User login
Acute hospital care is fast becoming acute geriatric care: people aged 65 years or older are only 13% of the population but account for 44% of days of care in nonfederal hospitals and 38% of discharges.1 In general, the elderly have longer hospital stays, incur greater costs, and have a higher risk of adverse outcomes than do their younger counterparts.2
Among the most common surgical procedures for patients older than 65 are percutaneous coronary intervention with stenting, coronary artery bypass graft surgery, and open reduction internal fixation for hip fracture; the latter is the most common operation in patients aged 85 years or older.3
Elderly patients frequently pose many challenges perioperatively that are not often seen in younger patients. Dementia, frailty, impaired ability to care for oneself, and malnourishment may be present at baseline and are likely to worsen postoperatively. The elderly are at increased risk of acute delirium and cognitive impairment postoperatively, which often complicates recovery and discharge placement.
This article uses a case study to review perioperative problems commonly encountered in elderly surgical patients, particularly those undergoing hip surgery. As the case is presented, I will review strategies to assess risks and prevent and mitigate postoperative cognitive dysfunction and other barriers to recovery.
CASE: AN 82-YEAR-OLD WOMAN WITH HIP FRACTURE
An 82-year-old woman is admitted to undergo open reduction internal fixation for hip fracture. She has a history of osteoarthritis, systolic hypertension, and visual impairment (20/70). Her medications include a beta-blocker, a thiazide diuretic, analgesics as needed, and a multivitamin. She was independent in all activities of daily living before the fracture. She is a social drinker and does not smoke. She has no known cardiovascular, lung, or renal disease.
Her laboratory test results are as follows:
- Blood urea nitrogen (BUN), 24 mg/dL
- Creatinine, 1.0 mg/dL
- Hemoglobin, 12.8 g/dL
- Albumin, 3.8 gm/dL
- Normal levels of thyroid-stimulating hormone and vitamin B12.
Thus, her lab results are normal except for the BUN:creatinine ratio being a bit high, at 24:1 (normal is 10:1, with ratios greater than 18:1 being associated with an increased risk of delirium4).
ASSESSING COGNITIVE RISK: POSTOPERATIVE COGNITIVE DYSFUNCTION VS DELIRIUM
Question: Which of these statements about this patient is most correct?
A. She is at high risk (> 40%) of postoperative cognitive dysfunction
B. Her risk of postoperative delirium is 5% to 10%
C. Postoperative delirium cannot be prevented
D. Preoperative haloperidol (1.5 mg/day for 3 days) will reduce the risk of delirium by 25%
The best answer is A. Postoperative cognitive dysfunction is different from delirium, though it is part of a spectrum of cognitive impairment that may occur after surgery and even persist for a prolonged period. The patient’s risk of postoperative delirium is actually a bit higher than 10% (see “Estimating the risk of delirium” below). Some evidence shows that postoperative delirium can be prevented, at least in hip fracture patients. Kalisvaart et al found that preoperative treatment with low-dose haloperidol reduced the duration and severity of delirium in elderly patients following hip surgery but did not reduce its incidence.5
Cognitive dysfunction often follows surgery
Postoperative cognitive dysfunction has long been recognized and was first described in patients after cardiac surgery, especially following coronary artery bypass graft procedures. In the last several years, we have recognized that it also occurs in patients who undergo noncardiac surgery. Postoperative cognitive dysfunction, which may persist for weeks to months, may not be obvious but can be detected by standard neuropsychological testing.6
Postoperative cognitive dysfunction is different from the “emergence delirium” that may immediately follow surgery and that is often associated with the wearing off of anesthesia. It is also distinct from “incident delirium,” which sometimes occurs over the first few postoperative days (discussed below).
Postoperative dysfunction is especially persistent in the elderly
A recent study found cognitive dysfunction to be common at hospital discharge after major noncardiac surgery in adults of all ages: rates at discharge were 36.6% in patients aged 18 to 39 years, 30.4% in those aged 40 to 59, and 41.4% in those 60 or older.7 Notably, however, the oldest group was most likely to have persistent symptoms. Three months after surgery, 12.7% of patients aged 60 or older continued to have postoperative cognitive dysfunction, which was more than double the rates in the young and middle-aged patient groups (5.7% and 5.6%, respectively).7
Although the cause of postoperative cognitive dysfunction is not well understood, predisposing factors in addition to advanced age include metabolic problems, lower educational level, and previous cerebral vascular accident.7 When elective surgery is considered by elderly patients, the decision should take into account their risk of postoperative cognitive dysfunction and the impact it may have on their quality of life.
PREDICTING AND PREVENTING DELIRIUM
Delirium is easily recognized
Delirium is a common complication of surgery. Unlike postoperative cognitive dysfunction, delirium is easy to detect clinically. It is a disorder of attention and cognition and classically presents as an acute change in mental status accompanied by the following8:
- Fluctuation in awareness
- Memory impairment
- Inattention (inability to stay on task, distractibility)
- Disorganized or illogical thinking
- Altered level of consciousness—ie, hyperalertness (agitation, pulling out intravenous lines, etc) or hypoalertness (“quiet delirium”).
Estimating the risk of delirium
Marcantonio and colleagues developed a model to predict the likelihood that delirium will develop in patients undergoing elective surgery.9 The model assigns points to various risk factors as follows:
- Age ≥ 70 years (1 point)
- History of alcohol abuse (1 point)
- Baseline cognitive impairment (1 point)
- Severe physical impairment (reduced ability to walk or perform daily activities) (1 point)
- Abnormal preoperative blood levels of electrolytes or glucose (1 point)
- Noncardiac thoracic surgery (1 point)
- Abdominal aortic aneurysm surgery (2 points).
The study to validate this model found that a score of 0 points is associated with only a 2% risk of developing postoperative delirium. A score of 3 or more points is associated with a 50% risk of postoperative delirium. A score of 1 or 2 points (as for the patient in our case study) is associated with an 11% risk, according to this Marcantonio model.9
Additionally, well-designed cohort studies of medical patients10 have identified four major independent predictors of incident delirium:
- Severe illness (eg, high fever, complicated infections)
- Baseline dementia
- Dehydration (high BUN:creatinine ratio)
- Sensory impairments (particularly visual).
Kalisvaart et al conducted a prospective cohort study to determine whether these risk factors in medical patients are applicable to elderly patients undergoing hip surgery.11 They found that the incidence of delirium was low (4%) in hip surgery patients with none of these factors, increased to 11% in patients with one or two of these factors, and increased to 37% in patients with three or four factors. These findings suggest that hip surgery patients (like our case patient) may be at greater risk of postoperative delirium than is reflected in the Marcantonio model discussed above,9 which was validated in a study of patients undergoing elective (not emergent) surgery.
Several drug classes raise dementia risk
Anticholinergic medications and other drugs with anticholinergic properties, ie, benzodiazepines and the opioid agent meperidine, also raise the risk for delirium. In general, the older an elderly patient is, the less appropriate these agents are. Many drugs that are not typically recognized as anticholinergics may have potent anticholinergic activity, including tricyclic antidepressants, first-generation antihistamines (eg, diphenhydramine), and high-dose H2-receptor blockers (particularly cimetidine); these agents too should be avoided in elderly patients.12
Strategies to reduce postoperative delirium risk
How can we lower the risk of postoperative delirium in elderly hip fracture patients? Marcantonio et al13 randomized 126 patients undergoing hip fracture repair to receive usual care alone or supplemented with the following additional measures:
- Supplemental oxygen during surgery
- Optimization of electrolytes and blood glucose preoperatively
- Discontinuation of high-risk medications
- Adequate nutritional intake (by parenteral route if necessary)
- Encouragement to get out of bed on the first postoperative day
- Treatment of severe pain.
The incidence of delirium was reduced from 50% in the usual-care group to 32% in the intervention group, and the incidence of severe delirium was reduced even more, from 29% to 12%, respectively.13
OTHER BEST PRACTICES IN PERIOPERATIVE HIP FRACTURE MANAGEMENT
In a systematic literature review to identify best practices for perioperative management of elderly patients with hip fracture, Beaupre et al14 found the following measures to be among those with the strongest evidence of benefit:
- Use of spinal or local anesthesia rather than general anesthesia
- Use of pressure-relieving mattresses to prevent pressure ulcers
- Perioperative administration of antibiotics
- Deep vein thrombosis prophylaxis.
The review concluded that providing nutritional supplementation also is probably helpful although the evidence is not robust. Additionally, it was unclear whether minimizing the delay between hospital admission and surgery has any impact on mortality.14
Is early surgery better?
Early studies suggested that the sooner a hip fracture patient goes to surgery, the lower the mortality, but this has not been supported in well-controlled trials: no difference in mortality has been found whether the patient’s conditions are first optimized to reduce the risk of surgery or if the operation commences within 24 hours.
Although mortality does not appear to be affected, avoiding delay of hip fracture repair yields improvement in other outcomes. In a well-designed prospective cohort study, Orosz et al found that medically stable patients with hip fracture (mean age, 82 years) who underwent surgery within 24 hours had fewer days of pain and less intense pain postoperatively than those whose surgery was delayed beyond 24 hours.15 The early-surgery group also had a 1.94-day reduction in average length of stay compared with the late-surgery group.
