertility preservation options in the United States include egg, embryo, and ovarian tissue banking and ovarian transposition and ovarian transplantation.5
Oncofertility: Maximizing reproductive potential in cancer patients
In 2006, Dr. Teresa Woodruff of the Feinberg School of Medicine at Northwestern University coined the term oncofertility. Oncofertility is defined by the Merriam-Webster dictionary as “a field concerned with minimizing the negative effects of cancer treatment (such as chemotherapy or radiation) on the reproductive system and fertility and with assisting individuals with reproductive impairments resulting from cancer therapy.”
Recognition of the many barriers to fertility preservation led to the establishment of the Oncofertility Consortium, a multi-institution group that includes Northwestern University, the University of California San Diego, the University of Pennsylvania, the University of Missouri, and Oregon Health and Science University. The Consortium facilitates collaboration between biomedical and social scientists, pediatricians, oncologists, reproductive specialists, educators, social workers, and medical ethicists in an effort to assess the impact of cancer and its treatment on future fertility and reproductive health and to advance knowledge. The Consortium also is a valuable information resource on fertility preservation options for patients, their families, and providers.6
The oncofertility program at Northwestern University was established as an interdisciplinary team of oncologists, reproductive health specialists, supportive care staff, and researchers. Reproductive-age women with cancer can participate in a comprehensive interdisciplinary approach to the management of their malignancy with strict planning and coordination of care, if they wish to maintain fertility following treatment. Many hospitals and health care systems have established such programs, recognizing that the need to preserve fertility potential is an essential part of the comprehensive care of a reproductive-age woman undergoing treatment. When a cancer diagnosis is made, prompt referral to a fertility specialist and a multidisciplinary approach to treatment planning are critical to mitigate the negative impact of cancer treatment on fertility and the potential risk of ovarian damage.
Barriers to oncofertility care
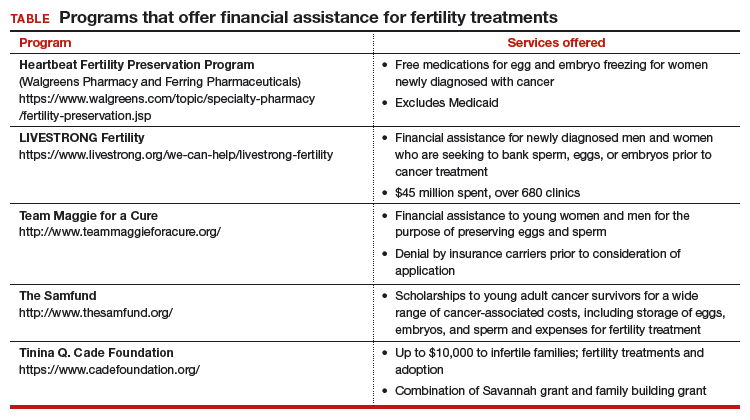
Timely referral to fertility specialists may not occur because of lack of a formal oncofertility program or unawareness of available therapeutic options. In some instances, delaying cancer treatment is not feasible. Additionally, many other factors must be considered regarding societal, ethical, and legal implications. But most concerning is the lack of consistent and timely access to funding for fertility preservation by third-party payers. Although some funding options exist, these require both patient awareness and effort to pursue (TABLE).
National legislation does not include provision for this aspect of women’s health, and as of 2017 insurance coverage for oncofertility was mandated only in 2 states, Connecticut and Rhode Island. In New York, Governor Cuomo directed the Department of Financial Services to study how to ensure that New Yorkers can have access to oncofertility services, and legislation is pending in the New York state legislature.7
Recently, Cardozo and colleagues reported that 15 states currently require insurers to provide some form of infertility coverage.8 By contrast, RESOLVE: The National Infertility Association, reports information on fertility coverage and the status of bills by state on its website (https://resolve.org). For example, in California, Hawaii, Illinois, and Maryland, bills have been proposed and are in various stages of assessment. Connecticut and Rhode Island mandate coverage. As always, details matter. Cardozo and colleagues eloquently point out limitations of coverage based on age and definition of infertility, and potential financial impact.8
An actuarial consulting company called NovaRest prepared a document for the state of Maryland in which the estimated expected number of “cases” would amount to 1,327 women and 731 men aged 10 to 44.9 These individuals might require oncofertility services. NovaRest estimated that clients could experience up to a 0.4% increase in insurance premiums annually if this program was offered. Similar estimates are reported by other states. In Kentucky and Mississippi, such bills “died in committee.” The American Society for Reproductive Medicine (ASRM) is actively lobbying with partners, including the Coalition to Protect Parenthood After Cancer, to advocate for preservation of fertility.

