User login
Because of the complex anatomy of the ankle joint and foot, the wide array of possible bone and soft-tissue injuries, and the uncommon occurrence of tumors at these sites, osteoid osteomas (OOs) are often not included in the differential diagnosis of foot and ankle pain.1,2 Patients with OO usually complain of severe pain that is worse at night and is relieved with use of nonsteroidal anti-inflammatory drugs (NSAIDs).1-4 This classic clinical presentation, combined with the characteristic imaging features, facilitates making an accurate diagnosis.
OOs were first described in 1935 by Jaffe,5 who characterized them as benign, solitary, osteoblastic tumors consisting of atypical bone and osteoid. On radiographs and thin-slice computed tomography (CT), these tumors are small osteolytic lesions surrounded by a larger region of cortical thickening, medullary sclerosis, and benign periosteal new bone formation.4,6,7 They often contain a central focus of calcification—the nidus. OOs typically occur in children and young adults; the majority of patients are younger than 25 years. OOs show a predilection for the appendicular skeleton, with the majority of the lesions in the femur and tibia.4,6,7 OOs infrequently occur in the bones of the hands and feet.8-12 Previous studies of foot and ankle OOs have been predominantly limited to case reports; the largest study, conducted almost 20 years ago, included only 10 patients.1
We conducted a study to evaluate the epidemiology and radiographic features of foot and ankle OOs, to evaluate surgical treatment options and outcomes in patients with foot and ankle OOs, and to evaluate the disease course of patients with foot and ankle OOs treated surgically or with radiofrequency ablation (RFA).
Materials and Methods
After obtaining approval from our institutional review board, we retrospectively reviewed all cases of patients who underwent a surgical or an interventional radiologic procedure and had a preoperative diagnosis of a lower extremity OO between 1990 and 2010. Only patients with a histologically confirmed diagnosis of OO were included in the review of foot and ankle cases.
The medical records of patients with a diagnosis of foot or ankle OO were reviewed for patient sex, age, OO site, clinical presentation, radiographic studies, pain characteristics, treatment modality, histologic diagnosis, and clinical outcome of the surgical or RFA procedure. Preoperative and postoperative clinical outcome scores were calculated using American Orthopedic Foot and Ankle Society (AOFAS) scores.
Whether to perform surgical excision or RFA was discussed between the treating surgeon and the radiologist before treatment. The goal was to treat each lesion while minimizing damage to normal, surrounding structures. If there was any question whether a lesion could be something other than OO based on radiographic features, the lesion was treated with surgical excision. Surgical excision consisted of curettage and bone grafting or en bloc removal. Surgical hardware was placed only when an osteotomy was needed to access the lesion. RFA was performed by consultant musculoskeletal radiologists. Before ablation, a CT-guided needle biopsy of the lesion was performed to obtain tissue for pathologic diagnosis. Recurrence was defined as return of preoperative symptoms after treatment, along with radiographic features of recurrence.
Statistical analysis was done with SPSS software (IBM, New York, New York) using unpaired Student t tests and Fisher exact tests. Statistical significance was set at P < .05.
Results
Of the 117 patients with a lower extremity OO, 13 (11%) had it in the bones of the foot or ankle (Table). Mean age at presentation was 20.1 years (range, 9-38 years). There was no statistically significant difference in age between patients with foot or ankle OO and patients with OO of the long bones of the lower extremity (P = .27). Of the 13 patients, 12 were male and 1 was female (Table). The foot and ankle OO sites were the talus (n = 5), the distal tibia/plafond (n = 3), the calcaneus (n = 2), the tarsal bones (n = 2), and the phalanx (n = 1). All 13 foot and ankle lesions were histologically confirmed as OO.
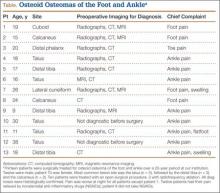
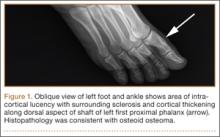
The 13 patients’ primary complaint was foot or ankle pain. Ten of the 13 were referred to our institution for clinical workup and management of foot or ankle pain and for assessment of radiographic features of OO (Figure 1). For all patients in the study, preoperative plain film radiographs of the affected extremity were obtained. Nine patients (69%) had a CT scan (Figure 2), 6 (46%) had a magnetic resonance imaging (MRI) scan, and 2 (15%) had a bone scan. Despite undergoing advanced imaging (1 CT, 1 MRI), 2 patients (15%) did not get a differential diagnosis of OO before being treated. The same 2 patients did not have radiographic images available for review to determine why a differential diagnosis of OO was not included based on imaging features prior to surgery. For the patients who did not have a diagnosis of OO before being evaluated at our institution, preliminary diagnoses included osteomyelitis and painful osteophytes. Twelve of the 13 patients complained of pain that was worse at night and was not relieved with use of NSAIDs. Mean time from symptom onset to presentation at our institution was 14.4 months (range, 3-42 months). All patients reported pain relief after the procedure. There was a significant (P = .0001) increase in AOFAS scores after surgery. Mean AOFAS score was 65.42 (range, 54-80) before surgery and 97.91 (range, 90-100) after surgery.
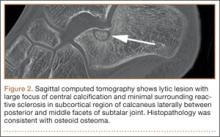
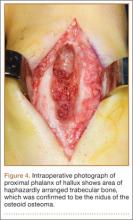
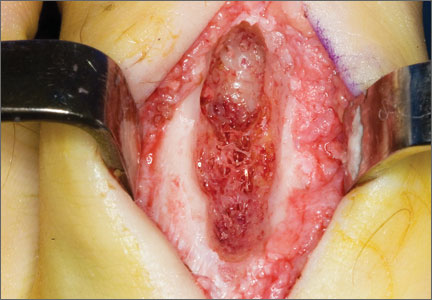
Before 1998, all foot and ankle OOs (n = 6) were treated with surgical excision. After RFA was introduced at our institution, 3 foot and ankle OOs (43%) were treated with RFA (Figures 3A, 3B), and 4 (57%) were treated with surgical curettage (Figure 4). The 4 surgical patients’ OOs were not amenable to RFA primarily because of anatomical considerations: In 2 patients, the OO was too near the articular surface; in another patient, the lesion was in intimate contact with a neurovascular bundle; in the fourth patient, the lesion was amenable to RFA, but the patient’s family selected surgical curettage instead.
Mean tumor nidus size was 7.5 mm (range, 3-12 mm). Bone graft was placed in 3 patients (30%), and surgical hardware was placed to repair a medial malleolar osteotomy in 1 (10%) of the patients treated surgically. The majority of the lesions (8) were in cancellous bone in a subcortical location. Three lesions were intracortical. Seven lesions were intra-articular, and 4 were extra-articular. Two patients did not have radiographic images available for review.
One patient had a recurrence of OO and underwent a repeat procedure 4 months after the initial one. At final follow-up, on average 1 year after the initial procedure (range, 2 weeks–3 years), there were no reported recurrences. One patient underwent a procedure to remove painful hardware that had been implanted, during the primary procedure, to repair the medial malleolar osteotomy used to access the lesion. Recurrence rates for RFA (n = 1) and surgical excision (n = 0) were similar.