A role for clinical pathways
To determine how the application of evidence-based perioperative practices affects actual outcomes in elderly hip fracture patients, Beaupre et al used a pre/post study design to evaluate the impact of an evidence-based clinical pathway at their institution.16 Though there were no differences in in-hospital mortality or the overall costs of inpatient care in elderly hip surgery patients before and after pathway implementation, the patients undergoing surgery after pathway implementation were significantly less likely to have postoperative delirium, heart failure, pressure ulcers, and urinary tract infections compared with those undergoing surgery before implementation. The outcomes benefits of this type of multimodal intervention are likely to extend to abdominal surgical procedures as well.
CASE CONTINUED: POSTOP DAY 2―PATIENT IS CONFUSED AND CRYING IN PAIN
On the second postoperative day, our patient appears weak and slightly confused. She is not eating and is crying in pain. Her neurological exam is normal.
Question: Which is the most appropriate next step?
A. Increase physical therapy
B. Begin an antidepressant
C. Insert a nasoenteric feeding tube
D. Increase doses of analgesics
The best answer is D. With no prior history of depression, an antidepressant would probably not be useful. It is premature to recommend nasoenteric feeding. Because pain hampers physical therapy, an increase in physical therapy would likewise be premature. Because we know the patient is in pain, the correct answer perhaps seems obvious. But keep in mind that relieving pain also has many other positive ramifications: intense pain can be a cause of delirium or at least worsen its symptoms, and pain relief is a prerequisite for the physical therapy that this patient needs.
Strategies for pain control
In general, the treatment of choice for postoperative pain is low-dose morphine sulfate (eg, 1–4 mg every 2 hours, titrated as needed). Acetaminophen can be given safely to virtually all patients. Patient-controlled analgesia is reasonable for select patients but not for older patients with cognitive impairment. Nonsteroidal anti-inflammatory drugs might be helpful in younger patients and even in robust elderly patients, but they must be used very cautiously in the older population because of the risk of gastric ulcers and bleeding, acute kidney injury, fluid retention, and exacerbation of congestive heart failure.
POSTOP DAY 3: PATIENT REPORTS LONG-STANDING FATIGUE
On postoperative day 3, the patient is weak and complains of fatigue. She says that before the fracture, she was experiencing mild weight loss, fatigue, and reduced activity.
Question: What is the most likely reason for her symptoms before the fracture?
A. Frailty
B. Occult heart failure
C. Adverse drug reaction to her beta-blocker
D. Clinical depression
The best answer is A. Occult heart failure is a reasonable second choice, as it is very common in older patients and the diagnosis is easy to miss unless florid pulmonary edema or associated symptoms (eg, chest pain) are present. But this patient had no history of heart disease and was only on medications for hypertension. An adverse drug reaction, such as to the beta-blocker, is unlikely and would probably not cause weight loss. The patient had no history of depression, so clinical depression is unlikely. That said, all the choices are reasonable to consider in elderly patients reporting fatigue and weakness.
Frailty is important to recognize
It is important to identify frailty and to aggressively manage frail patients postoperatively. Although frailty is not clearly defined, Fried et al17 identified five clinical features that correlate with its underlying pathophysiology:
- Minimal physical activity (ie, “doing less”)
- Generalized (not focal) muscle weakness
- Slowed performance (eg, walking short distances takes longer)
- Fatigue or poor endurance
- Unintentional weight loss.
The presence of three or more of these features meets the criteria for frailty and is associated with increased risk for mortality over the next 3 years with or without surgery,17 although surgery probably increases the risk.
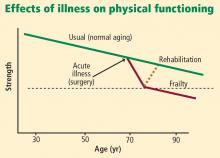
Frailty is believed to be a failure over time of the homeostatic mechanisms that keep our organ systems functioning in the face of a stress. Decline in the ability of organ systems to maintain normal function is probably caused by inflammation, chronic disease, and normal aging, and has been termed homeostenosis. As a person ages and physiologic reserves are reduced, adding a stress such as surgery or severe infection can result in organ failure—usually multiple-system organ failure. In any intensive care unit, one is likely to see elderly patients who were admitted with one medical or surgical problem and soon end up having renal, liver, or brain dysfunction as well.
Physical therapy immediately after hip fracture surgery is associated with significantly better locomotion 2 months later.18 A number of exercises are effective: range-of-motion exercises, low-impact aerobic activities, and exercises starting with low-intensity resistance (using bands, tubes, and weights) and progressing as tolerated to high-intensity resistance (with machines and pulleys) for an extended period of time.
Nutrition supplementation
Malnourishment can contribute to frailty, yet evidence for the benefits of supplementing nutrition is not strong, as noted above. However, meta-analyses of studies of nutritional interventions with meal supplementation (usually canned supplements) show that meal supplementation can improve mortality risk and reduce morbidity such as pressure ulcers in hospitalized elderly patients.19,20 The patients most likely to benefit are those who are undernourished at baseline and aged 75 years or older.
CASE CONTINUED: WHAT HAPPENS POST-DISCHARGE?
Following surgery, our patient wonders, “Where will I go next? What will my lifestyle be like?”
These are important questions to consider when first evaluating whether an elderly patient should undergo surgery. In the case of hip fracture, standard thinking is that without surgery, the patient will never recover the ability to independently walk and perform activities of daily living. But we also must recognize the considerable risks of surgery in the elderly population, particularly those aged 75 years or older.
Comprehensive discharge planning
Early and intensive discharge management enhances quality of life and may help reduce hospital costs. A good model of care involves collaboration of orthopedic surgeons, hospitalists, general internists, geriatricians, and dietitians to address procedures, diet and nutrition, mobility and activities of daily living, and pain medications.21 A case manager such as a social worker should start addressing care transition the day after surgery—planning ahead is imperative.
Following hip surgery, patients are routinely sent to skilled nursing facilities as soon as possible so they can start intensive physical therapy. Patients with significant functional impairment or who had delirium are more likely to require a prolonged hospital stay.
Naylor et al examined the effectiveness of comprehensive discharge planning in a study that randomized hospitalized patients (including surgical patients) 65 years or older to either usual discharge planning or intensive discharge planning with advanced practice nurses beginning early in hospitalization.22 The intervention group was followed by home care nurses for up to 4 weeks and had continuous telephone access to the nurses. Patients who received the intervention had a significantly lower risk of hospital readmission, and those who were readmitted had significantly shorter hospital stays. The total cost of care was also significantly lower in the intervention group.
Family conferences aid decision making
Family conferences can be very useful for working through the many questions and challenges that surgery in an elderly person can pose, including whether the patient should undergo the operation, postoperative management, and postdischarge placement.23 For patients with an uncertain prognosis because of unclear or multiple concurrent diseases, a family conference can help clarify the goals of therapy, inform the family about likely outcomes, and help determine the patient’s wishes and values. Such issues should be revisited as the postoperative course proceeds.
Family conferences also provide a good opportunity to review advanced directives, the need for life support, and possible transfers to intensive care. Family conferences can also help resolve conflicts in care management, as family members may not agree with the need for surgery, how aggressive treatment should be, or where to send the patient for rehabilitation. Differences among family members on these questions are especially common with elderly patients. Working out such issues will improve patient care, especially when done early in the hospitalization.
DISCUSSION
Question from the audience: In our preoperative clinic, we are trying to intervene to reduce delirium and postoperative cognitive dysfunction. How can we quickly screen for the most important predictors and act to reduce the risk?
Dr. Palmer: The most important risk factor for delirium is age, which obviously can’t be changed. Ask patients about alcohol use and depression. Check on nutritional status and begin supplementation if indicated. Discontinue high-risk medications. Check on electrolytes and their state of hydration; ideally, an electrolyte imbalance can be corrected preoperatively. In addition, other than in patients with end-stage renal disease, try to keep the hemoglobin above 7.5 g/dL, which appears to be associated with better outcomes and less risk of delirium.
It’s also important to remind the family to bring in the patient’s visual aids, hearing devices, and cane or walker so that they’re available right after the operation.
Intraoperative factors that are important for preventing delirium include maintaining good blood pressure levels, giving supplemental oxygen, minimizing the time under general anesthesia, and using local anesthesia if possible.
Question from the audience: How strong is the evidence for using spinal anesthesia as opposed to general anesthesia in preventing postoperative cognitive dysfunction and delirium, especially in the setting of hip fracture repair?
Dr. Palmer: The evidence is fairly soft. For patients undergoing either hip or knee arthroplasty who were randomized to receive either spinal (or local) or general anesthesia, the risk of delirium was similar, but complications such as prolonged bed rest, pressure ulcers, and catheter-related urinary tract infections were somewhat reduced in the spinal/local group.14 The relative risk of developing postoperative cognitive dysfunction is unclear—no randomized controlled trials have been conducted to answer that question.
Question from the audience: How do you use antipsychotic drugs, especially with the concerns from epidemiologic studies about an increased risk of death?
Dr. Palmer: No antipsychotic agents, including haloperidol, have a specific Food and Drug Administration–approved indication for treating agitation, dementia, or delirium. In general, they should not be used without a clear indication. That said, the usual off-label use is for patients who are severely agitated and are at risk of harming themselves or others. In an ICU setting, where patients have multiple lines, the use of these agents can be considered for a very agitated patient. Alternatives exist, but antipsychotics like haloperidol have the advantage that they can be given in small increments very rapidly and achieve good control of severe agitation.