Drs. Ursillo and Chalas bring attention to an important issue. As technology advances, so do treatment and coverage needs, and so does the need for ongoing physician and patient education.
In 1990, the US Congress passed the Breast and Cervical Cancer Mortality Prevention Act to help ensure that low-income women would have access to screening for these diseases. It took 10 years before Congress passed the Breast and Cervical Cancer Prevention and Treatment Act so that women detected with breast or cervical cancer could be treated. A curious delay, I know.
Today, we seem to be in a similar situation regarding fertility preservation. Cancer treatment is advanced, coverage is available. Fertility-related treatment is now possible, but coverage is nearly absent.
In my research for this commentary, I learned (a little) about ovarian transplantation and translocation. Even that little was enough to see that we live in an amazing new world. Drs. Ursillo and Chalas put out an important call for physicians to learn, to teach their patients, and, especially, to consider fertility preservation options before (when possible) initiating cancer treatment. It also is imperative to consider fertility preservation in young patients who have not yet reached their fertile years. Cancer treatment begun before fertility preservation may mean future irreversible infertility.
They also call for insurers and public programs to cover fertility and fertility preservation as “essential in the comprehensive care” of cancer patients. To the American College of Obstetricians and Gynecologists (ACOG), that means a federal policy that would ensure public and private coverage for every woman, no matter where she lives, her income level, or her employer.
In many ways, this is a difficult time in public policy related to women’s health. With ACOG’s leadership, our physician colleague organizations and patient advocacy groups are fighting hard to retain women’s health protections already in law. At this moment, opportunities are rare for consideration of expansion. But a national solution is the right solution.
Until we reach that goal, we support state efforts to require private health insurers to cover fertility preservation. As Drs. Ursillo and Chalas point out, only 2 states require private insurers to cover fertility preservation treatment. State-by-state efforts are notoriously difficult, unique, and inequitable to patients. Patients in some states simply are luckier than patients in other states. That is not how to solve a health care problem.
As is often the case, employers—in this case big, cutting-edge companies—are leading the way. Recently, an article in the Wall Street Journal (February 7, 2018) described companies that offer fertility treatment coverage to attract potential employees, such as Pinterest, American Express, and Foursquare. This is an important first step that we can build upon, ensuring that coverage includes fertility protection and then leveraging employer coverage experience to influence coverage more broadly.
Big employers may help us find our way, showing just how little inclusion of this coverage relates to premiums; by some estimates, only 0.4%. That is a small investment for enormous results in a patient’s future.
My takeaways from this thoughtful editorial:
- Physicians should educate themselves about fertility preservation options.
- Physicians should educate their patients about the same.
- Physicians should consider these options before initiating treatment.
- We all should advocate for our patients, in this case, national, state, and employer coverage of fertility treatment, including preservation.
Ms. DiVenere is Officer, Government and Political Affairs, at the American College of Obstetricians and Gynecologists in Washington, DC. She is an OBG Management Contributing Editor.
The author reports no financial relationships relevant to this article.
We need a joint effort
Most recent statistics support an increase in cancer survivorship over the past decade.10 This trend likely will continue thanks to greater application of screening and more effective therapies. The use of targeted therapy is on the rise, but it is not applicable for most malignancies at this time, and its effect on fertility is largely unknown. Millennials now constitute the largest group in our population, and delaying childbearing to the late second and third decades is now common. These medical and societal trends will result in more women being interested in fertility preservation.
The ASRM and other organizations are lobbying to support legislation to mandate coverage for oncofertility on a state-by-state basis. Major limitations of this approach include inability to address oncofertility unless such legislation already has been introduced, the lack of impact on individuals residing in other states, and inefficiency of regional lobbying. In addition, those who are self-insured are not subject to state mandates and therefore will not benefit from such coverage mandates. Finally, nuances in the definition of infertility or age-based restrictions may limit access to these services even when mandated.
A cancer diagnosis is always potentially life-threatening and is often perceived as devastating on a personal level. In women of reproductive age, it represents a threat to their future ability to bear children and to ovarian function. These women deserve to have the opportunity to consider all options to maintain fertility, and they should not struggle with difficult financial choices at a time of such extreme stress.