Discussion
OOs are relatively common bone tumors that account for about 13% of all benign bone tumors.4,13 OOs typically occur in children or young adults—the majority of patients are younger than 25 years—and are 3 times more common in males than females.4,13 Our findings for all patients with a lower extremity OO are consistent with those previously reported: male predominance (75 males, 42 females) and mean age under 25 years (mean age, 18.7 years). In patients with foot or ankle OO, male predominance was substantially greater (12 males, 1 female), though mean age at presentation (20.1 years) was similar.
Local pain is the most common complaint in patients who present with OO.4,13 Pain is thought to be generated by a combination of multiple nerve endings in the tumor14 and prostaglandin production by the tumor nidus (prostaglandins E2 and I2)3 causing an inflammatory reaction.6 In accord with previous studies,4 localized foot or ankle pain was the most common complaint at time of presentation in our study; 100% of our patients had it. All but 1 patient (92%) in our study described pain that was worse at night and relieved by aspirin or other nonsteroidal anti-inflammatory medications. Pain reduction after NSAID use was observed in 92% (12/13) of our patients as well; the 1 patient who did not report pain relief had not used NSAIDs before being evaluated at our institution. Our patient population reported night pain and pain relief with NSAID use more frequently than patients in other studies did.15,16
The bone most commonly involved in our patients’ foot and ankle OOs was the talus (5/13, 38%). This is in accord with 1 study1 but contradicts another, in which the most common foot and ankle site was the calcaneus.17 The site of the lesion in the bone can be subclassified as cortical, cancellous, or subperiosteal.11,12 Cortical OOs were the most common in our study, but in previous reports the most common were subperiosteal and cancellous.1,11 As all our OOs were cortical, we classified them (on the basis of the relationship of the nidus to the cortex) as intracortical, periosteal, or subcortical (endosteal) instead of subperiosteal or cancellous. Three of our patients’ lesions were intracortical, 8 were subcortical, and 2 patients did not have radiographs available for review at the time of the study.
Although the classic clinical presentation of OO is often sufficient to raise suspicion for the diagnosis, imaging studies play a crucial role in accurate diagnosis. An accurate diagnosis of OO in the long bones can be made if the lesion presents with characteristic imaging features, as a small round lytic lesion with associated cortical thickening, medullary sclerosis, and chronic benign periosteal new bone formation.15 In some cases, however, the nidus may be obscured by the extensive associated reactive changes on the radiographs, and therefore the differential diagnosis may also include stress fracture, Brodie abscess, or even osteosarcoma. High-resolution CT is the imaging modality of choice for accurate diagnosis of OO, and it often plays an instrumental role in making the diagnosis and excluding other diagnostic possibilities.15-17
As foot OOs often occur near the joint (7 intra-articular lesions in our study), they often lack the exuberant periosteal reaction, cortical thickening, and reactive medullary sclerosis that characterize these lesions in the appendicular skeleton.17 In addition, the anatomical complexity of the small bones of the foot and ankle, particularly the hindfoot, where the bones are flat and irregular, makes identifying the lesions difficult.17 Conventional radiographs are the initial imaging modality of choice for evaluating patients with a clinical suspicion of OO, and they may identify the tumor. However, if radiographs are nondiagnostic, and the diagnosis of OO is suspected, high-resolution CT should be performed.
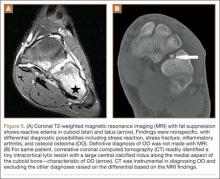
MRI is commonly used to assess for ligamentous, tendinous, and articular cartilage injuries in patients with ankle and hindfoot pain. However, as already discussed, and as reported in previous studies,17 accurate diagnosis of OO can be challenging with MRI (Figure 5A), and often the patients who had MRI scans then underwent CT (Table) for the definitive diagnosis (Figure 5B). In only 1 patient in our study was MRI used to make the preoperative diagnosis of OO (Table). In 2 patients (15%), even advanced imaging did not result in OO being included in the differential diagnosis. This is consistent with other reports, which found that a diagnosis was not made in 11% of patients.16 Although almost a quarter of patients did not have radiographic features diagnostic of OO, CT is the modality of choice for all patients who have clinical features suggestive of a diagnosis of OO.
Surgical treatment of OO is effective when the entire nidus is removed, with excision providing rapid pain relief.4,6,7,11,12 Historically, the tumor was often treated with wide, en bloc resection, but this is a large operation involving removal of a substantial amount of surrounding normal bone, as the lesion is often difficult to identify intraoperatively without preoperative localization.4,6,13 Curettage was performed on the lesion to reduce the amount of bone removed.4 Both techniques are reportedly very successful in treating OOs, with recurrence rates ranging from 0% to 15%.18,19 In our study, none of the surgically treated lesions recurred, and their AOFAS score improved from 67.11 (range, 54-80) before surgery to 98.33 (range, 93-100) after surgery. However, all surgically treated patients required a mean of 3 weeks (0-2.5 months) of either partial weight-bearing or non-weight-bearing of the affected extremity. A variety of treatment techniques have been used as alternatives to surgical resection in an attempt to treat OOs effectively and minimize damage to the surrounding normal bone.4,6,13 These techniques have included percutaneous CT-guided tumor excision with a trephine; percutaneous or surgical ablation using laser, cryotherapy, or ethanol; CT-guided localization followed by operative excision; and CT-guided percutaneous RFA.4,6,13,20 Over the past 2 decades, CT-guided percutaneous RFA has evolved to become the treatment of choice for painful OOs of the appendicular skeleton.15,21,22 The success of this procedure depends on accurate preprocedure diagnosis and precise anatomical localization with CT. Our results correlate with those in series reported in the literature, showing no significant difference in tumor recurrence rates between this technique and surgical excision.22
In our study, 3 patients were treated with CT-guided RFA. Because of recurrent pain, 1 of these patients had a repeat RFA 4 months after the initial procedure. After the second procedure, the patient was asymptomatic. Pain recurrence rates have ranged from 2% to 11% in large series of treated nonspinal OOs.21-23 Our RFA patients’ mean AOFAS score notably improved from 60.33 (range, 60-61) before surgery to 96.66 (range, 90-100) after surgery.
One of the distinct advantages of CT-guided RFA of OO is that it provides a minimally invasive technique for curative treatment with minimal damage to the adjacent normal bone by providing selective and controlled ablation of the tumor nidus.15 Additional advantages are that it can be performed as an outpatient procedure, and patients convalesce quickly with unrestricted weight-bearing and immediate return to activities of daily living.21-23 In addition, when RFA and surgical excision were compared on their average costs of hospitalization and treatment for OO, RFA was found to be less expensive.24
There were no RFA-related complications in our study population, but complications have been reported (albeit rarely) in other large studies of using RFA throughout the appendicular skeleton.21,25 Reported complications include skin burns, nerve damage, reflex sympathetic dystrophy, cellulitis, and thrombophlebitis.21,25 To reduce the risk for these complications, the investigators emphasized the importance of avoiding use of RFA for lesions near a neurovascular bundle (<1.5 cm away) or in a superficial location near the surface of the skin (<1.0 cm away).21,25
We believe that surgical resection and RFA provide equally effective treatment outcomes for patients with foot and ankle OOs. The major contraindication to RFA is anatomical proximity (<1.5 cm) to a major neurovascular bundle. Theoretically, articular cartilage can be damaged during RFA.21,25 To our knowledge, there have been no reported complications involving articular cartilage damage. However, surgeons should carefully measure the distance from lesion to articular cartilage and select the treatment option that will cause the least amount of damage to the cartilage.
