User login
Although pulmonary complications are not as well studied as cardiac complications in the postoperative setting, they are just as common following noncardiac surgery and are even more costly. It is worthwhile to identify surgical patients most at risk of postoperative pulmonary complications and take measures known to mitigate risk. This paper discusses important risk factors to identify during a preoperative pulmonary evaluation and then focuses on recent advances in strategies for reducing postoperative pulmonary complications. Teaching questions are included throughout, along with the rationale behind their answers.
POSTOPERATIVE PULMONARY COMPLICATIONS: WHAT ARE WE TRYING TO PREVENT AND WHY?
The definition of postoperative pulmonary complications is more variable and less intuitive than that of cardiac complications. Cardiac complications—postoperative myocardial infarction, cardiac death, and pulmonary edema—are more consistently defined and measured in clinical trials. Studies of postoperative pulmonary complications often group together pneumonia, respiratory failure, atelectasis, bronchospasm, and exacerbation of chronic obstructive pulmonary disease (COPD), making it more difficult to individually evaluate risk factors for different outcomes.
There are several reasons why it is important to consider pulmonary risk when evaluating patients preoperatively:
Pulmonary complications are as common as cardiac complications following noncardiac surgery. For example, in a secondary analysis of the cohort of noncardiac surgical patients used to validate the Revised Cardiac Risk Index,1 Fleischmann et al found that the incidence of pulmonary complications (2.7%) was highly comparable to that of cardiac complications (2.5%).2
Respiratory failure is a marker of ill health and predicts further complications. Postoperative respiratory failure (often defined as the need for ventilation for more than 48 hours after surgery) is an extremely morbid event. Johnson et al compared the outcomes of patients with and without respiratory failure as a complication of surgery.3 Among patients with respiratory failure, 26% died within 30 days, 6% had a myocardial infarction, 35% developed pneumonia, 10% developed acute renal failure, and 3% developed a deep vein thrombosis or pulmonary embolism; in contrast, rates of each of these events were lower than 2% among patients without respiratory failure.
Pulmonary complications are expensive and require lengthy hospitalization. The National Surgical Quality Improvement Program (NSQIP) compared hospitalization costs and length of stay among patients with various postoperative complications.4 Among infectious, cardiovascular, venous thromboembolic, and pulmonary complications, pulmonary complications were by far the most costly and, along with venous thromboembolic complications, required the longest mean hospital stay.
For these reasons, identifying patients at risk for pulmonary complications and developing a strategy to reduce the risk is clearly worthwhile.
IDENTIFYING RISK FOR PULMONARY COMPLICATIONS
Question: Which of the following is the most important risk factor for postoperative pulmonary complications?
A. High-risk surgical site
B. General anesthesia
C. COPD
D. Obesity
The correct answer is A. Pulmonary complications differ from cardiac complications in an important way: procedure-related factors are more predictive of pulmonary complications than are patient-related factors. Even healthy patients undergoing high-risk surgery are at risk for pulmonary complications. As for the other answer choices, general anesthesia and COPD are risk factors but are not as important as surgical site, and obesity has not been shown to be a risk factor at all.
Take-home points from the 2006 ACP guideline
Patient-related risk factors. As noted in Table 1, the most important patient-related risk factors identified in the ACP guideline are increasing age and increasing American Society of Anesthesiologists (ASA) classification of comorbidity.
The effect of advanced age becomes particularly notable around age 60 years and escalates from there. This effect of age differs from that for cardiac complications, for which age drops out as a risk factor after adjustment for other diseases and risk factors. For pulmonary complications, in contrast, even older patients who are healthy are at increased risk.
The ASA classification is a general index of overall morbidity that ranges from class 1 (normal healthy patient) to class 5 (moribund patient who is not expected to survive without the operation).
Notably, COPD and smoking were only minor risk factors in the ACP analysis.
Procedure-related risk factors. Surgical site was found to be the most important of any of the patient- or procedure-related risk factors. The closer the incision is to the diaphragm, the greater the risk for pulmonary complications. Aortic, thoracic, and abdominal procedures carry the highest risk (Table 1), and among abdominal procedures, upper abdominal surgery (eg, cholecystectomy) is riskier than lower abdominal surgery (eg, gynecologic).