Antipsychotic agents should only be used with great caution and for the shortest duration needed. As delirium resolves, they should be tapered fairly rapidly over a few days and ideally should be discontinued by the time of hospital discharge.
None of the antipsychotic agents—including those in the first generation and the newer atypical agents—is free of this risk of increased mortality. The mechanism is not understood; it may be torsades de pointes or hypotension leading to stroke or sudden cardiac death.
Question from the audience: What is the most efficient way to assess cognitive and physical functioning preoperatively?
Dr. Palmer: There may be a documented history of dementia, or family members may tell you if there has been memory loss or some decline in the patient’s self-care abilities. For patients without dementia, you can ask them directly if they can perform basic activities of daily living, such as getting out of bed or dressing. To assess higher-level function, ask if they can manage their own medications, pay bills, or handle their finances. If not, they might have cognitive impairment and are at higher risk for postoperative delirium. These are rather sensitive measures. There are instruments to assess this more precisely, but few clinicians have time to use them.
Quick bedside tests can help assess for delirium postoperatively. We see if patients are “alert and oriented times three” (“Do you know who you are, where you are, and the date?”). We test for attention by asking them to repeat a random string of numbers spoken 1 second apart in monotone; people who are delirious and many patients with severe dementia can’t repeat more than three numbers. A patient who is alert and oriented, has a good attention span (more than three numbers in correct order), and has no history of dementia probably doesn’t have delirium or dementia.
For physical function, ask if they can walk, get out of bed to a chair, and ambulate. If they don’t give clear answers, observe them get out of bed or a chair, walk 10 feet, and return to bed. If they can do that with good balance, especially within 10 to 15 seconds, they probably have reasonably normal mobility and are at lower risk for postoperative complications such as falls with injury.
- DeFrances CJ, Cullen KA, Kozak LJ. National Hospital Discharge Survey: 2005 annual summary with detailed diagnosis and procedure data. Vital Health Stat (Series 13) 2007; Dec(165):1–209.
- Welcome to HCUPnet: a tool for identifying, tracking, and analyzing national hospital statistics. Agency for Healthcare Research and Quality Web site. http://hcupnet.ahrq.gov. Accessed February 23, 2009.
- Kozak LJ, Owings MF, Hall MJ. National Hospital Discharge Survey: 2002 annual summary with detailed diagnosis and procedure data. Vital Health Stat (Series 13) 2005; Mar(158):1–199.
- Inouye SK, Viscoli CM, Horwitz RI, Hurst LD, Tinetti ME. A predictive model for delirium in hospitalized elderly medical patients based on admission characteristics. Ann Intern Med 1993; 119:474–481.
- Kalisvaart KJ, de Jonghe JF, Bogaards MJ, et al. Haloperidol prophylaxis for elderly hip-surgery patients at risk for delirium: a randomized placebo-controlled study. J Am Geriatr Soc 2005; 53:1658–1666.
- Silverstein JH, Timberger M, Reich DL, Uysal S. Central nervous system dysfunction after noncardiac surgery and anesthesia in the elderly. Anesthesiology 2007; 106:622–628.
- Monk TG, Weldon BC, Garvan CW, et al. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology 2008; 108:18–30.
- Inouye SK. Delirium in older persons. N Engl J Med 2006; 354:1157–1165.
- Marcantonio ER, Goldman L, Mangione CM, et al. A clinical prediction rule for delirium after elective noncardiac surgery. JAMA 1994; 271:134–139.
- Inouye SK. Predisposing and precipitating factors for delirium in hospitalized older patients. Dement Geriatr Cogn Disord 1999; 10:393–400.
- Kalisvaart KJ, Vreeswijk R, de Jonghe JF, et al. Risk factors and prediction of postoperative delirium in elderly hip-surgery patients: implementation and validation of a medical risk factor model. J Am Geriatr Soc 2006; 54:817–822.
- Fick DM, Cooper JW, Wade WE, et al. Updating the Beers criteria for potentially inappropriate medication use in older adults: results of a US consensus panel of experts. Arch Intern Med 2003; 163:2716–2724.
- Marcantonio ER, Flacker JM, Wright RJ, et al. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc 2001; 49:516–522.
- Beaupre LA, Jones CA, Saunders LD, Johnston DW, Buckingham J, Majumdar SR. Best practices for elderly hip fracture patients: a systematic overview of the evidence. J Gen Intern Med 2005; 20:1019–1025.
- Orosz GM, Magaziner J, Hannan EL, et al. Association of timing of surgery for hip fracture and patient outcomes. JAMA 2004; 291:1738–1743.
- Beaupre LA, Cinats JG, Senthilselvan A, et al. Reduced morbidity for elderly patients with a hip fracture after implementation of a perioperative evidence-based clinical pathway. Qual Saf Health Care 2006; 15:375–379.
- Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56:M146–M156.
- Penrod JD, Boockvar KS, Litke A, et al. Physical therapy and mobility 2 and 6 months after hip fracture. J Am Geriatr Soc 2004; 52:1114–1120.
- Milne AC, Avenell A, Potter J. Meta-analysis: protein and energy supplementation in older people. Ann Intern Med 2006; 144:37–48.
- Avenell A, Handoll HH. Nutritional supplementation for hip fracture aftercare in older people. Cochrane Database Syst Rev 2006; (4):CD001880.
- Miura LN, DiPiero AR, Homer LD. Effects of a geriatrician-led hip fracture program: improvements in clinical and economic outcomes. J Am Geriatr Soc 2009; 57:159–167.
- Naylor MD, Brooten D, Campbell R, et al. Comprehensive discharge planning and home follow-up of hospitalized elders: a randomized clinical trial. JAMA 1999; 281:613–620.
- Palmer RM. Acute hospital care of the elderly: making a difference. Hospitalist 2004; (suppl):4–7.
Acute hospital care is fast becoming acute geriatric care: people aged 65 years or older are only 13% of the population but account for 44% of days of care in nonfederal hospitals and 38% of discharges.1 In general, the elderly have longer hospital stays, incur greater costs, and have a higher risk of adverse outcomes than do their younger counterparts.2
Among the most common surgical procedures for patients older than 65 are percutaneous coronary intervention with stenting, coronary artery bypass graft surgery, and open reduction internal fixation for hip fracture; the latter is the most common operation in patients aged 85 years or older.3
Elderly patients frequently pose many challenges perioperatively that are not often seen in younger patients. Dementia, frailty, impaired ability to care for oneself, and malnourishment may be present at baseline and are likely to worsen postoperatively. The elderly are at increased risk of acute delirium and cognitive impairment postoperatively, which often complicates recovery and discharge placement.
This article uses a case study to review perioperative problems commonly encountered in elderly surgical patients, particularly those undergoing hip surgery. As the case is presented, I will review strategies to assess risks and prevent and mitigate postoperative cognitive dysfunction and other barriers to recovery.
CASE: AN 82-YEAR-OLD WOMAN WITH HIP FRACTURE
An 82-year-old woman is admitted to undergo open reduction internal fixation for hip fracture. She has a history of osteoarthritis, systolic hypertension, and visual impairment (20/70). Her medications include a beta-blocker, a thiazide diuretic, analgesics as needed, and a multivitamin. She was independent in all activities of daily living before the fracture. She is a social drinker and does not smoke. She has no known cardiovascular, lung, or renal disease.
Her laboratory test results are as follows:
- Blood urea nitrogen (BUN), 24 mg/dL
- Creatinine, 1.0 mg/dL
- Hemoglobin, 12.8 g/dL
- Albumin, 3.8 gm/dL
- Normal levels of thyroid-stimulating hormone and vitamin B12.
Thus, her lab results are normal except for the BUN:creatinine ratio being a bit high, at 24:1 (normal is 10:1, with ratios greater than 18:1 being associated with an increased risk of delirium4).
ASSESSING COGNITIVE RISK: POSTOPERATIVE COGNITIVE DYSFUNCTION VS DELIRIUM
Question: Which of these statements about this patient is most correct?
A. She is at high risk (> 40%) of postoperative cognitive dysfunction
B. Her risk of postoperative delirium is 5% to 10%
C. Postoperative delirium cannot be prevented
D. Preoperative haloperidol (1.5 mg/day for 3 days) will reduce the risk of delirium by 25%
The best answer is A. Postoperative cognitive dysfunction is different from delirium, though it is part of a spectrum of cognitive impairment that may occur after surgery and even persist for a prolonged period. The patient’s risk of postoperative delirium is actually a bit higher than 10% (see “Estimating the risk of delirium” below). Some evidence shows that postoperative delirium can be prevented, at least in hip fracture patients. Kalisvaart et al found that preoperative treatment with low-dose haloperidol reduced the duration and severity of delirium in elderly patients following hip surgery but did not reduce its incidence.5
Cognitive dysfunction often follows surgery
Postoperative cognitive dysfunction has long been recognized and was first described in patients after cardiac surgery, especially following coronary artery bypass graft procedures. In the last several years, we have recognized that it also occurs in patients who undergo noncardiac surgery. Postoperative cognitive dysfunction, which may persist for weeks to months, may not be obvious but can be detected by standard neuropsychological testing.6
Postoperative cognitive dysfunction is different from the “emergence delirium” that may immediately follow surgery and that is often associated with the wearing off of anesthesia. It is also distinct from “incident delirium,” which sometimes occurs over the first few postoperative days (discussed below).