Two limitations of this study are its retrospective nature and relatively small number of patients. As all the lesions in the study were treated surgically or with RFA, we are unable to comment on the natural history of untreated foot and ankle OOs. Although there were no recurrences, late recurrence is possible with longer follow-up. However, we think this study will not only increase familiarity with the imaging features of OOs involving the bones of the foot and ankle, but it will help clinicians formulate optimal treatment plans.
Overall, OOs are relatively common benign bone tumors, with limited reports of their occurrence in the foot and ankle. There should be a high index of suspicion for the diagnosis if a patient presents with the symptoms classically associated with the tumor, but in some cases the diagnosis can be challenging. Proper imaging is essential for prompt and accurate diagnosis.
1. Shereff MJ, Cullivan WT, Johnson KA. Osteoid-osteoma of the foot. J Bone Joint Surg Am. 1983;65(5):638-641.
2. Snow SW, Sobel M, DiCarlo EF, Thompson FM, Deland JT. Chronic ankle pain caused by osteoid osteoma of the neck of the talus. Foot Ankle Int. 1997;18(2):98-101.
3. Greco F, Tamburrelli F, Ciabattoni G. Prostaglandins in osteoid osteoma. Int Orthop. 1991;15(1):35-37.
4. Lee EH, Shafi M, Hui JH. Osteoid osteoma: a current review. J Ped Orthop. 2006;26(5):695-700.
5. Jaffe HL. Osteoid-osteoma: a benign osteoblastic tumour composed of osteoid and atypical bone. Arch Surg. 1935;31:19.
6. Ghanem I. The management of osteoid osteoma: updates and controversies. Curr Opin Pediatr. 2006;18(1):36-41.
7. Klein MH, Shankman S. Osteoid osteoma: radiologic and pathologic correlation. Skeletal Radiol. 1992;21(1):23-31.
8. Casadei R, Ferraro A, Ferruzzi A, Biagini R, Ruggieri P. Bone tumors of the foot: epidemiology and diagnosis. Chir Organi Mov. 1991;76(1):47-62.
9. Ebrahimzadeh MH, Ahmadzadeh-Chabock H, Ebrahimzadeh AR. Osteoid osteoma: a diagnosis for radicular pain of extremities. Orthopedics. 2009;32(11):821.
10. Lander PH, Azouz EM, Marton D. Subperiosteal osteoid osteoma of the talus. Clin Radiol. 1986;37(5):491-493.
11. Oztürk A, Yalçinkaya U, Ozkan Y, Yalçin N. Subperiosteal osteoid osteoma in the hallux of a 9-year-old female. J Foot Ankle Surg. 2008;47(6):579-582.
12. Sproule JA, Khan F, Fogarty EE. Osteoid osteoma: painful enlargement of the second toe. Arch Orthop Trauma Surg. 2004;124(5):354-356.
13. Atesok KI, Alman BA, Schemitsch EH, Peyser A, Mankin H. Osteoid osteoma and osteoblastoma. J Am Acad Orthop Surg. 2011;19(11):678-689.
14. Schulman L, Dorfman HD. Nerve fibers in osteoid osteoma. J Bone Joint Surg Am. 1970;52(7):1351-1356.
15. Rosenthal DI, Alexander A, Rosenberg AE, Springfield D. Ablation of osteoid osteomas with a percutaneously placed electrode: a new procedure. Radiology. 1992;183(1):29-33.
16. Gamba JL, Martinez S, Apple J, Harrelson JM, Nunley JA. Computed tomography of axial skeletal osteoid osteomas. AJR Am J Roentgenol. 1984;142(4):769-772.
17. Shukla S, Clarke AW, Saifuddin A. Imaging features of foot osteoid osteoma. Skeletal Radiol. 2010;39(7):683-689.
18. Sluga M, Windhager R, Pfeiffer M, Dominkus M, Kotz R. Peripheral osteoid osteoma. Is there still a place for traditional surgery? J Bone Joint Surg Br. 2002;84(2):249-251.
19. Ward WG, Eckardt JJ, Shayestehfar S, et al. Osteoid osteoma diagnosis and management with low morbidity. Clin Orthop. 1993;(291):229-235.
20. Donahue F, Ahmad A, Mnaymneh W, Pevsner NH. Osteoid osteoma. Computed tomography guided percutaneous excision. Clin Orthop. 1999;(366):191-196.
21. Rosenthal DI, Hornicek FJ, Torriani M, Gebhardt MC, Mankin HJ. Osteoid osteoma: percutaneous treatment with radiofrequency energy. Radiology. 2003;229(1):171-175.
22. Rosenthal DI, Hornicek FJ, Wolfe MW, et al. Percutaneous radiofrequency coagulation of osteoid osteoma compared with operative treatment. J Bone Joint Surg Am. 1998;80(6):815-821.
23. Rosenthal DI, Hornicek FJ, Wolfe MW, Jennings LC, Gebhardt MC, Mankin HJ. Decreasing length of hospital stay in treatment of osteoid osteoma. Clin Orthop. 1999;(361):186-191.
24. Lindner NJ, Scarborough M, Ciccarelli JM, Enneking WF. CT-controlled thermocoagulation of osteoid osteoma in comparison with traditional methods [in German]. Z Orthop Ihre Grenzgeb. 1997;135(6):522-527.
25. Rimondi E, Mavrogenis AF, Rossi G, et al. Radiofrequency ablation for non-spinal osteoid osteomas in 557 patients. Eur Radiol. 2012;22(1):181-188.
Because of the complex anatomy of the ankle joint and foot, the wide array of possible bone and soft-tissue injuries, and the uncommon occurrence of tumors at these sites, osteoid osteomas (OOs) are often not included in the differential diagnosis of foot and ankle pain.1,2 Patients with OO usually complain of severe pain that is worse at night and is relieved with use of nonsteroidal anti-inflammatory drugs (NSAIDs).1-4 This classic clinical presentation, combined with the characteristic imaging features, facilitates making an accurate diagnosis.
OOs were first described in 1935 by Jaffe,5 who characterized them as benign, solitary, osteoblastic tumors consisting of atypical bone and osteoid. On radiographs and thin-slice computed tomography (CT), these tumors are small osteolytic lesions surrounded by a larger region of cortical thickening, medullary sclerosis, and benign periosteal new bone formation.4,6,7 They often contain a central focus of calcification—the nidus. OOs typically occur in children and young adults; the majority of patients are younger than 25 years. OOs show a predilection for the appendicular skeleton, with the majority of the lesions in the femur and tibia.4,6,7 OOs infrequently occur in the bones of the hands and feet.8-12 Previous studies of foot and ankle OOs have been predominantly limited to case reports; the largest study, conducted almost 20 years ago, included only 10 patients.1
We conducted a study to evaluate the epidemiology and radiographic features of foot and ankle OOs, to evaluate surgical treatment options and outcomes in patients with foot and ankle OOs, and to evaluate the disease course of patients with foot and ankle OOs treated surgically or with radiofrequency ablation (RFA).
Materials and Methods
After obtaining approval from our institutional review board, we retrospectively reviewed all cases of patients who underwent a surgical or an interventional radiologic procedure and had a preoperative diagnosis of a lower extremity OO between 1990 and 2010. Only patients with a histologically confirmed diagnosis of OO were included in the review of foot and ankle cases.