Other procedure-related risk factors identified were emergency surgery, surgery lasting more than 3 hours, use of general anesthesia, and multiple transfusions (Table 1).
Newly identified risk factors
Question: Which of the following has recently been identified as a risk factor for postoperative pulmonary complications?
A. Epidural anesthesia
B. Insulin-treated diabetes
C. Obstructive sleep apnea
D. Immobility
The correct answer is C. There is no evidence that epidural anesthesia or insulin-treated diabetes are risk factors. Immobility seems intuitively correct but has not emerged as a risk factor among high-quality studies in the literature.
Obstructive sleep apnea. The role of obstructive sleep apnea was unclear prior to publication of new data in the last couple of years. Hwang et al enrolled 172 patients who were soon to have elective surgery and had at least two of four clinical features of obstructive sleep apnea (snoring, daytime somnolence, witnessed apnea event, or crowded oropharynx).8 Patients underwent nocturnal oximetry before surgery and were divided into two groups based on number of desaturation episodes per hour. Patients with five or more desaturations had markedly higher rates of postoperative respiratory complications (8 complications among 98 patients) than did patients with fewer than five desaturations (1 complication among 74 patients). The presence of five or more desaturations was also associated with higher rates of cardiac, gastrointestinal, and bleeding complications. Though this was a small study, its results suggest a significant association between obstructive sleep apnea and pulmonary complications.
The issue of whether to screen patients for obstructive sleep apnea before major noncardiac surgery is still unresolved.
Pulmonary hypertension has also been identified as a risk factor in recent years with the publication of two studies that estimated its impact on morbidity and mortality after major noncardiac surgery.9,10 One of the studies, a retrospective database review, found a 28% incidence of respiratory failure among 145 surgical patients with pulmonary hypertension.9 In the other study, a prospective case-control trial, respiratory failure occurred in 21% of patients with pulmonary hypertension compared with only 3% of matched controls.10 In the case-control study, pulmonary hypertension was also associated with significantly elevated rates of heart failure and in-hospital death.
The results of these studies do not support preoperative screening for undiagnosed pulmonary hypertension, but they do underscore the need to recognize established pulmonary hypertension as an important risk factor for postoperative complications.
AN UPDATED INDEX FOR RESPIRATORY FAILURE
Several years ago, investigators from the Veterans Affairs Medical Centers developed a respiratory failure index using a design similar to those of well-established indices for cardiac risk.11 The same group also developed a separate risk index for pneumonia.12
This respiratory failure index was recently updated3 to reflect experience from private and academic hospitals, making the results more generally applicable. The researchers evaluated data from 180,000 patients undergoing major general or vascular surgery (defined according to the NSQIP) over a 3-year period. Respiratory failure was defined as requiring at least 48 hours of ventilation or unplanned reintubation.
Comparison and contrast with the ACP guideline
Question: How does the updated respiratory failure index differ most significantly from the 2006 ACP guideline?
A. New index places greater emphasis on ASA class
B. New index offers a simplified weighted point scheme
C. New index ranks low albumin as a less important risk factor
D. New index attributes low risk to cigarette use
The correct answer is C: low albumin is a minor risk factor in the respiratory failure index, whereas it was one of the single most important predictors in the ACP guideline. As for the other answer choices, the new index places about the same emphasis on ASA class and cigarette use as does the ACP guideline, and it does not offer a simplified approach, as it incorporates 28 different factors.
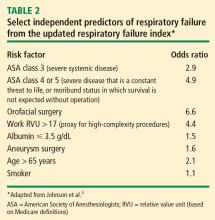
Overall, most risk factors were similar in the updated respiratory failure index and the ACP guideline, but the index differs in several important ways:
- The index assigns less risk to low albumin, functional dependence, and congestive heart failure
- The index assigns greater risk to orofacial surgery
- The index identifies several new risk factors—high-complexity surgery, preoperative sepsis, ascites, and hypernatremia (serum sodium > 145 mmol/L).