To address this important issue, a 3-pronged approach is called for:
- All providers caring for cancer patients of reproductive age must be aware of fertility preservation and inform patients of these options.
- Cancer survivors and their caretakers must assist in legislative advocacy efforts.
- Nationally mandated coverage must be sought.
A joint effort by the medical community and women advocates is critical to bring attention to this issue in a national forum and provide a solution that benefits all women.
Acknowledgement
The authors express gratitude to Erin Kramer, Government Affairs, American Society for Reproductive Medicine, and Christa Christakis, Executive Director, American College of Obstetricians and Gynecologists District II, for their assistance.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Woodruff TK. The Oncofertility Consortium—addressing fertility in young people with cancer. Nat Rev Clin Oncol. 2010;7(8):466–475.
- Pereira N, Schattman GL. Fertility preservation and sexual health after cancer therapy. J Oncol Pract. 2017;13(10):643–651.
- Soleimani R, Heytens E, Darzynkiewicz Z, Oktay K. Mechanisms of chemotherapy-induced human ovarian aging: double strand DNA breaks and microvascular compromise. Aging (Albany NY). 2011;3(8):782–793.
- Green DM, Nolan VG, Kawashima T, et al. Decreased fertility among female childhood cancer survivors who received 22-27 Gr hypothalamic/pituitary irradiation: a report from the Child Cancer Survivor Study. Fertil Steril. 2011;95(6):1922–1927.
- Oktay K, Harvey BE, Partridge AH, et al. Fertility preservation in patients with cancer: ASCO clinical practice guideline update [published online April 5, 2018]. J Clin Oncol. doi:10.1200/JCO.2018.78.1914.
- Woodruff TK, Snyder KA. Oncofertility: fertility preservation for cancer survivors. Chicago, IL: Springer; 2007.
- New York State Council on Women and Girls. Report on the status of New York women and girls: 2018 outlook. https://www.ny.gov/sites/ny.gov/files/atoms/files/StatusNYWomenGirls2018Outlook.pdf. Accessed April 16, 2018.
- Cardozo ER, Huber WJ, Stuckey AR, Alvero RJ. Mandating coverage for fertility preservation—a step in the right direction. N Engl J Med. 2017;377(17):1607–1609.
- NovaRest Actuarial Consulting. Annual mandate report: coverage for iatrogenic infertility. http://mhcc.maryland.gov/mhcc/pages/plr/plr/documents/NovaRest_Evaluation_of_%20Proposed_Mandated_Services_Iatrogenic_Infertility_FINAL_11-20-17.pdf. Published November 16, 2017. Accessed April 13, 2018.
- Siegel R, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30.
Fertility preservation and sexual health are main concerns in reproductive-age cancer survivors. Approximately 1% of cancer survivors are younger than age 20 and up to 10% are estimated to be younger than age 45.1 For many of these survivors, a cancer diagnosis may have occurred prior to their completion of childbearing.
Infertility or premature ovarian failure has been reported in 40% to 80% of cancer survivors due to chemotoxicity-induced accelerated loss of oocytes.2 Most gonadotoxic chemotherapeutic agents cause DNA double-strand breaks that cannot be adequately repaired, eventually leading to apoptotic cell death.3 Therefore, any chemotherapeutic agent that induces apoptotic death will cause irreversible depletion of ovarian reserve, since primordial follicles cannot be regenerated.
Alkylating agents, such as cyclophosphamide, have been shown to be most cytotoxic, and young cancer survivors who have received a combination of alkylating agents and abdominopelvic radiation—such as those with Hodgkin’s lymphoma—are at higher risk. Other poor prognostic factors for fertility include a hypothalamic-pituitary radiation dose greater than 30 Gy, an ovarian-uterine radiation dose greater than 5 Gy, summed alkylating agent dose score of 3 to 4 for each agent, and treatment with lomustine or cyclophosphamide.4
In general, a woman’s age (which reflects her existing ovarian reserve), type of therapeutic agents used, and duration of therapy impact the posttreatment viability of ovarian function. Despite conflicting information in published literature, medical suppression by gonadotropin-releasing hormone agonists is not effective.
Fertility preservation options in the United States include egg, embryo, and ovarian tissue banking and ovarian transposition and ovarian transplantation.5
Oncofertility: Maximizing reproductive potential in cancer patients
In 2006, Dr. Teresa Woodruff of the Feinberg School of Medicine at Northwestern University coined the term oncofertility. Oncofertility is defined by the Merriam-Webster dictionary as “a field concerned with minimizing the negative effects of cancer treatment (such as chemotherapy or radiation) on the reproductive system and fertility and with assisting individuals with reproductive impairments resulting from cancer therapy.”
