User login
Head-to-head comparisons are lacking, but similar results have been reported
BY MICHAEL D. DAKE, M.D.
Well, at least one thing is for sure – we would not have been having this discussion a mere 10 years ago.
I remained sheepishly silent for most of my early career as well-intentioned invasive and noninvasive specialists criticized the state of evidence supporting the legitimacy of endovascular interventions as a competitive strategy to manage infrainguinal peripheral arterial disease. Good data from well-controlled randomized clinical trials were not available to make a case for endovascular therapies.
Over the recent decade and a half, however, a number of contributing factors have influenced thinking and what we now consider standard of care for symptomatic disease of the superficial femoral artery (SFA). The proposal of an “endovascular first” interventional approach has evolved to a consensually agreed upon management strategy by all interested disciplines.
This did not occur on a whim. Rather, out of the shadows of relative ignorance there slowly emerged a welcomed accumulation of a large number of publications that detail the outcomes of a wide variety of randomized trials with a range of endovascular devices. This has allowed us to enter an era where valid comparisons between interventional therapies is not only possible, but allows us to more appropriately offer care to vascular patients with more nuanced strategies. These are strategies that recognize subtleties between subgroups of individuals stratified on the basis of patient demographics and lesion characteristics in a way not appreciated prior to the recent spate of endovascular device studies.
Thus, thanks to the dedication and hard work of many, we are now at a stage where we can have meaningful dialogues on a variety of endovascular topics, such as the one at hand, and proponents can argue their perspectives armed with objective evidence to support their positions. In this discussion regarding covered stent grafts and drug-eluting stents, we wish we had even more data.
Specifically, we are missing direct head-to-head comparisons between the two devices in patients with long SFA lesions. So, what do we know?
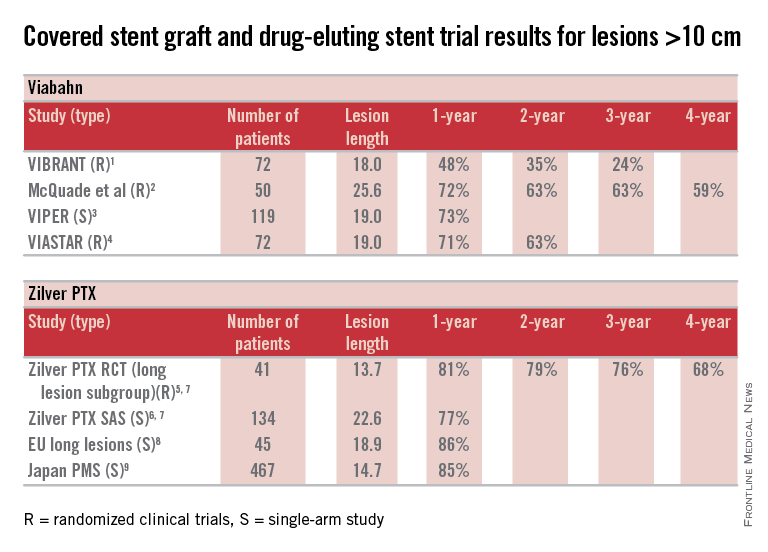
Here are some fundamental facts: The most commonly used covered stent graft for management of femoropopliteal occlusive disease is the Viabahn endoprosthesis (W. L. Gore and Associates, Flagstaff, Ariz.). The prosthesis is composed of a self-expanding nitinol stent framework and expanded polytetrafluoroethylene (ePTFE) graft with its surface lined with a coating of covalently bound heparin (Propaten bioactive surface).The only approved drug-eluting stent with significant safety and effectiveness data available is the Zilver PTX paclitaxel-eluting, self-expanding nitinol stent (Cook Medical Inc., Bloomington, Ind.).
Now in terms of the proposition, we need to discuss the meaning of the word “long” with reference to the SFA. Just what do we consider a long SFA lesion? I think all of us could agree that an arterial stenosis or occlusion of 6 cm or less is short. Lesions between 5 cm or 6 cm to 10 cm or 12 cm in length are moderately long, and disease greater than 10 cm or 12 cm is commonly characterized as long. Segments of disease greater than 20 cm long are typically considered very long or extremely long lesions from an endovascular interventional perspective.
So, how can currently available trial outcomes help us? Below, I have compiled a table that includes most of the recent clinical trial data for Viabahn and Zilver PTX in patients with long SFA occlusive disease.
OK, what can we honestly say about these data besides recognizing that we are at risk when we make any conclusions based upon cross-trial comparisons? Such an accounting of results is fraught with problems, but what we can say is that the table grossly confirms the current consensus that both devices enhance the standard of care for long lesions over traditional balloon angioplasty (PTA) and bare metal stent technologies.