Postoperative dysfunction is especially persistent in the elderly
A recent study found cognitive dysfunction to be common at hospital discharge after major noncardiac surgery in adults of all ages: rates at discharge were 36.6% in patients aged 18 to 39 years, 30.4% in those aged 40 to 59, and 41.4% in those 60 or older.7 Notably, however, the oldest group was most likely to have persistent symptoms. Three months after surgery, 12.7% of patients aged 60 or older continued to have postoperative cognitive dysfunction, which was more than double the rates in the young and middle-aged patient groups (5.7% and 5.6%, respectively).7
Although the cause of postoperative cognitive dysfunction is not well understood, predisposing factors in addition to advanced age include metabolic problems, lower educational level, and previous cerebral vascular accident.7 When elective surgery is considered by elderly patients, the decision should take into account their risk of postoperative cognitive dysfunction and the impact it may have on their quality of life.
PREDICTING AND PREVENTING DELIRIUM
Delirium is easily recognized
Delirium is a common complication of surgery. Unlike postoperative cognitive dysfunction, delirium is easy to detect clinically. It is a disorder of attention and cognition and classically presents as an acute change in mental status accompanied by the following8:
- Fluctuation in awareness
- Memory impairment
- Inattention (inability to stay on task, distractibility)
- Disorganized or illogical thinking
- Altered level of consciousness—ie, hyperalertness (agitation, pulling out intravenous lines, etc) or hypoalertness (“quiet delirium”).
Estimating the risk of delirium
Marcantonio and colleagues developed a model to predict the likelihood that delirium will develop in patients undergoing elective surgery.9 The model assigns points to various risk factors as follows:
- Age ≥ 70 years (1 point)
- History of alcohol abuse (1 point)
- Baseline cognitive impairment (1 point)
- Severe physical impairment (reduced ability to walk or perform daily activities) (1 point)
- Abnormal preoperative blood levels of electrolytes or glucose (1 point)
- Noncardiac thoracic surgery (1 point)
- Abdominal aortic aneurysm surgery (2 points).
The study to validate this model found that a score of 0 points is associated with only a 2% risk of developing postoperative delirium. A score of 3 or more points is associated with a 50% risk of postoperative delirium. A score of 1 or 2 points (as for the patient in our case study) is associated with an 11% risk, according to this Marcantonio model.9
Additionally, well-designed cohort studies of medical patients10 have identified four major independent predictors of incident delirium:
- Severe illness (eg, high fever, complicated infections)
- Baseline dementia
- Dehydration (high BUN:creatinine ratio)
- Sensory impairments (particularly visual).
Kalisvaart et al conducted a prospective cohort study to determine whether these risk factors in medical patients are applicable to elderly patients undergoing hip surgery.11 They found that the incidence of delirium was low (4%) in hip surgery patients with none of these factors, increased to 11% in patients with one or two of these factors, and increased to 37% in patients with three or four factors. These findings suggest that hip surgery patients (like our case patient) may be at greater risk of postoperative delirium than is reflected in the Marcantonio model discussed above,9 which was validated in a study of patients undergoing elective (not emergent) surgery.
Several drug classes raise dementia risk
Anticholinergic medications and other drugs with anticholinergic properties, ie, benzodiazepines and the opioid agent meperidine, also raise the risk for delirium. In general, the older an elderly patient is, the less appropriate these agents are. Many drugs that are not typically recognized as anticholinergics may have potent anticholinergic activity, including tricyclic antidepressants, first-generation antihistamines (eg, diphenhydramine), and high-dose H2-receptor blockers (particularly cimetidine); these agents too should be avoided in elderly patients.12
Strategies to reduce postoperative delirium risk
How can we lower the risk of postoperative delirium in elderly hip fracture patients? Marcantonio et al13 randomized 126 patients undergoing hip fracture repair to receive usual care alone or supplemented with the following additional measures:
- Supplemental oxygen during surgery
- Optimization of electrolytes and blood glucose preoperatively
- Discontinuation of high-risk medications
- Adequate nutritional intake (by parenteral route if necessary)
- Encouragement to get out of bed on the first postoperative day
- Treatment of severe pain.
The incidence of delirium was reduced from 50% in the usual-care group to 32% in the intervention group, and the incidence of severe delirium was reduced even more, from 29% to 12%, respectively.13
OTHER BEST PRACTICES IN PERIOPERATIVE HIP FRACTURE MANAGEMENT
In a systematic literature review to identify best practices for perioperative management of elderly patients with hip fracture, Beaupre et al14 found the following measures to be among those with the strongest evidence of benefit:
- Use of spinal or local anesthesia rather than general anesthesia
- Use of pressure-relieving mattresses to prevent pressure ulcers
- Perioperative administration of antibiotics
- Deep vein thrombosis prophylaxis.
The review concluded that providing nutritional supplementation also is probably helpful although the evidence is not robust. Additionally, it was unclear whether minimizing the delay between hospital admission and surgery has any impact on mortality.14
Is early surgery better?
Early studies suggested that the sooner a hip fracture patient goes to surgery, the lower the mortality, but this has not been supported in well-controlled trials: no difference in mortality has been found whether the patient’s conditions are first optimized to reduce the risk of surgery or if the operation commences within 24 hours.
Although mortality does not appear to be affected, avoiding delay of hip fracture repair yields improvement in other outcomes. In a well-designed prospective cohort study, Orosz et al found that medically stable patients with hip fracture (mean age, 82 years) who underwent surgery within 24 hours had fewer days of pain and less intense pain postoperatively than those whose surgery was delayed beyond 24 hours.15 The early-surgery group also had a 1.94-day reduction in average length of stay compared with the late-surgery group.
A role for clinical pathways
To determine how the application of evidence-based perioperative practices affects actual outcomes in elderly hip fracture patients, Beaupre et al used a pre/post study design to evaluate the impact of an evidence-based clinical pathway at their institution.16 Though there were no differences in in-hospital mortality or the overall costs of inpatient care in elderly hip surgery patients before and after pathway implementation, the patients undergoing surgery after pathway implementation were significantly less likely to have postoperative delirium, heart failure, pressure ulcers, and urinary tract infections compared with those undergoing surgery before implementation. The outcomes benefits of this type of multimodal intervention are likely to extend to abdominal surgical procedures as well.
CASE CONTINUED: POSTOP DAY 2―PATIENT IS CONFUSED AND CRYING IN PAIN
On the second postoperative day, our patient appears weak and slightly confused. She is not eating and is crying in pain. Her neurological exam is normal.
Question: Which is the most appropriate next step?
A. Increase physical therapy
B. Begin an antidepressant
C. Insert a nasoenteric feeding tube
D. Increase doses of analgesics
The best answer is D. With no prior history of depression, an antidepressant would probably not be useful. It is premature to recommend nasoenteric feeding. Because pain hampers physical therapy, an increase in physical therapy would likewise be premature. Because we know the patient is in pain, the correct answer perhaps seems obvious. But keep in mind that relieving pain also has many other positive ramifications: intense pain can be a cause of delirium or at least worsen its symptoms, and pain relief is a prerequisite for the physical therapy that this patient needs.
Strategies for pain control
In general, the treatment of choice for postoperative pain is low-dose morphine sulfate (eg, 1–4 mg every 2 hours, titrated as needed). Acetaminophen can be given safely to virtually all patients. Patient-controlled analgesia is reasonable for select patients but not for older patients with cognitive impairment. Nonsteroidal anti-inflammatory drugs might be helpful in younger patients and even in robust elderly patients, but they must be used very cautiously in the older population because of the risk of gastric ulcers and bleeding, acute kidney injury, fluid retention, and exacerbation of congestive heart failure.
POSTOP DAY 3: PATIENT REPORTS LONG-STANDING FATIGUE
On postoperative day 3, the patient is weak and complains of fatigue. She says that before the fracture, she was experiencing mild weight loss, fatigue, and reduced activity.
Question: What is the most likely reason for her symptoms before the fracture?
A. Frailty
B. Occult heart failure
C. Adverse drug reaction to her beta-blocker
D. Clinical depression
The best answer is A. Occult heart failure is a reasonable second choice, as it is very common in older patients and the diagnosis is easy to miss unless florid pulmonary edema or associated symptoms (eg, chest pain) are present. But this patient had no history of heart disease and was only on medications for hypertension. An adverse drug reaction, such as to the beta-blocker, is unlikely and would probably not cause weight loss. The patient had no history of depression, so clinical depression is unlikely. That said, all the choices are reasonable to consider in elderly patients reporting fatigue and weakness.
Frailty is important to recognize
It is important to identify frailty and to aggressively manage frail patients postoperatively. Although frailty is not clearly defined, Fried et al17 identified five clinical features that correlate with its underlying pathophysiology:
- Minimal physical activity (ie, “doing less”)
- Generalized (not focal) muscle weakness
- Slowed performance (eg, walking short distances takes longer)
- Fatigue or poor endurance
- Unintentional weight loss.