Recognition of the many barriers to fertility preservation led to the establishment of the Oncofertility Consortium, a multi-institution group that includes Northwestern University, the University of California San Diego, the University of Pennsylvania, the University of Missouri, and Oregon Health and Science University. The Consortium facilitates collaboration between biomedical and social scientists, pediatricians, oncologists, reproductive specialists, educators, social workers, and medical ethicists in an effort to assess the impact of cancer and its treatment on future fertility and reproductive health and to advance knowledge. The Consortium also is a valuable information resource on fertility preservation options for patients, their families, and providers.6
The oncofertility program at Northwestern University was established as an interdisciplinary team of oncologists, reproductive health specialists, supportive care staff, and researchers. Reproductive-age women with cancer can participate in a comprehensive interdisciplinary approach to the management of their malignancy with strict planning and coordination of care, if they wish to maintain fertility following treatment. Many hospitals and health care systems have established such programs, recognizing that the need to preserve fertility potential is an essential part of the comprehensive care of a reproductive-age woman undergoing treatment. When a cancer diagnosis is made, prompt referral to a fertility specialist and a multidisciplinary approach to treatment planning are critical to mitigate the negative impact of cancer treatment on fertility and the potential risk of ovarian damage.
Barriers to oncofertility care
Timely referral to fertility specialists may not occur because of lack of a formal oncofertility program or unawareness of available therapeutic options. In some instances, delaying cancer treatment is not feasible. Additionally, many other factors must be considered regarding societal, ethical, and legal implications. But most concerning is the lack of consistent and timely access to funding for fertility preservation by third-party payers. Although some funding options exist, these require both patient awareness and effort to pursue (TABLE).
National legislation does not include provision for this aspect of women’s health, and as of 2017 insurance coverage for oncofertility was mandated only in 2 states, Connecticut and Rhode Island. In New York, Governor Cuomo directed the Department of Financial Services to study how to ensure that New Yorkers can have access to oncofertility services, and legislation is pending in the New York state legislature.7
Recently, Cardozo and colleagues reported that 15 states currently require insurers to provide some form of infertility coverage.8 By contrast, RESOLVE: The National Infertility Association, reports information on fertility coverage and the status of bills by state on its website (https://resolve.org). For example, in California, Hawaii, Illinois, and Maryland, bills have been proposed and are in various stages of assessment. Connecticut and Rhode Island mandate coverage. As always, details matter. Cardozo and colleagues eloquently point out limitations of coverage based on age and definition of infertility, and potential financial impact.8
An actuarial consulting company called NovaRest prepared a document for the state of Maryland in which the estimated expected number of “cases” would amount to 1,327 women and 731 men aged 10 to 44.9 These individuals might require oncofertility services. NovaRest estimated that clients could experience up to a 0.4% increase in insurance premiums annually if this program was offered. Similar estimates are reported by other states. In Kentucky and Mississippi, such bills “died in committee.” The American Society for Reproductive Medicine (ASRM) is actively lobbying with partners, including the Coalition to Protect Parenthood After Cancer, to advocate for preservation of fertility.

Drs. Ursillo and Chalas bring attention to an important issue. As technology advances, so do treatment and coverage needs, and so does the need for ongoing physician and patient education.
In 1990, the US Congress passed the Breast and Cervical Cancer Mortality Prevention Act to help ensure that low-income women would have access to screening for these diseases. It took 10 years before Congress passed the Breast and Cervical Cancer Prevention and Treatment Act so that women detected with breast or cervical cancer could be treated. A curious delay, I know.
Today, we seem to be in a similar situation regarding fertility preservation. Cancer treatment is advanced, coverage is available. Fertility-related treatment is now possible, but coverage is nearly absent.
In my research for this commentary, I learned (a little) about ovarian transplantation and translocation. Even that little was enough to see that we live in an amazing new world. Drs. Ursillo and Chalas put out an important call for physicians to learn, to teach their patients, and, especially, to consider fertility preservation options before (when possible) initiating cancer treatment. It also is imperative to consider fertility preservation in young patients who have not yet reached their fertile years. Cancer treatment begun before fertility preservation may mean future irreversible infertility.