STRATEGIES FOR RISK REDUCTION
Postoperative CPAP: Good option when exercise ability is limited
Among the postoperative lung expansion modalities, continuous positive airway pressure (CPAP) is particularly useful for patients who are unable to perform deep breathing or incentive spirometry exercises. A recent systematic literature review identified nine randomized controlled trials of CPAP vs standard therapy in a total of 654 patients undergoing abdominal surgery.13 Meta-analysis of these studies showed that CPAP was associated with significant reductions in the risk of overall postoperative pulmonary complications (odds ratio [OR] = 0.66; 95% CI, 0.52–0.85), atelectasis (OR = 0.75; 95% CI, 0.58–0.97), and pneumonia (OR = 0.33; 95% CI, 0.14–0.75) relative to standard therapy.
Use nasogastric tubes selectively
Nasogastric tubes can be used either routinely following abdominal surgery or only in select patients—eg, those who have symptomatic abdominal distention or nausea. The difference is important since nasogastric tubes may potentially increase the risk of aspiration and thus lead to a pulmonary complication. Nelson et al conducted a meta-analysis of 24 studies that compared routine nasogastric tube use in abdominal surgery with selective use based on symptoms or abdominal distention.14 They found that routine use was associated with a significant increase in postoperative pulmonary complications (OR = 1.45; 95% CI, 1.08–1.93) relative to selective use, without achieving any of its intended goals.
Laparoscopic vs open surgery: Evidence begins to follow intuition
Intuitively, it seems that laparoscopic procedures should reduce risk for postoperative pulmonary complications compared with open surgical procedures, as they are associated with less postoperative pain, which should facilitate deep breathing and improve postoperative lung volumes. Nevertheless, evidence for whether laparoscopic surgery reduces the risk of pulmonary complications has been mixed until recently.
In 2008, however, Weller and Rosati published an analysis of a nationally representative database of 19,156 patients who underwent bariatric surgery in 2005.15 After adjusting for comorbidities, they found that the rate of postoperative pulmonary complications was nearly double if patients underwent open surgery as opposed to laparoscopic surgery (OR = 1.92; 95% CI, 1.54–2.38). Open surgery was also associated with significantly higher rates of sepsis, cardiovascular events, and reoperation compared with laparoscopic procedures. This study suggests that choosing laparoscopic procedures is another strategy that may reduce pulmonary complication rates, at least in the setting of bariatric surgery.
Postoperative thoracic epidural analgesia
Question: Thoracic epidural analgesia reduces rates of which of the following?
A. Pneumonia following abdominal aortic aneurysm repair
B. Pulmonary complications following coronary bypass surgery
C. Respiratory failure following abdominal surgery
D. All of the above
The correct answer is D. Thoracic epidural analgesia is another important strategy for reducing postoperative pulmonary complications, as demonstrated by a 2007 systematic literature review by Liu and Wu.16 Their analysis showed that rates of pneumonia, respiratory failure, and pulmonary complications overall were reduced by approximately one-third to more than one-half with the use of postoperative thoracic epidural analgesia in patients undergoing aortic aneurysm repair, coronary bypass surgery, and abdominal surgery.
Smoking cessation: The jury is still out
Whether preoperative cigarette cessation reduces pulmonary complication rates has been controversial over the past decade. Early reports showed that among patients who smoke, those who quit shortly before surgery actually had higher complication rates than patients who continued to smoke. The most reasonable explanation seems to be that many patients who stop smoking report increased coughing and sputum production for the first month or two. Selection bias also may have played a role in these findings.
More recently, two randomized trials studied the impact of perioperative smoking intervention programs involving counseling and nicotine replacement.17,18 Unfortunately, both studies primarily studied patients undergoing low-risk procedures and were insufficiently powered to show a difference in pulmonary complication rates. The question of whether smoking cessation is an effective strategy to reduce postoperative pulmonary risk remains unanswered.
Preoperative intensive lung expansion: A promising new intervention
While the effectiveness of postoperative lung expansion techniques is undisputed,7preoperative lung expansion—also known as inspiratory muscle training—has only recently been investigated. Hulzebos et al randomized 279 patients undergoing coronary artery bypass graft surgery who were at high risk for developing pulmonary complications to either usual care or inspiratory muscle training.19 The latter intervention involved 20 minutes per day of incentive spirometry, active breathing, and forced expiration techniques for at least 2 weeks prior to surgery. Rates of high-grade postoperative pulmonary complications were cut in half (OR = 0.52; 95% CI, 0.30–0.92) and rates of pneumonia were reduced by 60% (OR = 0.40; 95% CI, 0.19–0.84) in patients who received inspiratory muscle training relative to the usual-care group.