Beyond this, however, it is accepted that patency results with Viabahn are lesion-length immune – that is, outcomes in long and extremely long segments of disease are not very different from the patency achieved in short lesions. This is clearly different than what is traditionally found for interventions with PTA or bare metal stents. There is not enough controlled data for extremely long lesions to reach a conclusion on drug-eluting stents; however, there is an initial suggestion that they behave in a manner more similar to stent grafts than traditional devices.
Grossly, the table suggests that the midterm and available greater than 1-year patency results with Viabahn and Zilver PTX are relatively comparable. What about the price of the device? What role does it play in our selection of the current most cost-effective endovascular strategy for long SFA lesions?
In my institution Viabahn is more expensive than Zilver PTX with a relative cost premium of about 30%-50% depending on the treatment length. Of course, when treating long TASC C and D lesions any up-front difference in the costs of the devices used initially is more than made up for by any relative reduction in subsequent reinterventions.
So, there you have it. Look at the table as simply a current snapshot. In the future, we will benefit from additional trials and comparisons, not to mention better endovascular technologies to address symptomatic long SFA lesions.
Dr. Dake is the Thelma and Henry Doelger Professor of Cardiovascular Surgery at the Stanford (Calif.) School of Medicine. He disclosed that he is a member of the Peripheral Scientific Advisory Board: Abbott Vascular Member, is on the Aortic Medical Advisory Board: W. L. Gore, is a consultant for Cook Medical, Medtronic, and Surmodics Research, and receives grants/clinical trial support from W. L. Gore, Medtronic, and Novate.
References for table
1. J. Vasc. Surg. 2013;58:386-95.
2. J. Vasc. Surg. 2010;52:584-90.
3. J. Vasc. Interv. Radiol. 2013;24:165-73.
4. Cardiovasc. Interv. Radiol. 2015;38:25-32.
5. Circ. Cardiovasc. Interv. 2011;4:495-504.
6. J. Am. Coll. Cardiol. 2013;61:2417-27.
7. J. Endovasc. Ther. 2011;18:613-23.
8. Zeller T. Oral presentations. 2014.
9. Yokoi H. Oral presentations. 2014.
Covered stent grafts in the SFA are still the endovascular champion in long lesions
BY DENNIS GABLE, M.D.
There remains a continued debate among investigators as to the best modality for treatment of stenosis/occlusion of the SFA especially with the recent advent of drug-eluting technology. However, I suggest that for long lesions over 15 cm only covered stent grafts continue to outperform the competition.
If we review the numerous studies available on treatment of SFA disease, the Viabahn-covered stent device (W. L. Gore & Associates, Flagstaff, Ariz.) is by far the most studied modality. There are currently 22 independent studies available providing data on 1,473 limbs. Several of these reports are multicenter studies and many of them are prospective randomized trials. Two of the most recent are the VIPER (J. Vasc. Intervent. Radiol. 2013;24:165-73) and VIASTAR study (JACC 2013;62:1320-27).
The VIPER study prospectively enrolled 119 patients (72 with TASC II C/D disease; mean lesion length 190 mm). Primary patency was reported at 73% at 1 year but in patients with less than 20% oversizing, as is recommended by the IFU, patency as high as 88% was noted. Additionally, there was no difference in patency in smaller-diameter vessels (5 mm) versus larger-diameter vessels (6-7 mm).
In a head-to-head randomized controlled trial of Viabahn to bare metal stents (BMS), the VIASTAR study enrolled 141 patients (72 in covered stent arm; mean lesion length 190 mm). On a per protocol evaluation, the patency at 1 year was 78% and 71% on an intention-to-treat evaluation with a patency of 70% and 63% respectively at 2 years. There was no statistical difference between the two evaluations on intention to treat vs. per protocol but there was clear superiority demonstrated against BMS.
Furthermore, in a prospective, randomized, head-to-head comparison of Viabahn to prosthetic above knee femoral popliteal bypass, it was shown that there was no difference in primary or secondary patency between the two groups out to 4 years follow-up (J. Vasc. Surg. 2010;52:584-91). This included an average lesion length of 25.6 cm with a primary and secondary patency of 59% and 74% in the Viabahn group and 58% and 71% in the surgery group. When compared to a large meta-analysis for femoral popliteal bypass outcomes reported on by Bates and AbuRahma in 2004 (J. Endovasc. Ther. 2004;11[suppl. II]:II-107–27), the patency for the surgical arm with prosthetic bypass in the above Viabahn study was similar at 4 years to the 38 peer-reviewed articles Bates et al. reviewed with over 4,000 limbs. The reported primary and secondary patency at 4 years for prosthetic femoral above knee popliteal bypass was 51% and 61%, respectively in his review. Although the above Viabahn study was not powered to formally demonstrate noninferiority to surgical bypass with prosthetic, it did strongly suggest and show just that.