They also call for insurers and public programs to cover fertility and fertility preservation as “essential in the comprehensive care” of cancer patients. To the American College of Obstetricians and Gynecologists (ACOG), that means a federal policy that would ensure public and private coverage for every woman, no matter where she lives, her income level, or her employer.
In many ways, this is a difficult time in public policy related to women’s health. With ACOG’s leadership, our physician colleague organizations and patient advocacy groups are fighting hard to retain women’s health protections already in law. At this moment, opportunities are rare for consideration of expansion. But a national solution is the right solution.
Until we reach that goal, we support state efforts to require private health insurers to cover fertility preservation. As Drs. Ursillo and Chalas point out, only 2 states require private insurers to cover fertility preservation treatment. State-by-state efforts are notoriously difficult, unique, and inequitable to patients. Patients in some states simply are luckier than patients in other states. That is not how to solve a health care problem.
As is often the case, employers—in this case big, cutting-edge companies—are leading the way. Recently, an article in the Wall Street Journal (February 7, 2018) described companies that offer fertility treatment coverage to attract potential employees, such as Pinterest, American Express, and Foursquare. This is an important first step that we can build upon, ensuring that coverage includes fertility protection and then leveraging employer coverage experience to influence coverage more broadly.
Big employers may help us find our way, showing just how little inclusion of this coverage relates to premiums; by some estimates, only 0.4%. That is a small investment for enormous results in a patient’s future.
My takeaways from this thoughtful editorial:
- Physicians should educate themselves about fertility preservation options.
- Physicians should educate their patients about the same.
- Physicians should consider these options before initiating treatment.
- We all should advocate for our patients, in this case, national, state, and employer coverage of fertility treatment, including preservation.
Ms. DiVenere is Officer, Government and Political Affairs, at the American College of Obstetricians and Gynecologists in Washington, DC. She is an OBG Management Contributing Editor.
The author reports no financial relationships relevant to this article.
We need a joint effort
Most recent statistics support an increase in cancer survivorship over the past decade.10 This trend likely will continue thanks to greater application of screening and more effective therapies. The use of targeted therapy is on the rise, but it is not applicable for most malignancies at this time, and its effect on fertility is largely unknown. Millennials now constitute the largest group in our population, and delaying childbearing to the late second and third decades is now common. These medical and societal trends will result in more women being interested in fertility preservation.
The ASRM and other organizations are lobbying to support legislation to mandate coverage for oncofertility on a state-by-state basis. Major limitations of this approach include inability to address oncofertility unless such legislation already has been introduced, the lack of impact on individuals residing in other states, and inefficiency of regional lobbying. In addition, those who are self-insured are not subject to state mandates and therefore will not benefit from such coverage mandates. Finally, nuances in the definition of infertility or age-based restrictions may limit access to these services even when mandated.
A cancer diagnosis is always potentially life-threatening and is often perceived as devastating on a personal level. In women of reproductive age, it represents a threat to their future ability to bear children and to ovarian function. These women deserve to have the opportunity to consider all options to maintain fertility, and they should not struggle with difficult financial choices at a time of such extreme stress.
To address this important issue, a 3-pronged approach is called for:
- All providers caring for cancer patients of reproductive age must be aware of fertility preservation and inform patients of these options.
- Cancer survivors and their caretakers must assist in legislative advocacy efforts.
- Nationally mandated coverage must be sought.
A joint effort by the medical community and women advocates is critical to bring attention to this issue in a national forum and provide a solution that benefits all women.
Acknowledgement
The authors express gratitude to Erin Kramer, Government Affairs, American Society for Reproductive Medicine, and Christa Christakis, Executive Director, American College of Obstetricians and Gynecologists District II, for their assistance.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
Fertility preservation and sexual health are main concerns in reproductive-age cancer survivors. Approximately 1% of cancer survivors are younger than age 20 and up to 10% are estimated to be younger than age 45.1 For many of these survivors, a cancer diagnosis may have occurred prior to their completion of childbearing.
Infertility or premature ovarian failure has been reported in 40% to 80% of cancer survivors due to chemotoxicity-induced accelerated loss of oocytes.2 Most gonadotoxic chemotherapeutic agents cause DNA double-strand breaks that cannot be adequately repaired, eventually leading to apoptotic cell death.3 Therefore, any chemotherapeutic agent that induces apoptotic death will cause irreversible depletion of ovarian reserve, since primordial follicles cannot be regenerated.