The presence of three or more of these features meets the criteria for frailty and is associated with increased risk for mortality over the next 3 years with or without surgery,17 although surgery probably increases the risk.
Frailty is believed to be a failure over time of the homeostatic mechanisms that keep our organ systems functioning in the face of a stress. Decline in the ability of organ systems to maintain normal function is probably caused by inflammation, chronic disease, and normal aging, and has been termed homeostenosis. As a person ages and physiologic reserves are reduced, adding a stress such as surgery or severe infection can result in organ failure—usually multiple-system organ failure. In any intensive care unit, one is likely to see elderly patients who were admitted with one medical or surgical problem and soon end up having renal, liver, or brain dysfunction as well.
Physical therapy immediately after hip fracture surgery is associated with significantly better locomotion 2 months later.18 A number of exercises are effective: range-of-motion exercises, low-impact aerobic activities, and exercises starting with low-intensity resistance (using bands, tubes, and weights) and progressing as tolerated to high-intensity resistance (with machines and pulleys) for an extended period of time.
Nutrition supplementation
Malnourishment can contribute to frailty, yet evidence for the benefits of supplementing nutrition is not strong, as noted above. However, meta-analyses of studies of nutritional interventions with meal supplementation (usually canned supplements) show that meal supplementation can improve mortality risk and reduce morbidity such as pressure ulcers in hospitalized elderly patients.19,20 The patients most likely to benefit are those who are undernourished at baseline and aged 75 years or older.
CASE CONTINUED: WHAT HAPPENS POST-DISCHARGE?
Following surgery, our patient wonders, “Where will I go next? What will my lifestyle be like?”
These are important questions to consider when first evaluating whether an elderly patient should undergo surgery. In the case of hip fracture, standard thinking is that without surgery, the patient will never recover the ability to independently walk and perform activities of daily living. But we also must recognize the considerable risks of surgery in the elderly population, particularly those aged 75 years or older.
Comprehensive discharge planning
Early and intensive discharge management enhances quality of life and may help reduce hospital costs. A good model of care involves collaboration of orthopedic surgeons, hospitalists, general internists, geriatricians, and dietitians to address procedures, diet and nutrition, mobility and activities of daily living, and pain medications.21 A case manager such as a social worker should start addressing care transition the day after surgery—planning ahead is imperative.
Following hip surgery, patients are routinely sent to skilled nursing facilities as soon as possible so they can start intensive physical therapy. Patients with significant functional impairment or who had delirium are more likely to require a prolonged hospital stay.
Naylor et al examined the effectiveness of comprehensive discharge planning in a study that randomized hospitalized patients (including surgical patients) 65 years or older to either usual discharge planning or intensive discharge planning with advanced practice nurses beginning early in hospitalization.22 The intervention group was followed by home care nurses for up to 4 weeks and had continuous telephone access to the nurses. Patients who received the intervention had a significantly lower risk of hospital readmission, and those who were readmitted had significantly shorter hospital stays. The total cost of care was also significantly lower in the intervention group.
Family conferences aid decision making
Family conferences can be very useful for working through the many questions and challenges that surgery in an elderly person can pose, including whether the patient should undergo the operation, postoperative management, and postdischarge placement.23 For patients with an uncertain prognosis because of unclear or multiple concurrent diseases, a family conference can help clarify the goals of therapy, inform the family about likely outcomes, and help determine the patient’s wishes and values. Such issues should be revisited as the postoperative course proceeds.
Family conferences also provide a good opportunity to review advanced directives, the need for life support, and possible transfers to intensive care. Family conferences can also help resolve conflicts in care management, as family members may not agree with the need for surgery, how aggressive treatment should be, or where to send the patient for rehabilitation. Differences among family members on these questions are especially common with elderly patients. Working out such issues will improve patient care, especially when done early in the hospitalization.
DISCUSSION
Question from the audience: In our preoperative clinic, we are trying to intervene to reduce delirium and postoperative cognitive dysfunction. How can we quickly screen for the most important predictors and act to reduce the risk?
Dr. Palmer: The most important risk factor for delirium is age, which obviously can’t be changed. Ask patients about alcohol use and depression. Check on nutritional status and begin supplementation if indicated. Discontinue high-risk medications. Check on electrolytes and their state of hydration; ideally, an electrolyte imbalance can be corrected preoperatively. In addition, other than in patients with end-stage renal disease, try to keep the hemoglobin above 7.5 g/dL, which appears to be associated with better outcomes and less risk of delirium.
It’s also important to remind the family to bring in the patient’s visual aids, hearing devices, and cane or walker so that they’re available right after the operation.
Intraoperative factors that are important for preventing delirium include maintaining good blood pressure levels, giving supplemental oxygen, minimizing the time under general anesthesia, and using local anesthesia if possible.
Question from the audience: How strong is the evidence for using spinal anesthesia as opposed to general anesthesia in preventing postoperative cognitive dysfunction and delirium, especially in the setting of hip fracture repair?
Dr. Palmer: The evidence is fairly soft. For patients undergoing either hip or knee arthroplasty who were randomized to receive either spinal (or local) or general anesthesia, the risk of delirium was similar, but complications such as prolonged bed rest, pressure ulcers, and catheter-related urinary tract infections were somewhat reduced in the spinal/local group.14 The relative risk of developing postoperative cognitive dysfunction is unclear—no randomized controlled trials have been conducted to answer that question.
Question from the audience: How do you use antipsychotic drugs, especially with the concerns from epidemiologic studies about an increased risk of death?
Dr. Palmer: No antipsychotic agents, including haloperidol, have a specific Food and Drug Administration–approved indication for treating agitation, dementia, or delirium. In general, they should not be used without a clear indication. That said, the usual off-label use is for patients who are severely agitated and are at risk of harming themselves or others. In an ICU setting, where patients have multiple lines, the use of these agents can be considered for a very agitated patient. Alternatives exist, but antipsychotics like haloperidol have the advantage that they can be given in small increments very rapidly and achieve good control of severe agitation.
Antipsychotic agents should only be used with great caution and for the shortest duration needed. As delirium resolves, they should be tapered fairly rapidly over a few days and ideally should be discontinued by the time of hospital discharge.
None of the antipsychotic agents—including those in the first generation and the newer atypical agents—is free of this risk of increased mortality. The mechanism is not understood; it may be torsades de pointes or hypotension leading to stroke or sudden cardiac death.
Question from the audience: What is the most efficient way to assess cognitive and physical functioning preoperatively?
Dr. Palmer: There may be a documented history of dementia, or family members may tell you if there has been memory loss or some decline in the patient’s self-care abilities. For patients without dementia, you can ask them directly if they can perform basic activities of daily living, such as getting out of bed or dressing. To assess higher-level function, ask if they can manage their own medications, pay bills, or handle their finances. If not, they might have cognitive impairment and are at higher risk for postoperative delirium. These are rather sensitive measures. There are instruments to assess this more precisely, but few clinicians have time to use them.
Quick bedside tests can help assess for delirium postoperatively. We see if patients are “alert and oriented times three” (“Do you know who you are, where you are, and the date?”). We test for attention by asking them to repeat a random string of numbers spoken 1 second apart in monotone; people who are delirious and many patients with severe dementia can’t repeat more than three numbers. A patient who is alert and oriented, has a good attention span (more than three numbers in correct order), and has no history of dementia probably doesn’t have delirium or dementia.
For physical function, ask if they can walk, get out of bed to a chair, and ambulate. If they don’t give clear answers, observe them get out of bed or a chair, walk 10 feet, and return to bed. If they can do that with good balance, especially within 10 to 15 seconds, they probably have reasonably normal mobility and are at lower risk for postoperative complications such as falls with injury.
Acute hospital care is fast becoming acute geriatric care: people aged 65 years or older are only 13% of the population but account for 44% of days of care in nonfederal hospitals and 38% of discharges.1 In general, the elderly have longer hospital stays, incur greater costs, and have a higher risk of adverse outcomes than do their younger counterparts.2
Among the most common surgical procedures for patients older than 65 are percutaneous coronary intervention with stenting, coronary artery bypass graft surgery, and open reduction internal fixation for hip fracture; the latter is the most common operation in patients aged 85 years or older.3
Elderly patients frequently pose many challenges perioperatively that are not often seen in younger patients. Dementia, frailty, impaired ability to care for oneself, and malnourishment may be present at baseline and are likely to worsen postoperatively. The elderly are at increased risk of acute delirium and cognitive impairment postoperatively, which often complicates recovery and discharge placement.
This article uses a case study to review perioperative problems commonly encountered in elderly surgical patients, particularly those undergoing hip surgery. As the case is presented, I will review strategies to assess risks and prevent and mitigate postoperative cognitive dysfunction and other barriers to recovery.
CASE: AN 82-YEAR-OLD WOMAN WITH HIP FRACTURE
An 82-year-old woman is admitted to undergo open reduction internal fixation for hip fracture. She has a history of osteoarthritis, systolic hypertension, and visual impairment (20/70). Her medications include a beta-blocker, a thiazide diuretic, analgesics as needed, and a multivitamin. She was independent in all activities of daily living before the fracture. She is a social drinker and does not smoke. She has no known cardiovascular, lung, or renal disease.