How do we put these data together with the Zilver data and how do we decide what is best for our patients? Some operators have expressed concern over a perceived risk for a “higher rate of amputation” or “a worse Rutherford level of ischemia on presentation” if patients with the Viabahn stent graft occlude post procedure. Commonly, this results from extrapolation of prior studies looking at results of occlusion with an ePTFE bypass. In fact, review of peer-reviewed data reveal none of the prospective studies outlined above, or those currently available, demonstrate that either of these perceptions are true and there are no published prospective data that support these fears either. In the studies listed above as well as all current prospective studies available evaluating Viabahn usage, the highest rate of amputation reported was 5% by Fisher in 2006 with all of the remaining studies reporting an amputation rate of 2% or less (when reported). Moreover, it has not been demonstrated that patients with this device present with an increased level of ischemia secondary to sudden occlusion.
There is one report used to argue against the use of the Viabahn stent graft (J. Vasc. Surg. 2008;47:967-74). This study evaluated prospectively 109 patients (71 for claudication; 38 for critical limb ischemia) treated for SFA occlusive disease (mean lesion length 15.7 cm). Only 19 of the 109 patients (17%) were treated with Viabahn (17 for claudication; 2 for critical limb ischemia). The remaining limbs were treated with various other BMS devices (n=10). The authors concluded that patients initially treated with Viabahn who presented back with occlusion had a higher chance of presenting with acute symptoms (i.e., a worse Rutherford score). The lesion length treated in the Viabahn group, however, was nearly twice as long as all the other stent platforms combined (25.4 cm vs. 13.7 cm) and there was a higher level of tibial artery deterioration with thrombosis of the BMS group, compared with the Viabahn group (7.7% vs. 5.3%). The number of Viabahn patients presenting with acute thrombosis was not defined. With the small number of limbs treated in the Viabahn group, the conclusions expressed cannot be statistically supported.
What about the in-vogue DES device?
Dr. Dake and his colleagues recently presented 5-year data on the Zilver DES platform at VIVA 2014. He reported a primary patency at 5 years of 66.4% showing superiority to angioplasty alone as well as angioplasty with provisional stenting. This study enrolled 479 patients into the randomization arm and also had a registry arm that although often included in reporting of patency, does not stand up to the scrutiny of peer review. Even though there were some patients with longer lesions, the randomized arm mean lesion length was only 66 mm, which does not compare to the published longer mean lesion length of the Viabahn device. Bosiers et al. (J. Cardiovasc. Surg. 2013 54:115-222) reviewed 135 patients treated with the Zilver device (a subgroup derived from the 787 patients enrolled in the registry data of the Zilver trial) with a mean lesion length of 226 mm. They reported 77.6% primary patency but only at 1 year. Again, however, this is registry derived data and does not have the scientific validity of a randomized trial.
So what can I conclude from these experiences? We know today that covered stent grafts have been widely used and reported on, including by Dr. Dake himself (Radiology 2000 October;217:95-104) and all appear to have had similar conclusions.
The mean lesion length treated in these studies of Viabahn is often longer than 15 cm and nearly all studies report primary patency outcomes. Zilver supporters on the other hand are prone to quote TLR which is an inferior endpoint (as recently noted in an editorial by Dr. Russell Samson (Vasc. Spec. 2015;11:2). Costs of both devices are an issue but may vary by region and institution. However, Viabahn does have the advantage of longer devices, compared with the Zilver (15 and 25 cm vs. 10 cm) so fewer devices may be required to treat long lesions. Although short lesions may be better addressed with BMS or DES, for longer SFA lesions over 12-15 cm there are very few truly comparable data that argue against the use of Viabahn.
Dr. Gable is chief of vascular and endovascular surgery at The Heart Hospital Baylor Plano (Tex.). He is also an associate medical editor for Vascular Specialist. He disclosed that he is a consultant, speaker, and receives research support from W. L. Gore and Medtronic.
Head-to-head comparisons are lacking, but similar results have been reported
BY MICHAEL D. DAKE, M.D.
Well, at least one thing is for sure – we would not have been having this discussion a mere 10 years ago.
I remained sheepishly silent for most of my early career as well-intentioned invasive and noninvasive specialists criticized the state of evidence supporting the legitimacy of endovascular interventions as a competitive strategy to manage infrainguinal peripheral arterial disease. Good data from well-controlled randomized clinical trials were not available to make a case for endovascular therapies.