In clinical practice, preoperative inspiratory muscle training can be done in a chest physical therapy outpatient setting or a pulmonary rehabilitation clinic in the hospital.
SUMMARY
There have been a number of significant recent developments in the perioperative management of pulmonary complications:
- Obstructive sleep apnea has been confirmed as a risk factor, and pulmonary hypertension has emerged as a novel risk factor.
- An updated respiratory failure index has emerged as a useful research tool to identify high-risk patients and to ensure uniform risk stratification in future research.
- Evidence has mounted for the effectiveness of several risk-reduction strategies, including the use of laparoscopic procedures for bariatric surgery; selective use of nasogastric tubes; postoperative thoracic epidural analgesia; and intensive preoperative inspiratory muscle training.
DISCUSSION
Question from the audience: I do preoperative evaluations in an orthopedic ambulatory surgery center. Our surgeons often tell me, “Just order preoperative pulmonary function tests,” or, “Get a blood gas.” How should I respond?
Dr. Smetana: This is an area of some controversy, but in general, spirometry does not add much to a preoperative risk assessment that is based on a history and physical exam. Usually if the spirometry is abnormal, it will not be a surprise after careful clinical assessment. Arterial blood gases have no role in routine preoperative assessment.
Question from the audience: A chest x-ray is often requested preoperatively, but is it a necessary study?
Dr. Smetana: The data for preoperative chest x-rays are fairly poor and don’t allow us to assess whether they accurately predict complication rates. Most studies on chest x-rays have looked at how they affect preoperative management—eg, whether they change the anesthesia or even the surgery—and have shown that preoperative management changes in only about 1% to 2% of cases. So the chest x-ray is a fairly low-yield test in this setting.
One could argue that a preoperative chest x-ray might provide a baseline for postoperative comparison, but actually it is not usually helpful in this regard. Having a baseline does not make it easier to correctly diagnose pneumonia postoperatively, for example. Abnormal chest x-rays correlate with higher risk, but most patients with abnormal films would be suspected of being at higher risk anyway based on findings from the clinical assessment.
Question from the audience: Many primary care doctors in my hospital screen patients for pulmonary hypertension, but this raises the question of what to do with any information gained. What do you tell patients? Anesthesiologists?
Dr. Smetana: I don’t recommend preoperative screening for pulmonary hypertension unless there is some specific clinical reason to look for it. We don’t know if the perioperative risks that I described for patients with diagnosed or symptomatic pulmonary hypertension would also apply to patients with unrecognized, asymptomatic pulmonary hypertension that happened to be identified by screening.
Patients with pulmonary hypertension are at very high risk, especially for respiratory failure. But we don’t have any risk-reduction strategies specific to these patients, although I would recommend applying the general risk-reduction strategies that I discussed.
Question from the audience: I saw a man at my high-risk preoperative clinic who scored normally on a 6-minute walk test but then was found sound asleep when I was ready to see him a little while later. I suspected he had undiagnosed sleep apnea, and therefore had an increased risk of postoperative pulmonary complications, but what evidence would I have to delay his surgery to diagnose the sleep apnea and stabilize him on CPAP?
Dr. Smetana: For a patient with clinically suspected but undiagnosed sleep apnea, we have some evidence that the diagnosis should be pursued before surgery is performed.8 If the surgery were elective, it would be appropriate to have the patient evaluated and, if obstructive sleep apnea were diagnosed, treated in the customary way with CPAP. For patients who are hospitalized after surgery, CPAP can be continued as soon as possible in the hospital.
I would not have made this recommendation a few years ago, but now the evidence is more compelling. However, at this point I would not recommend routine preoperative screening of all patients for sleep apnea. Ongoing research is looking at this question.
Follow-up question: How long should surgery be delayed to optimize the patient on CPAP?
Dr. Smetana: Risk for postoperative respiratory failure is reduced very quickly after initiating CPAP therapy. A week would probably be sufficient, but there are no good data to specifically address that question.
Question from the audience: What about patients with asthma who are undergoing surgery—which ones benefit from stress-level steroids and preoperative nebulizer therapy?