Her laboratory test results are as follows:
- Blood urea nitrogen (BUN), 24 mg/dL
- Creatinine, 1.0 mg/dL
- Hemoglobin, 12.8 g/dL
- Albumin, 3.8 gm/dL
- Normal levels of thyroid-stimulating hormone and vitamin B12.
Thus, her lab results are normal except for the BUN:creatinine ratio being a bit high, at 24:1 (normal is 10:1, with ratios greater than 18:1 being associated with an increased risk of delirium4).
ASSESSING COGNITIVE RISK: POSTOPERATIVE COGNITIVE DYSFUNCTION VS DELIRIUM
Question: Which of these statements about this patient is most correct?
A. She is at high risk (> 40%) of postoperative cognitive dysfunction
B. Her risk of postoperative delirium is 5% to 10%
C. Postoperative delirium cannot be prevented
D. Preoperative haloperidol (1.5 mg/day for 3 days) will reduce the risk of delirium by 25%
The best answer is A. Postoperative cognitive dysfunction is different from delirium, though it is part of a spectrum of cognitive impairment that may occur after surgery and even persist for a prolonged period. The patient’s risk of postoperative delirium is actually a bit higher than 10% (see “Estimating the risk of delirium” below). Some evidence shows that postoperative delirium can be prevented, at least in hip fracture patients. Kalisvaart et al found that preoperative treatment with low-dose haloperidol reduced the duration and severity of delirium in elderly patients following hip surgery but did not reduce its incidence.5
Cognitive dysfunction often follows surgery
Postoperative cognitive dysfunction has long been recognized and was first described in patients after cardiac surgery, especially following coronary artery bypass graft procedures. In the last several years, we have recognized that it also occurs in patients who undergo noncardiac surgery. Postoperative cognitive dysfunction, which may persist for weeks to months, may not be obvious but can be detected by standard neuropsychological testing.6
Postoperative cognitive dysfunction is different from the “emergence delirium” that may immediately follow surgery and that is often associated with the wearing off of anesthesia. It is also distinct from “incident delirium,” which sometimes occurs over the first few postoperative days (discussed below).
Postoperative dysfunction is especially persistent in the elderly
A recent study found cognitive dysfunction to be common at hospital discharge after major noncardiac surgery in adults of all ages: rates at discharge were 36.6% in patients aged 18 to 39 years, 30.4% in those aged 40 to 59, and 41.4% in those 60 or older.7 Notably, however, the oldest group was most likely to have persistent symptoms. Three months after surgery, 12.7% of patients aged 60 or older continued to have postoperative cognitive dysfunction, which was more than double the rates in the young and middle-aged patient groups (5.7% and 5.6%, respectively).7
Although the cause of postoperative cognitive dysfunction is not well understood, predisposing factors in addition to advanced age include metabolic problems, lower educational level, and previous cerebral vascular accident.7 When elective surgery is considered by elderly patients, the decision should take into account their risk of postoperative cognitive dysfunction and the impact it may have on their quality of life.
PREDICTING AND PREVENTING DELIRIUM
Delirium is easily recognized
Delirium is a common complication of surgery. Unlike postoperative cognitive dysfunction, delirium is easy to detect clinically. It is a disorder of attention and cognition and classically presents as an acute change in mental status accompanied by the following8:
- Fluctuation in awareness
- Memory impairment
- Inattention (inability to stay on task, distractibility)
- Disorganized or illogical thinking
- Altered level of consciousness—ie, hyperalertness (agitation, pulling out intravenous lines, etc) or hypoalertness (“quiet delirium”).
Estimating the risk of delirium
Marcantonio and colleagues developed a model to predict the likelihood that delirium will develop in patients undergoing elective surgery.9 The model assigns points to various risk factors as follows:
- Age ≥ 70 years (1 point)
- History of alcohol abuse (1 point)
- Baseline cognitive impairment (1 point)
- Severe physical impairment (reduced ability to walk or perform daily activities) (1 point)
- Abnormal preoperative blood levels of electrolytes or glucose (1 point)
- Noncardiac thoracic surgery (1 point)
- Abdominal aortic aneurysm surgery (2 points).
The study to validate this model found that a score of 0 points is associated with only a 2% risk of developing postoperative delirium. A score of 3 or more points is associated with a 50% risk of postoperative delirium. A score of 1 or 2 points (as for the patient in our case study) is associated with an 11% risk, according to this Marcantonio model.9
Additionally, well-designed cohort studies of medical patients10 have identified four major independent predictors of incident delirium:
- Severe illness (eg, high fever, complicated infections)
- Baseline dementia
- Dehydration (high BUN:creatinine ratio)
- Sensory impairments (particularly visual).
Kalisvaart et al conducted a prospective cohort study to determine whether these risk factors in medical patients are applicable to elderly patients undergoing hip surgery.11 They found that the incidence of delirium was low (4%) in hip surgery patients with none of these factors, increased to 11% in patients with one or two of these factors, and increased to 37% in patients with three or four factors. These findings suggest that hip surgery patients (like our case patient) may be at greater risk of postoperative delirium than is reflected in the Marcantonio model discussed above,9 which was validated in a study of patients undergoing elective (not emergent) surgery.
Several drug classes raise dementia risk
Anticholinergic medications and other drugs with anticholinergic properties, ie, benzodiazepines and the opioid agent meperidine, also raise the risk for delirium. In general, the older an elderly patient is, the less appropriate these agents are. Many drugs that are not typically recognized as anticholinergics may have potent anticholinergic activity, including tricyclic antidepressants, first-generation antihistamines (eg, diphenhydramine), and high-dose H2-receptor blockers (particularly cimetidine); these agents too should be avoided in elderly patients.12
Strategies to reduce postoperative delirium risk
How can we lower the risk of postoperative delirium in elderly hip fracture patients? Marcantonio et al13 randomized 126 patients undergoing hip fracture repair to receive usual care alone or supplemented with the following additional measures:
- Supplemental oxygen during surgery
- Optimization of electrolytes and blood glucose preoperatively
- Discontinuation of high-risk medications
- Adequate nutritional intake (by parenteral route if necessary)
- Encouragement to get out of bed on the first postoperative day
- Treatment of severe pain.
The incidence of delirium was reduced from 50% in the usual-care group to 32% in the intervention group, and the incidence of severe delirium was reduced even more, from 29% to 12%, respectively.13
OTHER BEST PRACTICES IN PERIOPERATIVE HIP FRACTURE MANAGEMENT
In a systematic literature review to identify best practices for perioperative management of elderly patients with hip fracture, Beaupre et al14 found the following measures to be among those with the strongest evidence of benefit:
- Use of spinal or local anesthesia rather than general anesthesia
- Use of pressure-relieving mattresses to prevent pressure ulcers
- Perioperative administration of antibiotics
- Deep vein thrombosis prophylaxis.
The review concluded that providing nutritional supplementation also is probably helpful although the evidence is not robust. Additionally, it was unclear whether minimizing the delay between hospital admission and surgery has any impact on mortality.14
Is early surgery better?
Early studies suggested that the sooner a hip fracture patient goes to surgery, the lower the mortality, but this has not been supported in well-controlled trials: no difference in mortality has been found whether the patient’s conditions are first optimized to reduce the risk of surgery or if the operation commences within 24 hours.
Although mortality does not appear to be affected, avoiding delay of hip fracture repair yields improvement in other outcomes. In a well-designed prospective cohort study, Orosz et al found that medically stable patients with hip fracture (mean age, 82 years) who underwent surgery within 24 hours had fewer days of pain and less intense pain postoperatively than those whose surgery was delayed beyond 24 hours.15 The early-surgery group also had a 1.94-day reduction in average length of stay compared with the late-surgery group.
A role for clinical pathways
To determine how the application of evidence-based perioperative practices affects actual outcomes in elderly hip fracture patients, Beaupre et al used a pre/post study design to evaluate the impact of an evidence-based clinical pathway at their institution.16 Though there were no differences in in-hospital mortality or the overall costs of inpatient care in elderly hip surgery patients before and after pathway implementation, the patients undergoing surgery after pathway implementation were significantly less likely to have postoperative delirium, heart failure, pressure ulcers, and urinary tract infections compared with those undergoing surgery before implementation. The outcomes benefits of this type of multimodal intervention are likely to extend to abdominal surgical procedures as well.
CASE CONTINUED: POSTOP DAY 2―PATIENT IS CONFUSED AND CRYING IN PAIN
On the second postoperative day, our patient appears weak and slightly confused. She is not eating and is crying in pain. Her neurological exam is normal.
Question: Which is the most appropriate next step?
A. Increase physical therapy
B. Begin an antidepressant
C. Insert a nasoenteric feeding tube
D. Increase doses of analgesics
The best answer is D. With no prior history of depression, an antidepressant would probably not be useful. It is premature to recommend nasoenteric feeding. Because pain hampers physical therapy, an increase in physical therapy would likewise be premature. Because we know the patient is in pain, the correct answer perhaps seems obvious. But keep in mind that relieving pain also has many other positive ramifications: intense pain can be a cause of delirium or at least worsen its symptoms, and pain relief is a prerequisite for the physical therapy that this patient needs.