Alkylating agents, such as cyclophosphamide, have been shown to be most cytotoxic, and young cancer survivors who have received a combination of alkylating agents and abdominopelvic radiation—such as those with Hodgkin’s lymphoma—are at higher risk. Other poor prognostic factors for fertility include a hypothalamic-pituitary radiation dose greater than 30 Gy, an ovarian-uterine radiation dose greater than 5 Gy, summed alkylating agent dose score of 3 to 4 for each agent, and treatment with lomustine or cyclophosphamide.4
In general, a woman’s age (which reflects her existing ovarian reserve), type of therapeutic agents used, and duration of therapy impact the posttreatment viability of ovarian function. Despite conflicting information in published literature, medical suppression by gonadotropin-releasing hormone agonists is not effective.
Fertility preservation options in the United States include egg, embryo, and ovarian tissue banking and ovarian transposition and ovarian transplantation.5
Oncofertility: Maximizing reproductive potential in cancer patients
In 2006, Dr. Teresa Woodruff of the Feinberg School of Medicine at Northwestern University coined the term oncofertility. Oncofertility is defined by the Merriam-Webster dictionary as “a field concerned with minimizing the negative effects of cancer treatment (such as chemotherapy or radiation) on the reproductive system and fertility and with assisting individuals with reproductive impairments resulting from cancer therapy.”
Recognition of the many barriers to fertility preservation led to the establishment of the Oncofertility Consortium, a multi-institution group that includes Northwestern University, the University of California San Diego, the University of Pennsylvania, the University of Missouri, and Oregon Health and Science University. The Consortium facilitates collaboration between biomedical and social scientists, pediatricians, oncologists, reproductive specialists, educators, social workers, and medical ethicists in an effort to assess the impact of cancer and its treatment on future fertility and reproductive health and to advance knowledge. The Consortium also is a valuable information resource on fertility preservation options for patients, their families, and providers.6
The oncofertility program at Northwestern University was established as an interdisciplinary team of oncologists, reproductive health specialists, supportive care staff, and researchers. Reproductive-age women with cancer can participate in a comprehensive interdisciplinary approach to the management of their malignancy with strict planning and coordination of care, if they wish to maintain fertility following treatment. Many hospitals and health care systems have established such programs, recognizing that the need to preserve fertility potential is an essential part of the comprehensive care of a reproductive-age woman undergoing treatment. When a cancer diagnosis is made, prompt referral to a fertility specialist and a multidisciplinary approach to treatment planning are critical to mitigate the negative impact of cancer treatment on fertility and the potential risk of ovarian damage.
Barriers to oncofertility care
Timely referral to fertility specialists may not occur because of lack of a formal oncofertility program or unawareness of available therapeutic options. In some instances, delaying cancer treatment is not feasible. Additionally, many other factors must be considered regarding societal, ethical, and legal implications. But most concerning is the lack of consistent and timely access to funding for fertility preservation by third-party payers. Although some funding options exist, these require both patient awareness and effort to pursue (TABLE).
National legislation does not include provision for this aspect of women’s health, and as of 2017 insurance coverage for oncofertility was mandated only in 2 states, Connecticut and Rhode Island. In New York, Governor Cuomo directed the Department of Financial Services to study how to ensure that New Yorkers can have access to oncofertility services, and legislation is pending in the New York state legislature.7
Recently, Cardozo and colleagues reported that 15 states currently require insurers to provide some form of infertility coverage.8 By contrast, RESOLVE: The National Infertility Association, reports information on fertility coverage and the status of bills by state on its website (https://resolve.org). For example, in California, Hawaii, Illinois, and Maryland, bills have been proposed and are in various stages of assessment. Connecticut and Rhode Island mandate coverage. As always, details matter. Cardozo and colleagues eloquently point out limitations of coverage based on age and definition of infertility, and potential financial impact.8
An actuarial consulting company called NovaRest prepared a document for the state of Maryland in which the estimated expected number of “cases” would amount to 1,327 women and 731 men aged 10 to 44.9 These individuals might require oncofertility services. NovaRest estimated that clients could experience up to a 0.4% increase in insurance premiums annually if this program was offered. Similar estimates are reported by other states. In Kentucky and Mississippi, such bills “died in committee.” The American Society for Reproductive Medicine (ASRM) is actively lobbying with partners, including the Coalition to Protect Parenthood After Cancer, to advocate for preservation of fertility.