The medical records of patients with a diagnosis of foot or ankle OO were reviewed for patient sex, age, OO site, clinical presentation, radiographic studies, pain characteristics, treatment modality, histologic diagnosis, and clinical outcome of the surgical or RFA procedure. Preoperative and postoperative clinical outcome scores were calculated using American Orthopedic Foot and Ankle Society (AOFAS) scores.
Whether to perform surgical excision or RFA was discussed between the treating surgeon and the radiologist before treatment. The goal was to treat each lesion while minimizing damage to normal, surrounding structures. If there was any question whether a lesion could be something other than OO based on radiographic features, the lesion was treated with surgical excision. Surgical excision consisted of curettage and bone grafting or en bloc removal. Surgical hardware was placed only when an osteotomy was needed to access the lesion. RFA was performed by consultant musculoskeletal radiologists. Before ablation, a CT-guided needle biopsy of the lesion was performed to obtain tissue for pathologic diagnosis. Recurrence was defined as return of preoperative symptoms after treatment, along with radiographic features of recurrence.
Statistical analysis was done with SPSS software (IBM, New York, New York) using unpaired Student t tests and Fisher exact tests. Statistical significance was set at P < .05.
Results
Of the 117 patients with a lower extremity OO, 13 (11%) had it in the bones of the foot or ankle (Table). Mean age at presentation was 20.1 years (range, 9-38 years). There was no statistically significant difference in age between patients with foot or ankle OO and patients with OO of the long bones of the lower extremity (P = .27). Of the 13 patients, 12 were male and 1 was female (Table). The foot and ankle OO sites were the talus (n = 5), the distal tibia/plafond (n = 3), the calcaneus (n = 2), the tarsal bones (n = 2), and the phalanx (n = 1). All 13 foot and ankle lesions were histologically confirmed as OO.
The 13 patients’ primary complaint was foot or ankle pain. Ten of the 13 were referred to our institution for clinical workup and management of foot or ankle pain and for assessment of radiographic features of OO (Figure 1). For all patients in the study, preoperative plain film radiographs of the affected extremity were obtained. Nine patients (69%) had a CT scan (Figure 2), 6 (46%) had a magnetic resonance imaging (MRI) scan, and 2 (15%) had a bone scan. Despite undergoing advanced imaging (1 CT, 1 MRI), 2 patients (15%) did not get a differential diagnosis of OO before being treated. The same 2 patients did not have radiographic images available for review to determine why a differential diagnosis of OO was not included based on imaging features prior to surgery. For the patients who did not have a diagnosis of OO before being evaluated at our institution, preliminary diagnoses included osteomyelitis and painful osteophytes. Twelve of the 13 patients complained of pain that was worse at night and was not relieved with use of NSAIDs. Mean time from symptom onset to presentation at our institution was 14.4 months (range, 3-42 months). All patients reported pain relief after the procedure. There was a significant (P = .0001) increase in AOFAS scores after surgery. Mean AOFAS score was 65.42 (range, 54-80) before surgery and 97.91 (range, 90-100) after surgery.
Before 1998, all foot and ankle OOs (n = 6) were treated with surgical excision. After RFA was introduced at our institution, 3 foot and ankle OOs (43%) were treated with RFA (Figures 3A, 3B), and 4 (57%) were treated with surgical curettage (Figure 4). The 4 surgical patients’ OOs were not amenable to RFA primarily because of anatomical considerations: In 2 patients, the OO was too near the articular surface; in another patient, the lesion was in intimate contact with a neurovascular bundle; in the fourth patient, the lesion was amenable to RFA, but the patient’s family selected surgical curettage instead.
Mean tumor nidus size was 7.5 mm (range, 3-12 mm). Bone graft was placed in 3 patients (30%), and surgical hardware was placed to repair a medial malleolar osteotomy in 1 (10%) of the patients treated surgically. The majority of the lesions (8) were in cancellous bone in a subcortical location. Three lesions were intracortical. Seven lesions were intra-articular, and 4 were extra-articular. Two patients did not have radiographic images available for review.
One patient had a recurrence of OO and underwent a repeat procedure 4 months after the initial one. At final follow-up, on average 1 year after the initial procedure (range, 2 weeks–3 years), there were no reported recurrences. One patient underwent a procedure to remove painful hardware that had been implanted, during the primary procedure, to repair the medial malleolar osteotomy used to access the lesion. Recurrence rates for RFA (n = 1) and surgical excision (n = 0) were similar.
Discussion
OOs are relatively common bone tumors that account for about 13% of all benign bone tumors.4,13 OOs typically occur in children or young adults—the majority of patients are younger than 25 years—and are 3 times more common in males than females.4,13 Our findings for all patients with a lower extremity OO are consistent with those previously reported: male predominance (75 males, 42 females) and mean age under 25 years (mean age, 18.7 years). In patients with foot or ankle OO, male predominance was substantially greater (12 males, 1 female), though mean age at presentation (20.1 years) was similar.
Local pain is the most common complaint in patients who present with OO.4,13 Pain is thought to be generated by a combination of multiple nerve endings in the tumor14 and prostaglandin production by the tumor nidus (prostaglandins E2 and I2)3 causing an inflammatory reaction.6 In accord with previous studies,4 localized foot or ankle pain was the most common complaint at time of presentation in our study; 100% of our patients had it. All but 1 patient (92%) in our study described pain that was worse at night and relieved by aspirin or other nonsteroidal anti-inflammatory medications. Pain reduction after NSAID use was observed in 92% (12/13) of our patients as well; the 1 patient who did not report pain relief had not used NSAIDs before being evaluated at our institution. Our patient population reported night pain and pain relief with NSAID use more frequently than patients in other studies did.15,16
The bone most commonly involved in our patients’ foot and ankle OOs was the talus (5/13, 38%). This is in accord with 1 study1 but contradicts another, in which the most common foot and ankle site was the calcaneus.17 The site of the lesion in the bone can be subclassified as cortical, cancellous, or subperiosteal.11,12 Cortical OOs were the most common in our study, but in previous reports the most common were subperiosteal and cancellous.1,11 As all our OOs were cortical, we classified them (on the basis of the relationship of the nidus to the cortex) as intracortical, periosteal, or subcortical (endosteal) instead of subperiosteal or cancellous. Three of our patients’ lesions were intracortical, 8 were subcortical, and 2 patients did not have radiographs available for review at the time of the study.
Although the classic clinical presentation of OO is often sufficient to raise suspicion for the diagnosis, imaging studies play a crucial role in accurate diagnosis. An accurate diagnosis of OO in the long bones can be made if the lesion presents with characteristic imaging features, as a small round lytic lesion with associated cortical thickening, medullary sclerosis, and chronic benign periosteal new bone formation.15 In some cases, however, the nidus may be obscured by the extensive associated reactive changes on the radiographs, and therefore the differential diagnosis may also include stress fracture, Brodie abscess, or even osteosarcoma. High-resolution CT is the imaging modality of choice for accurate diagnosis of OO, and it often plays an instrumental role in making the diagnosis and excluding other diagnostic possibilities.15-17
As foot OOs often occur near the joint (7 intra-articular lesions in our study), they often lack the exuberant periosteal reaction, cortical thickening, and reactive medullary sclerosis that characterize these lesions in the appendicular skeleton.17 In addition, the anatomical complexity of the small bones of the foot and ankle, particularly the hindfoot, where the bones are flat and irregular, makes identifying the lesions difficult.17 Conventional radiographs are the initial imaging modality of choice for evaluating patients with a clinical suspicion of OO, and they may identify the tumor. However, if radiographs are nondiagnostic, and the diagnosis of OO is suspected, high-resolution CT should be performed.