Strategies for pain control
In general, the treatment of choice for postoperative pain is low-dose morphine sulfate (eg, 1–4 mg every 2 hours, titrated as needed). Acetaminophen can be given safely to virtually all patients. Patient-controlled analgesia is reasonable for select patients but not for older patients with cognitive impairment. Nonsteroidal anti-inflammatory drugs might be helpful in younger patients and even in robust elderly patients, but they must be used very cautiously in the older population because of the risk of gastric ulcers and bleeding, acute kidney injury, fluid retention, and exacerbation of congestive heart failure.
POSTOP DAY 3: PATIENT REPORTS LONG-STANDING FATIGUE
On postoperative day 3, the patient is weak and complains of fatigue. She says that before the fracture, she was experiencing mild weight loss, fatigue, and reduced activity.
Question: What is the most likely reason for her symptoms before the fracture?
A. Frailty
B. Occult heart failure
C. Adverse drug reaction to her beta-blocker
D. Clinical depression
The best answer is A. Occult heart failure is a reasonable second choice, as it is very common in older patients and the diagnosis is easy to miss unless florid pulmonary edema or associated symptoms (eg, chest pain) are present. But this patient had no history of heart disease and was only on medications for hypertension. An adverse drug reaction, such as to the beta-blocker, is unlikely and would probably not cause weight loss. The patient had no history of depression, so clinical depression is unlikely. That said, all the choices are reasonable to consider in elderly patients reporting fatigue and weakness.
Frailty is important to recognize
It is important to identify frailty and to aggressively manage frail patients postoperatively. Although frailty is not clearly defined, Fried et al17 identified five clinical features that correlate with its underlying pathophysiology:
- Minimal physical activity (ie, “doing less”)
- Generalized (not focal) muscle weakness
- Slowed performance (eg, walking short distances takes longer)
- Fatigue or poor endurance
- Unintentional weight loss.
The presence of three or more of these features meets the criteria for frailty and is associated with increased risk for mortality over the next 3 years with or without surgery,17 although surgery probably increases the risk.
Frailty is believed to be a failure over time of the homeostatic mechanisms that keep our organ systems functioning in the face of a stress. Decline in the ability of organ systems to maintain normal function is probably caused by inflammation, chronic disease, and normal aging, and has been termed homeostenosis. As a person ages and physiologic reserves are reduced, adding a stress such as surgery or severe infection can result in organ failure—usually multiple-system organ failure. In any intensive care unit, one is likely to see elderly patients who were admitted with one medical or surgical problem and soon end up having renal, liver, or brain dysfunction as well.
Physical therapy immediately after hip fracture surgery is associated with significantly better locomotion 2 months later.18 A number of exercises are effective: range-of-motion exercises, low-impact aerobic activities, and exercises starting with low-intensity resistance (using bands, tubes, and weights) and progressing as tolerated to high-intensity resistance (with machines and pulleys) for an extended period of time.
Nutrition supplementation
Malnourishment can contribute to frailty, yet evidence for the benefits of supplementing nutrition is not strong, as noted above. However, meta-analyses of studies of nutritional interventions with meal supplementation (usually canned supplements) show that meal supplementation can improve mortality risk and reduce morbidity such as pressure ulcers in hospitalized elderly patients.19,20 The patients most likely to benefit are those who are undernourished at baseline and aged 75 years or older.
CASE CONTINUED: WHAT HAPPENS POST-DISCHARGE?
Following surgery, our patient wonders, “Where will I go next? What will my lifestyle be like?”
These are important questions to consider when first evaluating whether an elderly patient should undergo surgery. In the case of hip fracture, standard thinking is that without surgery, the patient will never recover the ability to independently walk and perform activities of daily living. But we also must recognize the considerable risks of surgery in the elderly population, particularly those aged 75 years or older.
Comprehensive discharge planning
Early and intensive discharge management enhances quality of life and may help reduce hospital costs. A good model of care involves collaboration of orthopedic surgeons, hospitalists, general internists, geriatricians, and dietitians to address procedures, diet and nutrition, mobility and activities of daily living, and pain medications.21 A case manager such as a social worker should start addressing care transition the day after surgery—planning ahead is imperative.
Following hip surgery, patients are routinely sent to skilled nursing facilities as soon as possible so they can start intensive physical therapy. Patients with significant functional impairment or who had delirium are more likely to require a prolonged hospital stay.
Naylor et al examined the effectiveness of comprehensive discharge planning in a study that randomized hospitalized patients (including surgical patients) 65 years or older to either usual discharge planning or intensive discharge planning with advanced practice nurses beginning early in hospitalization.22 The intervention group was followed by home care nurses for up to 4 weeks and had continuous telephone access to the nurses. Patients who received the intervention had a significantly lower risk of hospital readmission, and those who were readmitted had significantly shorter hospital stays. The total cost of care was also significantly lower in the intervention group.
Family conferences aid decision making
Family conferences can be very useful for working through the many questions and challenges that surgery in an elderly person can pose, including whether the patient should undergo the operation, postoperative management, and postdischarge placement.23 For patients with an uncertain prognosis because of unclear or multiple concurrent diseases, a family conference can help clarify the goals of therapy, inform the family about likely outcomes, and help determine the patient’s wishes and values. Such issues should be revisited as the postoperative course proceeds.
Family conferences also provide a good opportunity to review advanced directives, the need for life support, and possible transfers to intensive care. Family conferences can also help resolve conflicts in care management, as family members may not agree with the need for surgery, how aggressive treatment should be, or where to send the patient for rehabilitation. Differences among family members on these questions are especially common with elderly patients. Working out such issues will improve patient care, especially when done early in the hospitalization.
DISCUSSION
Question from the audience: In our preoperative clinic, we are trying to intervene to reduce delirium and postoperative cognitive dysfunction. How can we quickly screen for the most important predictors and act to reduce the risk?
Dr. Palmer: The most important risk factor for delirium is age, which obviously can’t be changed. Ask patients about alcohol use and depression. Check on nutritional status and begin supplementation if indicated. Discontinue high-risk medications. Check on electrolytes and their state of hydration; ideally, an electrolyte imbalance can be corrected preoperatively. In addition, other than in patients with end-stage renal disease, try to keep the hemoglobin above 7.5 g/dL, which appears to be associated with better outcomes and less risk of delirium.
It’s also important to remind the family to bring in the patient’s visual aids, hearing devices, and cane or walker so that they’re available right after the operation.
Intraoperative factors that are important for preventing delirium include maintaining good blood pressure levels, giving supplemental oxygen, minimizing the time under general anesthesia, and using local anesthesia if possible.
Question from the audience: How strong is the evidence for using spinal anesthesia as opposed to general anesthesia in preventing postoperative cognitive dysfunction and delirium, especially in the setting of hip fracture repair?
Dr. Palmer: The evidence is fairly soft. For patients undergoing either hip or knee arthroplasty who were randomized to receive either spinal (or local) or general anesthesia, the risk of delirium was similar, but complications such as prolonged bed rest, pressure ulcers, and catheter-related urinary tract infections were somewhat reduced in the spinal/local group.14 The relative risk of developing postoperative cognitive dysfunction is unclear—no randomized controlled trials have been conducted to answer that question.
Question from the audience: How do you use antipsychotic drugs, especially with the concerns from epidemiologic studies about an increased risk of death?
Dr. Palmer: No antipsychotic agents, including haloperidol, have a specific Food and Drug Administration–approved indication for treating agitation, dementia, or delirium. In general, they should not be used without a clear indication. That said, the usual off-label use is for patients who are severely agitated and are at risk of harming themselves or others. In an ICU setting, where patients have multiple lines, the use of these agents can be considered for a very agitated patient. Alternatives exist, but antipsychotics like haloperidol have the advantage that they can be given in small increments very rapidly and achieve good control of severe agitation.
Antipsychotic agents should only be used with great caution and for the shortest duration needed. As delirium resolves, they should be tapered fairly rapidly over a few days and ideally should be discontinued by the time of hospital discharge.
None of the antipsychotic agents—including those in the first generation and the newer atypical agents—is free of this risk of increased mortality. The mechanism is not understood; it may be torsades de pointes or hypotension leading to stroke or sudden cardiac death.
Question from the audience: What is the most efficient way to assess cognitive and physical functioning preoperatively?
Dr. Palmer: There may be a documented history of dementia, or family members may tell you if there has been memory loss or some decline in the patient’s self-care abilities. For patients without dementia, you can ask them directly if they can perform basic activities of daily living, such as getting out of bed or dressing. To assess higher-level function, ask if they can manage their own medications, pay bills, or handle their finances. If not, they might have cognitive impairment and are at higher risk for postoperative delirium. These are rather sensitive measures. There are instruments to assess this more precisely, but few clinicians have time to use them.
Quick bedside tests can help assess for delirium postoperatively. We see if patients are “alert and oriented times three” (“Do you know who you are, where you are, and the date?”). We test for attention by asking them to repeat a random string of numbers spoken 1 second apart in monotone; people who are delirious and many patients with severe dementia can’t repeat more than three numbers. A patient who is alert and oriented, has a good attention span (more than three numbers in correct order), and has no history of dementia probably doesn’t have delirium or dementia.