Drs. Ursillo and Chalas bring attention to an important issue. As technology advances, so do treatment and coverage needs, and so does the need for ongoing physician and patient education.
In 1990, the US Congress passed the Breast and Cervical Cancer Mortality Prevention Act to help ensure that low-income women would have access to screening for these diseases. It took 10 years before Congress passed the Breast and Cervical Cancer Prevention and Treatment Act so that women detected with breast or cervical cancer could be treated. A curious delay, I know.
Today, we seem to be in a similar situation regarding fertility preservation. Cancer treatment is advanced, coverage is available. Fertility-related treatment is now possible, but coverage is nearly absent.
In my research for this commentary, I learned (a little) about ovarian transplantation and translocation. Even that little was enough to see that we live in an amazing new world. Drs. Ursillo and Chalas put out an important call for physicians to learn, to teach their patients, and, especially, to consider fertility preservation options before (when possible) initiating cancer treatment. It also is imperative to consider fertility preservation in young patients who have not yet reached their fertile years. Cancer treatment begun before fertility preservation may mean future irreversible infertility.
They also call for insurers and public programs to cover fertility and fertility preservation as “essential in the comprehensive care” of cancer patients. To the American College of Obstetricians and Gynecologists (ACOG), that means a federal policy that would ensure public and private coverage for every woman, no matter where she lives, her income level, or her employer.
In many ways, this is a difficult time in public policy related to women’s health. With ACOG’s leadership, our physician colleague organizations and patient advocacy groups are fighting hard to retain women’s health protections already in law. At this moment, opportunities are rare for consideration of expansion. But a national solution is the right solution.
Until we reach that goal, we support state efforts to require private health insurers to cover fertility preservation. As Drs. Ursillo and Chalas point out, only 2 states require private insurers to cover fertility preservation treatment. State-by-state efforts are notoriously difficult, unique, and inequitable to patients. Patients in some states simply are luckier than patients in other states. That is not how to solve a health care problem.
As is often the case, employers—in this case big, cutting-edge companies—are leading the way. Recently, an article in the Wall Street Journal (February 7, 2018) described companies that offer fertility treatment coverage to attract potential employees, such as Pinterest, American Express, and Foursquare. This is an important first step that we can build upon, ensuring that coverage includes fertility protection and then leveraging employer coverage experience to influence coverage more broadly.
Big employers may help us find our way, showing just how little inclusion of this coverage relates to premiums; by some estimates, only 0.4%. That is a small investment for enormous results in a patient’s future.
My takeaways from this thoughtful editorial:
- Physicians should educate themselves about fertility preservation options.
- Physicians should educate their patients about the same.
- Physicians should consider these options before initiating treatment.
- We all should advocate for our patients, in this case, national, state, and employer coverage of fertility treatment, including preservation.
Ms. DiVenere is Officer, Government and Political Affairs, at the American College of Obstetricians and Gynecologists in Washington, DC. She is an OBG Management Contributing Editor.
The author reports no financial relationships relevant to this article.
We need a joint effort
Most recent statistics support an increase in cancer survivorship over the past decade.10 This trend likely will continue thanks to greater application of screening and more effective therapies. The use of targeted therapy is on the rise, but it is not applicable for most malignancies at this time, and its effect on fertility is largely unknown. Millennials now constitute the largest group in our population, and delaying childbearing to the late second and third decades is now common. These medical and societal trends will result in more women being interested in fertility preservation.
The ASRM and other organizations are lobbying to support legislation to mandate coverage for oncofertility on a state-by-state basis. Major limitations of this approach include inability to address oncofertility unless such legislation already has been introduced, the lack of impact on individuals residing in other states, and inefficiency of regional lobbying. In addition, those who are self-insured are not subject to state mandates and therefore will not benefit from such coverage mandates. Finally, nuances in the definition of infertility or age-based restrictions may limit access to these services even when mandated.
A cancer diagnosis is always potentially life-threatening and is often perceived as devastating on a personal level. In women of reproductive age, it represents a threat to their future ability to bear children and to ovarian function. These women deserve to have the opportunity to consider all options to maintain fertility, and they should not struggle with difficult financial choices at a time of such extreme stress.
To address this important issue, a 3-pronged approach is called for:
- All providers caring for cancer patients of reproductive age must be aware of fertility preservation and inform patients of these options.
- Cancer survivors and their caretakers must assist in legislative advocacy efforts.