MRI is commonly used to assess for ligamentous, tendinous, and articular cartilage injuries in patients with ankle and hindfoot pain. However, as already discussed, and as reported in previous studies,17 accurate diagnosis of OO can be challenging with MRI (Figure 5A), and often the patients who had MRI scans then underwent CT (Table) for the definitive diagnosis (Figure 5B). In only 1 patient in our study was MRI used to make the preoperative diagnosis of OO (Table). In 2 patients (15%), even advanced imaging did not result in OO being included in the differential diagnosis. This is consistent with other reports, which found that a diagnosis was not made in 11% of patients.16 Although almost a quarter of patients did not have radiographic features diagnostic of OO, CT is the modality of choice for all patients who have clinical features suggestive of a diagnosis of OO.
Surgical treatment of OO is effective when the entire nidus is removed, with excision providing rapid pain relief.4,6,7,11,12 Historically, the tumor was often treated with wide, en bloc resection, but this is a large operation involving removal of a substantial amount of surrounding normal bone, as the lesion is often difficult to identify intraoperatively without preoperative localization.4,6,13 Curettage was performed on the lesion to reduce the amount of bone removed.4 Both techniques are reportedly very successful in treating OOs, with recurrence rates ranging from 0% to 15%.18,19 In our study, none of the surgically treated lesions recurred, and their AOFAS score improved from 67.11 (range, 54-80) before surgery to 98.33 (range, 93-100) after surgery. However, all surgically treated patients required a mean of 3 weeks (0-2.5 months) of either partial weight-bearing or non-weight-bearing of the affected extremity. A variety of treatment techniques have been used as alternatives to surgical resection in an attempt to treat OOs effectively and minimize damage to the surrounding normal bone.4,6,13 These techniques have included percutaneous CT-guided tumor excision with a trephine; percutaneous or surgical ablation using laser, cryotherapy, or ethanol; CT-guided localization followed by operative excision; and CT-guided percutaneous RFA.4,6,13,20 Over the past 2 decades, CT-guided percutaneous RFA has evolved to become the treatment of choice for painful OOs of the appendicular skeleton.15,21,22 The success of this procedure depends on accurate preprocedure diagnosis and precise anatomical localization with CT. Our results correlate with those in series reported in the literature, showing no significant difference in tumor recurrence rates between this technique and surgical excision.22
In our study, 3 patients were treated with CT-guided RFA. Because of recurrent pain, 1 of these patients had a repeat RFA 4 months after the initial procedure. After the second procedure, the patient was asymptomatic. Pain recurrence rates have ranged from 2% to 11% in large series of treated nonspinal OOs.21-23 Our RFA patients’ mean AOFAS score notably improved from 60.33 (range, 60-61) before surgery to 96.66 (range, 90-100) after surgery.
One of the distinct advantages of CT-guided RFA of OO is that it provides a minimally invasive technique for curative treatment with minimal damage to the adjacent normal bone by providing selective and controlled ablation of the tumor nidus.15 Additional advantages are that it can be performed as an outpatient procedure, and patients convalesce quickly with unrestricted weight-bearing and immediate return to activities of daily living.21-23 In addition, when RFA and surgical excision were compared on their average costs of hospitalization and treatment for OO, RFA was found to be less expensive.24
There were no RFA-related complications in our study population, but complications have been reported (albeit rarely) in other large studies of using RFA throughout the appendicular skeleton.21,25 Reported complications include skin burns, nerve damage, reflex sympathetic dystrophy, cellulitis, and thrombophlebitis.21,25 To reduce the risk for these complications, the investigators emphasized the importance of avoiding use of RFA for lesions near a neurovascular bundle (<1.5 cm away) or in a superficial location near the surface of the skin (<1.0 cm away).21,25
We believe that surgical resection and RFA provide equally effective treatment outcomes for patients with foot and ankle OOs. The major contraindication to RFA is anatomical proximity (<1.5 cm) to a major neurovascular bundle. Theoretically, articular cartilage can be damaged during RFA.21,25 To our knowledge, there have been no reported complications involving articular cartilage damage. However, surgeons should carefully measure the distance from lesion to articular cartilage and select the treatment option that will cause the least amount of damage to the cartilage.
Two limitations of this study are its retrospective nature and relatively small number of patients. As all the lesions in the study were treated surgically or with RFA, we are unable to comment on the natural history of untreated foot and ankle OOs. Although there were no recurrences, late recurrence is possible with longer follow-up. However, we think this study will not only increase familiarity with the imaging features of OOs involving the bones of the foot and ankle, but it will help clinicians formulate optimal treatment plans.
Overall, OOs are relatively common benign bone tumors, with limited reports of their occurrence in the foot and ankle. There should be a high index of suspicion for the diagnosis if a patient presents with the symptoms classically associated with the tumor, but in some cases the diagnosis can be challenging. Proper imaging is essential for prompt and accurate diagnosis.
Because of the complex anatomy of the ankle joint and foot, the wide array of possible bone and soft-tissue injuries, and the uncommon occurrence of tumors at these sites, osteoid osteomas (OOs) are often not included in the differential diagnosis of foot and ankle pain.1,2 Patients with OO usually complain of severe pain that is worse at night and is relieved with use of nonsteroidal anti-inflammatory drugs (NSAIDs).1-4 This classic clinical presentation, combined with the characteristic imaging features, facilitates making an accurate diagnosis.
OOs were first described in 1935 by Jaffe,5 who characterized them as benign, solitary, osteoblastic tumors consisting of atypical bone and osteoid. On radiographs and thin-slice computed tomography (CT), these tumors are small osteolytic lesions surrounded by a larger region of cortical thickening, medullary sclerosis, and benign periosteal new bone formation.4,6,7 They often contain a central focus of calcification—the nidus. OOs typically occur in children and young adults; the majority of patients are younger than 25 years. OOs show a predilection for the appendicular skeleton, with the majority of the lesions in the femur and tibia.4,6,7 OOs infrequently occur in the bones of the hands and feet.8-12 Previous studies of foot and ankle OOs have been predominantly limited to case reports; the largest study, conducted almost 20 years ago, included only 10 patients.1
We conducted a study to evaluate the epidemiology and radiographic features of foot and ankle OOs, to evaluate surgical treatment options and outcomes in patients with foot and ankle OOs, and to evaluate the disease course of patients with foot and ankle OOs treated surgically or with radiofrequency ablation (RFA).
Materials and Methods
After obtaining approval from our institutional review board, we retrospectively reviewed all cases of patients who underwent a surgical or an interventional radiologic procedure and had a preoperative diagnosis of a lower extremity OO between 1990 and 2010. Only patients with a histologically confirmed diagnosis of OO were included in the review of foot and ankle cases.