Over the recent decade and a half, however, a number of contributing factors have influenced thinking and what we now consider standard of care for symptomatic disease of the superficial femoral artery (SFA). The proposal of an “endovascular first” interventional approach has evolved to a consensually agreed upon management strategy by all interested disciplines.
This did not occur on a whim. Rather, out of the shadows of relative ignorance there slowly emerged a welcomed accumulation of a large number of publications that detail the outcomes of a wide variety of randomized trials with a range of endovascular devices. This has allowed us to enter an era where valid comparisons between interventional therapies is not only possible, but allows us to more appropriately offer care to vascular patients with more nuanced strategies. These are strategies that recognize subtleties between subgroups of individuals stratified on the basis of patient demographics and lesion characteristics in a way not appreciated prior to the recent spate of endovascular device studies.
Thus, thanks to the dedication and hard work of many, we are now at a stage where we can have meaningful dialogues on a variety of endovascular topics, such as the one at hand, and proponents can argue their perspectives armed with objective evidence to support their positions. In this discussion regarding covered stent grafts and drug-eluting stents, we wish we had even more data.
Specifically, we are missing direct head-to-head comparisons between the two devices in patients with long SFA lesions. So, what do we know?

Here are some fundamental facts: The most commonly used covered stent graft for management of femoropopliteal occlusive disease is the Viabahn endoprosthesis (W. L. Gore and Associates, Flagstaff, Ariz.). The prosthesis is composed of a self-expanding nitinol stent framework and expanded polytetrafluoroethylene (ePTFE) graft with its surface lined with a coating of covalently bound heparin (Propaten bioactive surface).The only approved drug-eluting stent with significant safety and effectiveness data available is the Zilver PTX paclitaxel-eluting, self-expanding nitinol stent (Cook Medical Inc., Bloomington, Ind.).
Now in terms of the proposition, we need to discuss the meaning of the word “long” with reference to the SFA. Just what do we consider a long SFA lesion? I think all of us could agree that an arterial stenosis or occlusion of 6 cm or less is short. Lesions between 5 cm or 6 cm to 10 cm or 12 cm in length are moderately long, and disease greater than 10 cm or 12 cm is commonly characterized as long. Segments of disease greater than 20 cm long are typically considered very long or extremely long lesions from an endovascular interventional perspective.
So, how can currently available trial outcomes help us? Below, I have compiled a table that includes most of the recent clinical trial data for Viabahn and Zilver PTX in patients with long SFA occlusive disease.
OK, what can we honestly say about these data besides recognizing that we are at risk when we make any conclusions based upon cross-trial comparisons? Such an accounting of results is fraught with problems, but what we can say is that the table grossly confirms the current consensus that both devices enhance the standard of care for long lesions over traditional balloon angioplasty (PTA) and bare metal stent technologies.
Beyond this, however, it is accepted that patency results with Viabahn are lesion-length immune – that is, outcomes in long and extremely long segments of disease are not very different from the patency achieved in short lesions. This is clearly different than what is traditionally found for interventions with PTA or bare metal stents. There is not enough controlled data for extremely long lesions to reach a conclusion on drug-eluting stents; however, there is an initial suggestion that they behave in a manner more similar to stent grafts than traditional devices.
Grossly, the table suggests that the midterm and available greater than 1-year patency results with Viabahn and Zilver PTX are relatively comparable. What about the price of the device? What role does it play in our selection of the current most cost-effective endovascular strategy for long SFA lesions?
In my institution Viabahn is more expensive than Zilver PTX with a relative cost premium of about 30%-50% depending on the treatment length. Of course, when treating long TASC C and D lesions any up-front difference in the costs of the devices used initially is more than made up for by any relative reduction in subsequent reinterventions.
So, there you have it. Look at the table as simply a current snapshot. In the future, we will benefit from additional trials and comparisons, not to mention better endovascular technologies to address symptomatic long SFA lesions.
Dr. Dake is the Thelma and Henry Doelger Professor of Cardiovascular Surgery at the Stanford (Calif.) School of Medicine. He disclosed that he is a member of the Peripheral Scientific Advisory Board: Abbott Vascular Member, is on the Aortic Medical Advisory Board: W. L. Gore, is a consultant for Cook Medical, Medtronic, and Surmodics Research, and receives grants/clinical trial support from W. L. Gore, Medtronic, and Novate.
References for table
1. J. Vasc. Surg. 2013;58:386-95.
2. J. Vasc. Surg. 2010;52:584-90.
3. J. Vasc. Interv. Radiol. 2013;24:165-73.
4. Cardiovasc. Interv. Radiol. 2015;38:25-32.
5. Circ. Cardiovasc. Interv. 2011;4:495-504.
6. J. Am. Coll. Cardiol. 2013;61:2417-27.
7. J. Endovasc. Ther. 2011;18:613-23.
8. Zeller T. Oral presentations. 2014.
9. Yokoi H. Oral presentations. 2014.
Covered stent grafts in the SFA are still the endovascular champion in long lesions
BY DENNIS GABLE, M.D.