Dr. Smetana: Surprisingly, asthma—if well controlled—is not a risk factor for postoperative pulmonary complications. Patients within 80% of their predicted or personal best peak flow appear to have a risk similar to that of patients without asthma. For patients with uncontrolled or poorly controlled asthma, the general rule is the same as for patients with COPD: treat them the same as if they weren’t having surgery. If a patient with asthma has a clinical indication for corticosteroids based on his or her condition, give corticosteroids whether or not surgery is planned. Corticosteroids are safe and do not raise the risk of postoperative wound complications. But we have no evidence to support routine use of steroids for all patients with asthma simply because elective surgery is planned.
Follow-up question: Do you optimize poorly controlled patients with oral prednisone for several days preoperatively, or do you use a stress protocol?
Dr. Smetana: For a patient whom you would normally treat with an outpatient course of prednisone, you should do just that. For a patient with an exacerbation severe enough to require admission for intravenous steroids and inhaled nebulizer therapy, then you should use that strategy. If the surgery is elective, it should be delayed until the patient is at his or her personal best.
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Fleischmann KE, Goldman L, Young B, et al. Association between cardiac and noncardiac complications in patients undergoing noncardiac surgery: outcomes and effects on length of stay. Am J Med 2003; 115:515–520.
- Johnson RG, Arozullah AM, Neumayer L, et al. Multivariable predictors of postoperative respiratory failure after general and vascular surgery: results from the Patient Safety in Surgery Study. J Am Coll Surg 2007; 204:1188–1198.
- Dimick JB, Chen SL, Taheri PA, et al. Hospital costs associated with surgical complications: a report from the private-sector National Surgical Quality Improvement Program. J Am Coll Surg 2004; 199:531–537.
- Qaseem A, Snow V, Fitterman N, et al. Risk assessment for and strategies to reduce perioperative pulmonary complications for patients undergoing noncardiothoracic surgery: a guideline from the American College of Physicians. Ann Intern Med 2006; 144:575–580.
- Smetana GW, Lawrence VA, Cornell JE. Preoperative pulmonary risk stratification for noncardiothoracic surgery: systematic review for the American College of Physicians. Ann Intern Med 2006; 144:581–595.
- Lawrence VA, Cornell JE, Smetana GW; American College of Physicians. Strategies to reduce postoperative pulmonary complications after noncardiothoracic surgery: systematic review for the American College of Physicians. Ann Intern Med 2006; 144:596–608.
- Hwang D, Shakir N, Limann B, et al. Association of sleep-disordered breathing with postoperative complications. Chest 2008; 133:1128–1134.
- Ramakrishna G, Sprung J, Ravi BS, et al. Impact of pulmonary hypertension on the outcomes of noncardiac surgery: predictors of perioperative morbidity and mortality. J Am Coll Cardiol 2005; 45:1691–1699.
- Lai HC, Lai HC, Wang KY, Lee WL, Ting CT, Liu TJ. Severe pulmonary hypertension complicates postoperative outcome of noncardiac surgery. Br J Anaesth 2007; 99:184–190.
- Arozullah AM, Daley J, Henderson WG, Khuri SF. Multifactorial risk index for predicting postoperative respiratory failure in men after major noncardiac surgery. The National Veterans Administration Surgical Quality Improvement Program. Ann Surg 2000; 232:242–253.
- Arozullah AM, Khuri SF, Henderson WG, et al. Development and validation of a multifactorial risk index for predicting postoperative pneumonia after major noncardiac surgery. Ann Intern Med 2001; 135:847–857.
- Ferreyra GP, Baussano I, Squadrone V, et al. Continuous positive airway pressure for treatment of respiratory complications after abdominal surgery: a systematic review and meta-analysis. Ann Surg 2008; 247:617–626.
- Nelson R, Edwards S, Tse B. Prophylactic nasogastric decompression after abdominal surgery. Cochrane Database Syst Rev 2007; Jul 18 (3):CD004929.
- Weller WE, Rosati C. Comparing outcomes of laparoscopic versus open bariatric surgery. Ann Surg 2008; 248:10–15.
- Liu SS, Wu CL. Effect of postoperative analgesia on major postoperative complications: a systematic update of the evidence. Anesth Analg 2007; 104:689–702.
- Møller AM, Villebro N, Pedersen T, Tønnesen H. Effect of preoperative smoking intervention on postoperative complications: a randomised clinical trial. Lancet 2002; 359:114–117.