The medical records of patients with a diagnosis of foot or ankle OO were reviewed for patient sex, age, OO site, clinical presentation, radiographic studies, pain characteristics, treatment modality, histologic diagnosis, and clinical outcome of the surgical or RFA procedure. Preoperative and postoperative clinical outcome scores were calculated using American Orthopedic Foot and Ankle Society (AOFAS) scores.
Whether to perform surgical excision or RFA was discussed between the treating surgeon and the radiologist before treatment. The goal was to treat each lesion while minimizing damage to normal, surrounding structures. If there was any question whether a lesion could be something other than OO based on radiographic features, the lesion was treated with surgical excision. Surgical excision consisted of curettage and bone grafting or en bloc removal. Surgical hardware was placed only when an osteotomy was needed to access the lesion. RFA was performed by consultant musculoskeletal radiologists. Before ablation, a CT-guided needle biopsy of the lesion was performed to obtain tissue for pathologic diagnosis. Recurrence was defined as return of preoperative symptoms after treatment, along with radiographic features of recurrence.
Statistical analysis was done with SPSS software (IBM, New York, New York) using unpaired Student t tests and Fisher exact tests. Statistical significance was set at P < .05.
Results
Of the 117 patients with a lower extremity OO, 13 (11%) had it in the bones of the foot or ankle (Table). Mean age at presentation was 20.1 years (range, 9-38 years). There was no statistically significant difference in age between patients with foot or ankle OO and patients with OO of the long bones of the lower extremity (P = .27). Of the 13 patients, 12 were male and 1 was female (Table). The foot and ankle OO sites were the talus (n = 5), the distal tibia/plafond (n = 3), the calcaneus (n = 2), the tarsal bones (n = 2), and the phalanx (n = 1). All 13 foot and ankle lesions were histologically confirmed as OO.
The 13 patients’ primary complaint was foot or ankle pain. Ten of the 13 were referred to our institution for clinical workup and management of foot or ankle pain and for assessment of radiographic features of OO (Figure 1). For all patients in the study, preoperative plain film radiographs of the affected extremity were obtained. Nine patients (69%) had a CT scan (Figure 2), 6 (46%) had a magnetic resonance imaging (MRI) scan, and 2 (15%) had a bone scan. Despite undergoing advanced imaging (1 CT, 1 MRI), 2 patients (15%) did not get a differential diagnosis of OO before being treated. The same 2 patients did not have radiographic images available for review to determine why a differential diagnosis of OO was not included based on imaging features prior to surgery. For the patients who did not have a diagnosis of OO before being evaluated at our institution, preliminary diagnoses included osteomyelitis and painful osteophytes. Twelve of the 13 patients complained of pain that was worse at night and was not relieved with use of NSAIDs. Mean time from symptom onset to presentation at our institution was 14.4 months (range, 3-42 months). All patients reported pain relief after the procedure. There was a significant (P = .0001) increase in AOFAS scores after surgery. Mean AOFAS score was 65.42 (range, 54-80) before surgery and 97.91 (range, 90-100) after surgery.
Before 1998, all foot and ankle OOs (n = 6) were treated with surgical excision. After RFA was introduced at our institution, 3 foot and ankle OOs (43%) were treated with RFA (Figures 3A, 3B), and 4 (57%) were treated with surgical curettage (Figure 4). The 4 surgical patients’ OOs were not amenable to RFA primarily because of anatomical considerations: In 2 patients, the OO was too near the articular surface; in another patient, the lesion was in intimate contact with a neurovascular bundle; in the fourth patient, the lesion was amenable to RFA, but the patient’s family selected surgical curettage instead.
Mean tumor nidus size was 7.5 mm (range, 3-12 mm). Bone graft was placed in 3 patients (30%), and surgical hardware was placed to repair a medial malleolar osteotomy in 1 (10%) of the patients treated surgically. The majority of the lesions (8) were in cancellous bone in a subcortical location. Three lesions were intracortical. Seven lesions were intra-articular, and 4 were extra-articular. Two patients did not have radiographic images available for review.
One patient had a recurrence of OO and underwent a repeat procedure 4 months after the initial one. At final follow-up, on average 1 year after the initial procedure (range, 2 weeks–3 years), there were no reported recurrences. One patient underwent a procedure to remove painful hardware that had been implanted, during the primary procedure, to repair the medial malleolar osteotomy used to access the lesion. Recurrence rates for RFA (n = 1) and surgical excision (n = 0) were similar.
Discussion
OOs are relatively common bone tumors that account for about 13% of all benign bone tumors.4,13 OOs typically occur in children or young adults—the majority of patients are younger than 25 years—and are 3 times more common in males than females.4,13 Our findings for all patients with a lower extremity OO are consistent with those previously reported: male predominance (75 males, 42 females) and mean age under 25 years (mean age, 18.7 years). In patients with foot or ankle OO, male predominance was substantially greater (12 males, 1 female), though mean age at presentation (20.1 years) was similar.
Local pain is the most common complaint in patients who present with OO.4,13 Pain is thought to be generated by a combination of multiple nerve endings in the tumor14 and prostaglandin production by the tumor nidus (prostaglandins E2 and I2)3 causing an inflammatory reaction.6 In accord with previous studies,4 localized foot or ankle pain was the most common complaint at time of presentation in our study; 100% of our patients had it. All but 1 patient (92%) in our study described pain that was worse at night and relieved by aspirin or other nonsteroidal anti-inflammatory medications. Pain reduction after NSAID use was observed in 92% (12/13) of our patients as well; the 1 patient who did not report pain relief had not used NSAIDs before being evaluated at our institution. Our patient population reported night pain and pain relief with NSAID use more frequently than patients in other studies did.15,16
The bone most commonly involved in our patients’ foot and ankle OOs was the talus (5/13, 38%). This is in accord with 1 study1 but contradicts another, in which the most common foot and ankle site was the calcaneus.17 The site of the lesion in the bone can be subclassified as cortical, cancellous, or subperiosteal.11,12 Cortical OOs were the most common in our study, but in previous reports the most common were subperiosteal and cancellous.1,11 As all our OOs were cortical, we classified them (on the basis of the relationship of the nidus to the cortex) as intracortical, periosteal, or subcortical (endosteal) instead of subperiosteal or cancellous. Three of our patients’ lesions were intracortical, 8 were subcortical, and 2 patients did not have radiographs available for review at the time of the study.
Although the classic clinical presentation of OO is often sufficient to raise suspicion for the diagnosis, imaging studies play a crucial role in accurate diagnosis. An accurate diagnosis of OO in the long bones can be made if the lesion presents with characteristic imaging features, as a small round lytic lesion with associated cortical thickening, medullary sclerosis, and chronic benign periosteal new bone formation.15 In some cases, however, the nidus may be obscured by the extensive associated reactive changes on the radiographs, and therefore the differential diagnosis may also include stress fracture, Brodie abscess, or even osteosarcoma. High-resolution CT is the imaging modality of choice for accurate diagnosis of OO, and it often plays an instrumental role in making the diagnosis and excluding other diagnostic possibilities.15-17
As foot OOs often occur near the joint (7 intra-articular lesions in our study), they often lack the exuberant periosteal reaction, cortical thickening, and reactive medullary sclerosis that characterize these lesions in the appendicular skeleton.17 In addition, the anatomical complexity of the small bones of the foot and ankle, particularly the hindfoot, where the bones are flat and irregular, makes identifying the lesions difficult.17 Conventional radiographs are the initial imaging modality of choice for evaluating patients with a clinical suspicion of OO, and they may identify the tumor. However, if radiographs are nondiagnostic, and the diagnosis of OO is suspected, high-resolution CT should be performed.