There remains a continued debate among investigators as to the best modality for treatment of stenosis/occlusion of the SFA especially with the recent advent of drug-eluting technology. However, I suggest that for long lesions over 15 cm only covered stent grafts continue to outperform the competition.
If we review the numerous studies available on treatment of SFA disease, the Viabahn-covered stent device (W. L. Gore & Associates, Flagstaff, Ariz.) is by far the most studied modality. There are currently 22 independent studies available providing data on 1,473 limbs. Several of these reports are multicenter studies and many of them are prospective randomized trials. Two of the most recent are the VIPER (J. Vasc. Intervent. Radiol. 2013;24:165-73) and VIASTAR study (JACC 2013;62:1320-27).
The VIPER study prospectively enrolled 119 patients (72 with TASC II C/D disease; mean lesion length 190 mm). Primary patency was reported at 73% at 1 year but in patients with less than 20% oversizing, as is recommended by the IFU, patency as high as 88% was noted. Additionally, there was no difference in patency in smaller-diameter vessels (5 mm) versus larger-diameter vessels (6-7 mm).
In a head-to-head randomized controlled trial of Viabahn to bare metal stents (BMS), the VIASTAR study enrolled 141 patients (72 in covered stent arm; mean lesion length 190 mm). On a per protocol evaluation, the patency at 1 year was 78% and 71% on an intention-to-treat evaluation with a patency of 70% and 63% respectively at 2 years. There was no statistical difference between the two evaluations on intention to treat vs. per protocol but there was clear superiority demonstrated against BMS.
Furthermore, in a prospective, randomized, head-to-head comparison of Viabahn to prosthetic above knee femoral popliteal bypass, it was shown that there was no difference in primary or secondary patency between the two groups out to 4 years follow-up (J. Vasc. Surg. 2010;52:584-91). This included an average lesion length of 25.6 cm with a primary and secondary patency of 59% and 74% in the Viabahn group and 58% and 71% in the surgery group. When compared to a large meta-analysis for femoral popliteal bypass outcomes reported on by Bates and AbuRahma in 2004 (J. Endovasc. Ther. 2004;11[suppl. II]:II-107–27), the patency for the surgical arm with prosthetic bypass in the above Viabahn study was similar at 4 years to the 38 peer-reviewed articles Bates et al. reviewed with over 4,000 limbs. The reported primary and secondary patency at 4 years for prosthetic femoral above knee popliteal bypass was 51% and 61%, respectively in his review. Although the above Viabahn study was not powered to formally demonstrate noninferiority to surgical bypass with prosthetic, it did strongly suggest and show just that.
How do we put these data together with the Zilver data and how do we decide what is best for our patients? Some operators have expressed concern over a perceived risk for a “higher rate of amputation” or “a worse Rutherford level of ischemia on presentation” if patients with the Viabahn stent graft occlude post procedure. Commonly, this results from extrapolation of prior studies looking at results of occlusion with an ePTFE bypass. In fact, review of peer-reviewed data reveal none of the prospective studies outlined above, or those currently available, demonstrate that either of these perceptions are true and there are no published prospective data that support these fears either. In the studies listed above as well as all current prospective studies available evaluating Viabahn usage, the highest rate of amputation reported was 5% by Fisher in 2006 with all of the remaining studies reporting an amputation rate of 2% or less (when reported). Moreover, it has not been demonstrated that patients with this device present with an increased level of ischemia secondary to sudden occlusion.
There is one report used to argue against the use of the Viabahn stent graft (J. Vasc. Surg. 2008;47:967-74). This study evaluated prospectively 109 patients (71 for claudication; 38 for critical limb ischemia) treated for SFA occlusive disease (mean lesion length 15.7 cm). Only 19 of the 109 patients (17%) were treated with Viabahn (17 for claudication; 2 for critical limb ischemia). The remaining limbs were treated with various other BMS devices (n=10). The authors concluded that patients initially treated with Viabahn who presented back with occlusion had a higher chance of presenting with acute symptoms (i.e., a worse Rutherford score). The lesion length treated in the Viabahn group, however, was nearly twice as long as all the other stent platforms combined (25.4 cm vs. 13.7 cm) and there was a higher level of tibial artery deterioration with thrombosis of the BMS group, compared with the Viabahn group (7.7% vs. 5.3%). The number of Viabahn patients presenting with acute thrombosis was not defined. With the small number of limbs treated in the Viabahn group, the conclusions expressed cannot be statistically supported.