For physical function, ask if they can walk, get out of bed to a chair, and ambulate. If they don’t give clear answers, observe them get out of bed or a chair, walk 10 feet, and return to bed. If they can do that with good balance, especially within 10 to 15 seconds, they probably have reasonably normal mobility and are at lower risk for postoperative complications such as falls with injury.
- DeFrances CJ, Cullen KA, Kozak LJ. National Hospital Discharge Survey: 2005 annual summary with detailed diagnosis and procedure data. Vital Health Stat (Series 13) 2007; Dec(165):1–209.
- Welcome to HCUPnet: a tool for identifying, tracking, and analyzing national hospital statistics. Agency for Healthcare Research and Quality Web site. http://hcupnet.ahrq.gov. Accessed February 23, 2009.
- Kozak LJ, Owings MF, Hall MJ. National Hospital Discharge Survey: 2002 annual summary with detailed diagnosis and procedure data. Vital Health Stat (Series 13) 2005; Mar(158):1–199.
- Inouye SK, Viscoli CM, Horwitz RI, Hurst LD, Tinetti ME. A predictive model for delirium in hospitalized elderly medical patients based on admission characteristics. Ann Intern Med 1993; 119:474–481.
- Kalisvaart KJ, de Jonghe JF, Bogaards MJ, et al. Haloperidol prophylaxis for elderly hip-surgery patients at risk for delirium: a randomized placebo-controlled study. J Am Geriatr Soc 2005; 53:1658–1666.
- Silverstein JH, Timberger M, Reich DL, Uysal S. Central nervous system dysfunction after noncardiac surgery and anesthesia in the elderly. Anesthesiology 2007; 106:622–628.
- Monk TG, Weldon BC, Garvan CW, et al. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology 2008; 108:18–30.
- Inouye SK. Delirium in older persons. N Engl J Med 2006; 354:1157–1165.
- Marcantonio ER, Goldman L, Mangione CM, et al. A clinical prediction rule for delirium after elective noncardiac surgery. JAMA 1994; 271:134–139.
- Inouye SK. Predisposing and precipitating factors for delirium in hospitalized older patients. Dement Geriatr Cogn Disord 1999; 10:393–400.
- Kalisvaart KJ, Vreeswijk R, de Jonghe JF, et al. Risk factors and prediction of postoperative delirium in elderly hip-surgery patients: implementation and validation of a medical risk factor model. J Am Geriatr Soc 2006; 54:817–822.
- Fick DM, Cooper JW, Wade WE, et al. Updating the Beers criteria for potentially inappropriate medication use in older adults: results of a US consensus panel of experts. Arch Intern Med 2003; 163:2716–2724.
- Marcantonio ER, Flacker JM, Wright RJ, et al. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc 2001; 49:516–522.
- Beaupre LA, Jones CA, Saunders LD, Johnston DW, Buckingham J, Majumdar SR. Best practices for elderly hip fracture patients: a systematic overview of the evidence. J Gen Intern Med 2005; 20:1019–1025.
- Orosz GM, Magaziner J, Hannan EL, et al. Association of timing of surgery for hip fracture and patient outcomes. JAMA 2004; 291:1738–1743.
- Beaupre LA, Cinats JG, Senthilselvan A, et al. Reduced morbidity for elderly patients with a hip fracture after implementation of a perioperative evidence-based clinical pathway. Qual Saf Health Care 2006; 15:375–379.
- Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56:M146–M156.
- Penrod JD, Boockvar KS, Litke A, et al. Physical therapy and mobility 2 and 6 months after hip fracture. J Am Geriatr Soc 2004; 52:1114–1120.
- Milne AC, Avenell A, Potter J. Meta-analysis: protein and energy supplementation in older people. Ann Intern Med 2006; 144:37–48.
- Avenell A, Handoll HH. Nutritional supplementation for hip fracture aftercare in older people. Cochrane Database Syst Rev 2006; (4):CD001880.
- Miura LN, DiPiero AR, Homer LD. Effects of a geriatrician-led hip fracture program: improvements in clinical and economic outcomes. J Am Geriatr Soc 2009; 57:159–167.
- Naylor MD, Brooten D, Campbell R, et al. Comprehensive discharge planning and home follow-up of hospitalized elders: a randomized clinical trial. JAMA 1999; 281:613–620.
- Palmer RM. Acute hospital care of the elderly: making a difference. Hospitalist 2004; (suppl):4–7.
- DeFrances CJ, Cullen KA, Kozak LJ. National Hospital Discharge Survey: 2005 annual summary with detailed diagnosis and procedure data. Vital Health Stat (Series 13) 2007; Dec(165):1–209.
- Welcome to HCUPnet: a tool for identifying, tracking, and analyzing national hospital statistics. Agency for Healthcare Research and Quality Web site. http://hcupnet.ahrq.gov. Accessed February 23, 2009.
- Kozak LJ, Owings MF, Hall MJ. National Hospital Discharge Survey: 2002 annual summary with detailed diagnosis and procedure data. Vital Health Stat (Series 13) 2005; Mar(158):1–199.
- Inouye SK, Viscoli CM, Horwitz RI, Hurst LD, Tinetti ME. A predictive model for delirium in hospitalized elderly medical patients based on admission characteristics. Ann Intern Med 1993; 119:474–481.
- Kalisvaart KJ, de Jonghe JF, Bogaards MJ, et al. Haloperidol prophylaxis for elderly hip-surgery patients at risk for delirium: a randomized placebo-controlled study. J Am Geriatr Soc 2005; 53:1658–1666.
- Silverstein JH, Timberger M, Reich DL, Uysal S. Central nervous system dysfunction after noncardiac surgery and anesthesia in the elderly. Anesthesiology 2007; 106:622–628.
- Monk TG, Weldon BC, Garvan CW, et al. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology 2008; 108:18–30.
- Inouye SK. Delirium in older persons. N Engl J Med 2006; 354:1157–1165.
- Marcantonio ER, Goldman L, Mangione CM, et al. A clinical prediction rule for delirium after elective noncardiac surgery. JAMA 1994; 271:134–139.
- Inouye SK. Predisposing and precipitating factors for delirium in hospitalized older patients. Dement Geriatr Cogn Disord 1999; 10:393–400.
- Kalisvaart KJ, Vreeswijk R, de Jonghe JF, et al. Risk factors and prediction of postoperative delirium in elderly hip-surgery patients: implementation and validation of a medical risk factor model. J Am Geriatr Soc 2006; 54:817–822.
- Fick DM, Cooper JW, Wade WE, et al. Updating the Beers criteria for potentially inappropriate medication use in older adults: results of a US consensus panel of experts. Arch Intern Med 2003; 163:2716–2724.
- Marcantonio ER, Flacker JM, Wright RJ, et al. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc 2001; 49:516–522.
- Beaupre LA, Jones CA, Saunders LD, Johnston DW, Buckingham J, Majumdar SR. Best practices for elderly hip fracture patients: a systematic overview of the evidence. J Gen Intern Med 2005; 20:1019–1025.
- Orosz GM, Magaziner J, Hannan EL, et al. Association of timing of surgery for hip fracture and patient outcomes. JAMA 2004; 291:1738–1743.
- Beaupre LA, Cinats JG, Senthilselvan A, et al. Reduced morbidity for elderly patients with a hip fracture after implementation of a perioperative evidence-based clinical pathway. Qual Saf Health Care 2006; 15:375–379.
- Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56:M146–M156.
- Penrod JD, Boockvar KS, Litke A, et al. Physical therapy and mobility 2 and 6 months after hip fracture. J Am Geriatr Soc 2004; 52:1114–1120.
- Milne AC, Avenell A, Potter J. Meta-analysis: protein and energy supplementation in older people. Ann Intern Med 2006; 144:37–48.
- Avenell A, Handoll HH. Nutritional supplementation for hip fracture aftercare in older people. Cochrane Database Syst Rev 2006; (4):CD001880.
- Miura LN, DiPiero AR, Homer LD. Effects of a geriatrician-led hip fracture program: improvements in clinical and economic outcomes. J Am Geriatr Soc 2009; 57:159–167.
- Naylor MD, Brooten D, Campbell R, et al. Comprehensive discharge planning and home follow-up of hospitalized elders: a randomized clinical trial. JAMA 1999; 281:613–620.
- Palmer RM. Acute hospital care of the elderly: making a difference. Hospitalist 2004; (suppl):4–7.
KEY POINTS
- Postoperative cognitive dysfunction and delirium are distinct conditions, though both are common in the elderly. Postoperative cognitive dysfunction may persist for weeks to months and may not be obvious, whereas delirium, a disorder of attention and cognition, is easier to detect clinically.
- Major predictors of postoperative delirium are severe illness, baseline dementia, dehydration, and sensory impairment.
- Drugs that raise dementia risk include anticholinergics, benzodiazepines, meperidine, tricyclic antidepressants, first-generation antihistamines, and high-dose H2-receptor blockers.
- Early performance of hip fracture surgery in the elderly (ie, within 24 hours of admission) has not been shown to lower mortality but appears to improve other outcomes.
- Identifying and managing frail elderly patients is important. Signs of frailty are minimal activity, generalized muscle weakness, slowed performance, fatigue, and weight loss.