- Nationally mandated coverage must be sought.
A joint effort by the medical community and women advocates is critical to bring attention to this issue in a national forum and provide a solution that benefits all women.
Acknowledgement
The authors express gratitude to Erin Kramer, Government Affairs, American Society for Reproductive Medicine, and Christa Christakis, Executive Director, American College of Obstetricians and Gynecologists District II, for their assistance.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Woodruff TK. The Oncofertility Consortium—addressing fertility in young people with cancer. Nat Rev Clin Oncol. 2010;7(8):466–475.
- Pereira N, Schattman GL. Fertility preservation and sexual health after cancer therapy. J Oncol Pract. 2017;13(10):643–651.
- Soleimani R, Heytens E, Darzynkiewicz Z, Oktay K. Mechanisms of chemotherapy-induced human ovarian aging: double strand DNA breaks and microvascular compromise. Aging (Albany NY). 2011;3(8):782–793.
- Green DM, Nolan VG, Kawashima T, et al. Decreased fertility among female childhood cancer survivors who received 22-27 Gr hypothalamic/pituitary irradiation: a report from the Child Cancer Survivor Study. Fertil Steril. 2011;95(6):1922–1927.
- Oktay K, Harvey BE, Partridge AH, et al. Fertility preservation in patients with cancer: ASCO clinical practice guideline update [published online April 5, 2018]. J Clin Oncol. doi:10.1200/JCO.2018.78.1914.
- Woodruff TK, Snyder KA. Oncofertility: fertility preservation for cancer survivors. Chicago, IL: Springer; 2007.
- New York State Council on Women and Girls. Report on the status of New York women and girls: 2018 outlook. https://www.ny.gov/sites/ny.gov/files/atoms/files/StatusNYWomenGirls2018Outlook.pdf. Accessed April 16, 2018.
- Cardozo ER, Huber WJ, Stuckey AR, Alvero RJ. Mandating coverage for fertility preservation—a step in the right direction. N Engl J Med. 2017;377(17):1607–1609.
- NovaRest Actuarial Consulting. Annual mandate report: coverage for iatrogenic infertility. http://mhcc.maryland.gov/mhcc/pages/plr/plr/documents/NovaRest_Evaluation_of_%20Proposed_Mandated_Services_Iatrogenic_Infertility_FINAL_11-20-17.pdf. Published November 16, 2017. Accessed April 13, 2018.
- Siegel R, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30.
- Woodruff TK. The Oncofertility Consortium—addressing fertility in young people with cancer. Nat Rev Clin Oncol. 2010;7(8):466–475.
- Pereira N, Schattman GL. Fertility preservation and sexual health after cancer therapy. J Oncol Pract. 2017;13(10):643–651.
- Soleimani R, Heytens E, Darzynkiewicz Z, Oktay K. Mechanisms of chemotherapy-induced human ovarian aging: double strand DNA breaks and microvascular compromise. Aging (Albany NY). 2011;3(8):782–793.
- Green DM, Nolan VG, Kawashima T, et al. Decreased fertility among female childhood cancer survivors who received 22-27 Gr hypothalamic/pituitary irradiation: a report from the Child Cancer Survivor Study. Fertil Steril. 2011;95(6):1922–1927.
- Oktay K, Harvey BE, Partridge AH, et al. Fertility preservation in patients with cancer: ASCO clinical practice guideline update [published online April 5, 2018]. J Clin Oncol. doi:10.1200/JCO.2018.78.1914.
- Woodruff TK, Snyder KA. Oncofertility: fertility preservation for cancer survivors. Chicago, IL: Springer; 2007.
- New York State Council on Women and Girls. Report on the status of New York women and girls: 2018 outlook. https://www.ny.gov/sites/ny.gov/files/atoms/files/StatusNYWomenGirls2018Outlook.pdf. Accessed April 16, 2018.
- Cardozo ER, Huber WJ, Stuckey AR, Alvero RJ. Mandating coverage for fertility preservation—a step in the right direction. N Engl J Med. 2017;377(17):1607–1609.
- NovaRest Actuarial Consulting. Annual mandate report: coverage for iatrogenic infertility. http://mhcc.maryland.gov/mhcc/pages/plr/plr/documents/NovaRest_Evaluation_of_%20Proposed_Mandated_Services_Iatrogenic_Infertility_FINAL_11-20-17.pdf. Published November 16, 2017. Accessed April 13, 2018.
- Siegel R, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30.