What about the in-vogue DES device?
Dr. Dake and his colleagues recently presented 5-year data on the Zilver DES platform at VIVA 2014. He reported a primary patency at 5 years of 66.4% showing superiority to angioplasty alone as well as angioplasty with provisional stenting. This study enrolled 479 patients into the randomization arm and also had a registry arm that although often included in reporting of patency, does not stand up to the scrutiny of peer review. Even though there were some patients with longer lesions, the randomized arm mean lesion length was only 66 mm, which does not compare to the published longer mean lesion length of the Viabahn device. Bosiers et al. (J. Cardiovasc. Surg. 2013 54:115-222) reviewed 135 patients treated with the Zilver device (a subgroup derived from the 787 patients enrolled in the registry data of the Zilver trial) with a mean lesion length of 226 mm. They reported 77.6% primary patency but only at 1 year. Again, however, this is registry derived data and does not have the scientific validity of a randomized trial.
So what can I conclude from these experiences? We know today that covered stent grafts have been widely used and reported on, including by Dr. Dake himself (Radiology 2000 October;217:95-104) and all appear to have had similar conclusions.
The mean lesion length treated in these studies of Viabahn is often longer than 15 cm and nearly all studies report primary patency outcomes. Zilver supporters on the other hand are prone to quote TLR which is an inferior endpoint (as recently noted in an editorial by Dr. Russell Samson (Vasc. Spec. 2015;11:2). Costs of both devices are an issue but may vary by region and institution. However, Viabahn does have the advantage of longer devices, compared with the Zilver (15 and 25 cm vs. 10 cm) so fewer devices may be required to treat long lesions. Although short lesions may be better addressed with BMS or DES, for longer SFA lesions over 12-15 cm there are very few truly comparable data that argue against the use of Viabahn.
Dr. Gable is chief of vascular and endovascular surgery at The Heart Hospital Baylor Plano (Tex.). He is also an associate medical editor for Vascular Specialist. He disclosed that he is a consultant, speaker, and receives research support from W. L. Gore and Medtronic.
Head-to-head comparisons are lacking, but similar results have been reported
BY MICHAEL D. DAKE, M.D.
Well, at least one thing is for sure – we would not have been having this discussion a mere 10 years ago.
I remained sheepishly silent for most of my early career as well-intentioned invasive and noninvasive specialists criticized the state of evidence supporting the legitimacy of endovascular interventions as a competitive strategy to manage infrainguinal peripheral arterial disease. Good data from well-controlled randomized clinical trials were not available to make a case for endovascular therapies.
Over the recent decade and a half, however, a number of contributing factors have influenced thinking and what we now consider standard of care for symptomatic disease of the superficial femoral artery (SFA). The proposal of an “endovascular first” interventional approach has evolved to a consensually agreed upon management strategy by all interested disciplines.
This did not occur on a whim. Rather, out of the shadows of relative ignorance there slowly emerged a welcomed accumulation of a large number of publications that detail the outcomes of a wide variety of randomized trials with a range of endovascular devices. This has allowed us to enter an era where valid comparisons between interventional therapies is not only possible, but allows us to more appropriately offer care to vascular patients with more nuanced strategies. These are strategies that recognize subtleties between subgroups of individuals stratified on the basis of patient demographics and lesion characteristics in a way not appreciated prior to the recent spate of endovascular device studies.
Thus, thanks to the dedication and hard work of many, we are now at a stage where we can have meaningful dialogues on a variety of endovascular topics, such as the one at hand, and proponents can argue their perspectives armed with objective evidence to support their positions. In this discussion regarding covered stent grafts and drug-eluting stents, we wish we had even more data.
Specifically, we are missing direct head-to-head comparisons between the two devices in patients with long SFA lesions. So, what do we know?

Here are some fundamental facts: The most commonly used covered stent graft for management of femoropopliteal occlusive disease is the Viabahn endoprosthesis (W. L. Gore and Associates, Flagstaff, Ariz.). The prosthesis is composed of a self-expanding nitinol stent framework and expanded polytetrafluoroethylene (ePTFE) graft with its surface lined with a coating of covalently bound heparin (Propaten bioactive surface).The only approved drug-eluting stent with significant safety and effectiveness data available is the Zilver PTX paclitaxel-eluting, self-expanding nitinol stent (Cook Medical Inc., Bloomington, Ind.).
Now in terms of the proposition, we need to discuss the meaning of the word “long” with reference to the SFA. Just what do we consider a long SFA lesion? I think all of us could agree that an arterial stenosis or occlusion of 6 cm or less is short. Lesions between 5 cm or 6 cm to 10 cm or 12 cm in length are moderately long, and disease greater than 10 cm or 12 cm is commonly characterized as long. Segments of disease greater than 20 cm long are typically considered very long or extremely long lesions from an endovascular interventional perspective.