MRI is commonly used to assess for ligamentous, tendinous, and articular cartilage injuries in patients with ankle and hindfoot pain. However, as already discussed, and as reported in previous studies,17 accurate diagnosis of OO can be challenging with MRI (Figure 5A), and often the patients who had MRI scans then underwent CT (Table) for the definitive diagnosis (Figure 5B). In only 1 patient in our study was MRI used to make the preoperative diagnosis of OO (Table). In 2 patients (15%), even advanced imaging did not result in OO being included in the differential diagnosis. This is consistent with other reports, which found that a diagnosis was not made in 11% of patients.16 Although almost a quarter of patients did not have radiographic features diagnostic of OO, CT is the modality of choice for all patients who have clinical features suggestive of a diagnosis of OO.
Surgical treatment of OO is effective when the entire nidus is removed, with excision providing rapid pain relief.4,6,7,11,12 Historically, the tumor was often treated with wide, en bloc resection, but this is a large operation involving removal of a substantial amount of surrounding normal bone, as the lesion is often difficult to identify intraoperatively without preoperative localization.4,6,13 Curettage was performed on the lesion to reduce the amount of bone removed.4 Both techniques are reportedly very successful in treating OOs, with recurrence rates ranging from 0% to 15%.18,19 In our study, none of the surgically treated lesions recurred, and their AOFAS score improved from 67.11 (range, 54-80) before surgery to 98.33 (range, 93-100) after surgery. However, all surgically treated patients required a mean of 3 weeks (0-2.5 months) of either partial weight-bearing or non-weight-bearing of the affected extremity. A variety of treatment techniques have been used as alternatives to surgical resection in an attempt to treat OOs effectively and minimize damage to the surrounding normal bone.4,6,13 These techniques have included percutaneous CT-guided tumor excision with a trephine; percutaneous or surgical ablation using laser, cryotherapy, or ethanol; CT-guided localization followed by operative excision; and CT-guided percutaneous RFA.4,6,13,20 Over the past 2 decades, CT-guided percutaneous RFA has evolved to become the treatment of choice for painful OOs of the appendicular skeleton.15,21,22 The success of this procedure depends on accurate preprocedure diagnosis and precise anatomical localization with CT. Our results correlate with those in series reported in the literature, showing no significant difference in tumor recurrence rates between this technique and surgical excision.22
In our study, 3 patients were treated with CT-guided RFA. Because of recurrent pain, 1 of these patients had a repeat RFA 4 months after the initial procedure. After the second procedure, the patient was asymptomatic. Pain recurrence rates have ranged from 2% to 11% in large series of treated nonspinal OOs.21-23 Our RFA patients’ mean AOFAS score notably improved from 60.33 (range, 60-61) before surgery to 96.66 (range, 90-100) after surgery.
One of the distinct advantages of CT-guided RFA of OO is that it provides a minimally invasive technique for curative treatment with minimal damage to the adjacent normal bone by providing selective and controlled ablation of the tumor nidus.15 Additional advantages are that it can be performed as an outpatient procedure, and patients convalesce quickly with unrestricted weight-bearing and immediate return to activities of daily living.21-23 In addition, when RFA and surgical excision were compared on their average costs of hospitalization and treatment for OO, RFA was found to be less expensive.24
There were no RFA-related complications in our study population, but complications have been reported (albeit rarely) in other large studies of using RFA throughout the appendicular skeleton.21,25 Reported complications include skin burns, nerve damage, reflex sympathetic dystrophy, cellulitis, and thrombophlebitis.21,25 To reduce the risk for these complications, the investigators emphasized the importance of avoiding use of RFA for lesions near a neurovascular bundle (<1.5 cm away) or in a superficial location near the surface of the skin (<1.0 cm away).21,25
We believe that surgical resection and RFA provide equally effective treatment outcomes for patients with foot and ankle OOs. The major contraindication to RFA is anatomical proximity (<1.5 cm) to a major neurovascular bundle. Theoretically, articular cartilage can be damaged during RFA.21,25 To our knowledge, there have been no reported complications involving articular cartilage damage. However, surgeons should carefully measure the distance from lesion to articular cartilage and select the treatment option that will cause the least amount of damage to the cartilage.
Two limitations of this study are its retrospective nature and relatively small number of patients. As all the lesions in the study were treated surgically or with RFA, we are unable to comment on the natural history of untreated foot and ankle OOs. Although there were no recurrences, late recurrence is possible with longer follow-up. However, we think this study will not only increase familiarity with the imaging features of OOs involving the bones of the foot and ankle, but it will help clinicians formulate optimal treatment plans.
Overall, OOs are relatively common benign bone tumors, with limited reports of their occurrence in the foot and ankle. There should be a high index of suspicion for the diagnosis if a patient presents with the symptoms classically associated with the tumor, but in some cases the diagnosis can be challenging. Proper imaging is essential for prompt and accurate diagnosis.
1. Shereff MJ, Cullivan WT, Johnson KA. Osteoid-osteoma of the foot. J Bone Joint Surg Am. 1983;65(5):638-641.
2. Snow SW, Sobel M, DiCarlo EF, Thompson FM, Deland JT. Chronic ankle pain caused by osteoid osteoma of the neck of the talus. Foot Ankle Int. 1997;18(2):98-101.
3. Greco F, Tamburrelli F, Ciabattoni G. Prostaglandins in osteoid osteoma. Int Orthop. 1991;15(1):35-37.
4. Lee EH, Shafi M, Hui JH. Osteoid osteoma: a current review. J Ped Orthop. 2006;26(5):695-700.
5. Jaffe HL. Osteoid-osteoma: a benign osteoblastic tumour composed of osteoid and atypical bone. Arch Surg. 1935;31:19.
6. Ghanem I. The management of osteoid osteoma: updates and controversies. Curr Opin Pediatr. 2006;18(1):36-41.
7. Klein MH, Shankman S. Osteoid osteoma: radiologic and pathologic correlation. Skeletal Radiol. 1992;21(1):23-31.
8. Casadei R, Ferraro A, Ferruzzi A, Biagini R, Ruggieri P. Bone tumors of the foot: epidemiology and diagnosis. Chir Organi Mov. 1991;76(1):47-62.
9. Ebrahimzadeh MH, Ahmadzadeh-Chabock H, Ebrahimzadeh AR. Osteoid osteoma: a diagnosis for radicular pain of extremities. Orthopedics. 2009;32(11):821.
10. Lander PH, Azouz EM, Marton D. Subperiosteal osteoid osteoma of the talus. Clin Radiol. 1986;37(5):491-493.
11. Oztürk A, Yalçinkaya U, Ozkan Y, Yalçin N. Subperiosteal osteoid osteoma in the hallux of a 9-year-old female. J Foot Ankle Surg. 2008;47(6):579-582.
12. Sproule JA, Khan F, Fogarty EE. Osteoid osteoma: painful enlargement of the second toe. Arch Orthop Trauma Surg. 2004;124(5):354-356.
13. Atesok KI, Alman BA, Schemitsch EH, Peyser A, Mankin H. Osteoid osteoma and osteoblastoma. J Am Acad Orthop Surg. 2011;19(11):678-689.