So, how can currently available trial outcomes help us? Below, I have compiled a table that includes most of the recent clinical trial data for Viabahn and Zilver PTX in patients with long SFA occlusive disease.
OK, what can we honestly say about these data besides recognizing that we are at risk when we make any conclusions based upon cross-trial comparisons? Such an accounting of results is fraught with problems, but what we can say is that the table grossly confirms the current consensus that both devices enhance the standard of care for long lesions over traditional balloon angioplasty (PTA) and bare metal stent technologies.
Beyond this, however, it is accepted that patency results with Viabahn are lesion-length immune – that is, outcomes in long and extremely long segments of disease are not very different from the patency achieved in short lesions. This is clearly different than what is traditionally found for interventions with PTA or bare metal stents. There is not enough controlled data for extremely long lesions to reach a conclusion on drug-eluting stents; however, there is an initial suggestion that they behave in a manner more similar to stent grafts than traditional devices.
Grossly, the table suggests that the midterm and available greater than 1-year patency results with Viabahn and Zilver PTX are relatively comparable. What about the price of the device? What role does it play in our selection of the current most cost-effective endovascular strategy for long SFA lesions?
In my institution Viabahn is more expensive than Zilver PTX with a relative cost premium of about 30%-50% depending on the treatment length. Of course, when treating long TASC C and D lesions any up-front difference in the costs of the devices used initially is more than made up for by any relative reduction in subsequent reinterventions.
So, there you have it. Look at the table as simply a current snapshot. In the future, we will benefit from additional trials and comparisons, not to mention better endovascular technologies to address symptomatic long SFA lesions.
Dr. Dake is the Thelma and Henry Doelger Professor of Cardiovascular Surgery at the Stanford (Calif.) School of Medicine. He disclosed that he is a member of the Peripheral Scientific Advisory Board: Abbott Vascular Member, is on the Aortic Medical Advisory Board: W. L. Gore, is a consultant for Cook Medical, Medtronic, and Surmodics Research, and receives grants/clinical trial support from W. L. Gore, Medtronic, and Novate.
References for table
1. J. Vasc. Surg. 2013;58:386-95.
2. J. Vasc. Surg. 2010;52:584-90.
3. J. Vasc. Interv. Radiol. 2013;24:165-73.
4. Cardiovasc. Interv. Radiol. 2015;38:25-32.
5. Circ. Cardiovasc. Interv. 2011;4:495-504.
6. J. Am. Coll. Cardiol. 2013;61:2417-27.
7. J. Endovasc. Ther. 2011;18:613-23.
8. Zeller T. Oral presentations. 2014.
9. Yokoi H. Oral presentations. 2014.
Covered stent grafts in the SFA are still the endovascular champion in long lesions
BY DENNIS GABLE, M.D.
There remains a continued debate among investigators as to the best modality for treatment of stenosis/occlusion of the SFA especially with the recent advent of drug-eluting technology. However, I suggest that for long lesions over 15 cm only covered stent grafts continue to outperform the competition.
If we review the numerous studies available on treatment of SFA disease, the Viabahn-covered stent device (W. L. Gore & Associates, Flagstaff, Ariz.) is by far the most studied modality. There are currently 22 independent studies available providing data on 1,473 limbs. Several of these reports are multicenter studies and many of them are prospective randomized trials. Two of the most recent are the VIPER (J. Vasc. Intervent. Radiol. 2013;24:165-73) and VIASTAR study (JACC 2013;62:1320-27).
The VIPER study prospectively enrolled 119 patients (72 with TASC II C/D disease; mean lesion length 190 mm). Primary patency was reported at 73% at 1 year but in patients with less than 20% oversizing, as is recommended by the IFU, patency as high as 88% was noted. Additionally, there was no difference in patency in smaller-diameter vessels (5 mm) versus larger-diameter vessels (6-7 mm).
In a head-to-head randomized controlled trial of Viabahn to bare metal stents (BMS), the VIASTAR study enrolled 141 patients (72 in covered stent arm; mean lesion length 190 mm). On a per protocol evaluation, the patency at 1 year was 78% and 71% on an intention-to-treat evaluation with a patency of 70% and 63% respectively at 2 years. There was no statistical difference between the two evaluations on intention to treat vs. per protocol but there was clear superiority demonstrated against BMS.