14. Schulman L, Dorfman HD. Nerve fibers in osteoid osteoma. J Bone Joint Surg Am. 1970;52(7):1351-1356.
15. Rosenthal DI, Alexander A, Rosenberg AE, Springfield D. Ablation of osteoid osteomas with a percutaneously placed electrode: a new procedure. Radiology. 1992;183(1):29-33.
16. Gamba JL, Martinez S, Apple J, Harrelson JM, Nunley JA. Computed tomography of axial skeletal osteoid osteomas. AJR Am J Roentgenol. 1984;142(4):769-772.
17. Shukla S, Clarke AW, Saifuddin A. Imaging features of foot osteoid osteoma. Skeletal Radiol. 2010;39(7):683-689.
18. Sluga M, Windhager R, Pfeiffer M, Dominkus M, Kotz R. Peripheral osteoid osteoma. Is there still a place for traditional surgery? J Bone Joint Surg Br. 2002;84(2):249-251.
19. Ward WG, Eckardt JJ, Shayestehfar S, et al. Osteoid osteoma diagnosis and management with low morbidity. Clin Orthop. 1993;(291):229-235.
20. Donahue F, Ahmad A, Mnaymneh W, Pevsner NH. Osteoid osteoma. Computed tomography guided percutaneous excision. Clin Orthop. 1999;(366):191-196.
21. Rosenthal DI, Hornicek FJ, Torriani M, Gebhardt MC, Mankin HJ. Osteoid osteoma: percutaneous treatment with radiofrequency energy. Radiology. 2003;229(1):171-175.
22. Rosenthal DI, Hornicek FJ, Wolfe MW, et al. Percutaneous radiofrequency coagulation of osteoid osteoma compared with operative treatment. J Bone Joint Surg Am. 1998;80(6):815-821.
23. Rosenthal DI, Hornicek FJ, Wolfe MW, Jennings LC, Gebhardt MC, Mankin HJ. Decreasing length of hospital stay in treatment of osteoid osteoma. Clin Orthop. 1999;(361):186-191.
24. Lindner NJ, Scarborough M, Ciccarelli JM, Enneking WF. CT-controlled thermocoagulation of osteoid osteoma in comparison with traditional methods [in German]. Z Orthop Ihre Grenzgeb. 1997;135(6):522-527.
25. Rimondi E, Mavrogenis AF, Rossi G, et al. Radiofrequency ablation for non-spinal osteoid osteomas in 557 patients. Eur Radiol. 2012;22(1):181-188.
1. Shereff MJ, Cullivan WT, Johnson KA. Osteoid-osteoma of the foot. J Bone Joint Surg Am. 1983;65(5):638-641.
2. Snow SW, Sobel M, DiCarlo EF, Thompson FM, Deland JT. Chronic ankle pain caused by osteoid osteoma of the neck of the talus. Foot Ankle Int. 1997;18(2):98-101.
3. Greco F, Tamburrelli F, Ciabattoni G. Prostaglandins in osteoid osteoma. Int Orthop. 1991;15(1):35-37.
4. Lee EH, Shafi M, Hui JH. Osteoid osteoma: a current review. J Ped Orthop. 2006;26(5):695-700.
5. Jaffe HL. Osteoid-osteoma: a benign osteoblastic tumour composed of osteoid and atypical bone. Arch Surg. 1935;31:19.
6. Ghanem I. The management of osteoid osteoma: updates and controversies. Curr Opin Pediatr. 2006;18(1):36-41.
7. Klein MH, Shankman S. Osteoid osteoma: radiologic and pathologic correlation. Skeletal Radiol. 1992;21(1):23-31.
8. Casadei R, Ferraro A, Ferruzzi A, Biagini R, Ruggieri P. Bone tumors of the foot: epidemiology and diagnosis. Chir Organi Mov. 1991;76(1):47-62.
9. Ebrahimzadeh MH, Ahmadzadeh-Chabock H, Ebrahimzadeh AR. Osteoid osteoma: a diagnosis for radicular pain of extremities. Orthopedics. 2009;32(11):821.
10. Lander PH, Azouz EM, Marton D. Subperiosteal osteoid osteoma of the talus. Clin Radiol. 1986;37(5):491-493.
11. Oztürk A, Yalçinkaya U, Ozkan Y, Yalçin N. Subperiosteal osteoid osteoma in the hallux of a 9-year-old female. J Foot Ankle Surg. 2008;47(6):579-582.
12. Sproule JA, Khan F, Fogarty EE. Osteoid osteoma: painful enlargement of the second toe. Arch Orthop Trauma Surg. 2004;124(5):354-356.
13. Atesok KI, Alman BA, Schemitsch EH, Peyser A, Mankin H. Osteoid osteoma and osteoblastoma. J Am Acad Orthop Surg. 2011;19(11):678-689.
14. Schulman L, Dorfman HD. Nerve fibers in osteoid osteoma. J Bone Joint Surg Am. 1970;52(7):1351-1356.
15. Rosenthal DI, Alexander A, Rosenberg AE, Springfield D. Ablation of osteoid osteomas with a percutaneously placed electrode: a new procedure. Radiology. 1992;183(1):29-33.
16. Gamba JL, Martinez S, Apple J, Harrelson JM, Nunley JA. Computed tomography of axial skeletal osteoid osteomas. AJR Am J Roentgenol. 1984;142(4):769-772.
17. Shukla S, Clarke AW, Saifuddin A. Imaging features of foot osteoid osteoma. Skeletal Radiol. 2010;39(7):683-689.
18. Sluga M, Windhager R, Pfeiffer M, Dominkus M, Kotz R. Peripheral osteoid osteoma. Is there still a place for traditional surgery? J Bone Joint Surg Br. 2002;84(2):249-251.
19. Ward WG, Eckardt JJ, Shayestehfar S, et al. Osteoid osteoma diagnosis and management with low morbidity. Clin Orthop. 1993;(291):229-235.
20. Donahue F, Ahmad A, Mnaymneh W, Pevsner NH. Osteoid osteoma. Computed tomography guided percutaneous excision. Clin Orthop. 1999;(366):191-196.
21. Rosenthal DI, Hornicek FJ, Torriani M, Gebhardt MC, Mankin HJ. Osteoid osteoma: percutaneous treatment with radiofrequency energy. Radiology. 2003;229(1):171-175.
22. Rosenthal DI, Hornicek FJ, Wolfe MW, et al. Percutaneous radiofrequency coagulation of osteoid osteoma compared with operative treatment. J Bone Joint Surg Am. 1998;80(6):815-821.
23. Rosenthal DI, Hornicek FJ, Wolfe MW, Jennings LC, Gebhardt MC, Mankin HJ. Decreasing length of hospital stay in treatment of osteoid osteoma. Clin Orthop. 1999;(361):186-191.
24. Lindner NJ, Scarborough M, Ciccarelli JM, Enneking WF. CT-controlled thermocoagulation of osteoid osteoma in comparison with traditional methods [in German]. Z Orthop Ihre Grenzgeb. 1997;135(6):522-527.
25. Rimondi E, Mavrogenis AF, Rossi G, et al. Radiofrequency ablation for non-spinal osteoid osteomas in 557 patients. Eur Radiol. 2012;22(1):181-188.