Furthermore, in a prospective, randomized, head-to-head comparison of Viabahn to prosthetic above knee femoral popliteal bypass, it was shown that there was no difference in primary or secondary patency between the two groups out to 4 years follow-up (J. Vasc. Surg. 2010;52:584-91). This included an average lesion length of 25.6 cm with a primary and secondary patency of 59% and 74% in the Viabahn group and 58% and 71% in the surgery group. When compared to a large meta-analysis for femoral popliteal bypass outcomes reported on by Bates and AbuRahma in 2004 (J. Endovasc. Ther. 2004;11[suppl. II]:II-107–27), the patency for the surgical arm with prosthetic bypass in the above Viabahn study was similar at 4 years to the 38 peer-reviewed articles Bates et al. reviewed with over 4,000 limbs. The reported primary and secondary patency at 4 years for prosthetic femoral above knee popliteal bypass was 51% and 61%, respectively in his review. Although the above Viabahn study was not powered to formally demonstrate noninferiority to surgical bypass with prosthetic, it did strongly suggest and show just that.
How do we put these data together with the Zilver data and how do we decide what is best for our patients? Some operators have expressed concern over a perceived risk for a “higher rate of amputation” or “a worse Rutherford level of ischemia on presentation” if patients with the Viabahn stent graft occlude post procedure. Commonly, this results from extrapolation of prior studies looking at results of occlusion with an ePTFE bypass. In fact, review of peer-reviewed data reveal none of the prospective studies outlined above, or those currently available, demonstrate that either of these perceptions are true and there are no published prospective data that support these fears either. In the studies listed above as well as all current prospective studies available evaluating Viabahn usage, the highest rate of amputation reported was 5% by Fisher in 2006 with all of the remaining studies reporting an amputation rate of 2% or less (when reported). Moreover, it has not been demonstrated that patients with this device present with an increased level of ischemia secondary to sudden occlusion.
There is one report used to argue against the use of the Viabahn stent graft (J. Vasc. Surg. 2008;47:967-74). This study evaluated prospectively 109 patients (71 for claudication; 38 for critical limb ischemia) treated for SFA occlusive disease (mean lesion length 15.7 cm). Only 19 of the 109 patients (17%) were treated with Viabahn (17 for claudication; 2 for critical limb ischemia). The remaining limbs were treated with various other BMS devices (n=10). The authors concluded that patients initially treated with Viabahn who presented back with occlusion had a higher chance of presenting with acute symptoms (i.e., a worse Rutherford score). The lesion length treated in the Viabahn group, however, was nearly twice as long as all the other stent platforms combined (25.4 cm vs. 13.7 cm) and there was a higher level of tibial artery deterioration with thrombosis of the BMS group, compared with the Viabahn group (7.7% vs. 5.3%). The number of Viabahn patients presenting with acute thrombosis was not defined. With the small number of limbs treated in the Viabahn group, the conclusions expressed cannot be statistically supported.
What about the in-vogue DES device?
Dr. Dake and his colleagues recently presented 5-year data on the Zilver DES platform at VIVA 2014. He reported a primary patency at 5 years of 66.4% showing superiority to angioplasty alone as well as angioplasty with provisional stenting. This study enrolled 479 patients into the randomization arm and also had a registry arm that although often included in reporting of patency, does not stand up to the scrutiny of peer review. Even though there were some patients with longer lesions, the randomized arm mean lesion length was only 66 mm, which does not compare to the published longer mean lesion length of the Viabahn device. Bosiers et al. (J. Cardiovasc. Surg. 2013 54:115-222) reviewed 135 patients treated with the Zilver device (a subgroup derived from the 787 patients enrolled in the registry data of the Zilver trial) with a mean lesion length of 226 mm. They reported 77.6% primary patency but only at 1 year. Again, however, this is registry derived data and does not have the scientific validity of a randomized trial.
So what can I conclude from these experiences? We know today that covered stent grafts have been widely used and reported on, including by Dr. Dake himself (Radiology 2000 October;217:95-104) and all appear to have had similar conclusions.
The mean lesion length treated in these studies of Viabahn is often longer than 15 cm and nearly all studies report primary patency outcomes. Zilver supporters on the other hand are prone to quote TLR which is an inferior endpoint (as recently noted in an editorial by Dr. Russell Samson (Vasc. Spec. 2015;11:2). Costs of both devices are an issue but may vary by region and institution. However, Viabahn does have the advantage of longer devices, compared with the Zilver (15 and 25 cm vs. 10 cm) so fewer devices may be required to treat long lesions. Although short lesions may be better addressed with BMS or DES, for longer SFA lesions over 12-15 cm there are very few truly comparable data that argue against the use of Viabahn.
Dr. Gable is chief of vascular and endovascular surgery at The Heart Hospital Baylor Plano (Tex.). He is also an associate medical editor for Vascular Specialist. He disclosed that he is a consultant, speaker, and receives research support from W. L. Gore and Medtronic.