- Lindström D, Sadr Azodi O, Wladis A, et al. Effects of a perioperative smoking cessation intervention on postoperative complications: a randomized trial. Ann Surg 2008; 248:739–745.
- Hulzebos EH, Helders PJ, Favié NJ, et al. Preoperative intensive inspiratory muscle training to prevent postoperative pulmonary complications in high-risk patients undergoing CABG surgery: a randomized clinical trial. JAMA 2006; 296:1851–1857.
Although pulmonary complications are not as well studied as cardiac complications in the postoperative setting, they are just as common following noncardiac surgery and are even more costly. It is worthwhile to identify surgical patients most at risk of postoperative pulmonary complications and take measures known to mitigate risk. This paper discusses important risk factors to identify during a preoperative pulmonary evaluation and then focuses on recent advances in strategies for reducing postoperative pulmonary complications. Teaching questions are included throughout, along with the rationale behind their answers.
POSTOPERATIVE PULMONARY COMPLICATIONS: WHAT ARE WE TRYING TO PREVENT AND WHY?
The definition of postoperative pulmonary complications is more variable and less intuitive than that of cardiac complications. Cardiac complications—postoperative myocardial infarction, cardiac death, and pulmonary edema—are more consistently defined and measured in clinical trials. Studies of postoperative pulmonary complications often group together pneumonia, respiratory failure, atelectasis, bronchospasm, and exacerbation of chronic obstructive pulmonary disease (COPD), making it more difficult to individually evaluate risk factors for different outcomes.
There are several reasons why it is important to consider pulmonary risk when evaluating patients preoperatively:
Pulmonary complications are as common as cardiac complications following noncardiac surgery. For example, in a secondary analysis of the cohort of noncardiac surgical patients used to validate the Revised Cardiac Risk Index,1 Fleischmann et al found that the incidence of pulmonary complications (2.7%) was highly comparable to that of cardiac complications (2.5%).2
Respiratory failure is a marker of ill health and predicts further complications. Postoperative respiratory failure (often defined as the need for ventilation for more than 48 hours after surgery) is an extremely morbid event. Johnson et al compared the outcomes of patients with and without respiratory failure as a complication of surgery.3 Among patients with respiratory failure, 26% died within 30 days, 6% had a myocardial infarction, 35% developed pneumonia, 10% developed acute renal failure, and 3% developed a deep vein thrombosis or pulmonary embolism; in contrast, rates of each of these events were lower than 2% among patients without respiratory failure.
Pulmonary complications are expensive and require lengthy hospitalization. The National Surgical Quality Improvement Program (NSQIP) compared hospitalization costs and length of stay among patients with various postoperative complications.4 Among infectious, cardiovascular, venous thromboembolic, and pulmonary complications, pulmonary complications were by far the most costly and, along with venous thromboembolic complications, required the longest mean hospital stay.
For these reasons, identifying patients at risk for pulmonary complications and developing a strategy to reduce the risk is clearly worthwhile.
IDENTIFYING RISK FOR PULMONARY COMPLICATIONS
Question: Which of the following is the most important risk factor for postoperative pulmonary complications?
A. High-risk surgical site
B. General anesthesia
C. COPD
D. Obesity
The correct answer is A. Pulmonary complications differ from cardiac complications in an important way: procedure-related factors are more predictive of pulmonary complications than are patient-related factors. Even healthy patients undergoing high-risk surgery are at risk for pulmonary complications. As for the other answer choices, general anesthesia and COPD are risk factors but are not as important as surgical site, and obesity has not been shown to be a risk factor at all.
Take-home points from the 2006 ACP guideline
Patient-related risk factors. As noted in Table 1, the most important patient-related risk factors identified in the ACP guideline are increasing age and increasing American Society of Anesthesiologists (ASA) classification of comorbidity.
The effect of advanced age becomes particularly notable around age 60 years and escalates from there. This effect of age differs from that for cardiac complications, for which age drops out as a risk factor after adjustment for other diseases and risk factors. For pulmonary complications, in contrast, even older patients who are healthy are at increased risk.
The ASA classification is a general index of overall morbidity that ranges from class 1 (normal healthy patient) to class 5 (moribund patient who is not expected to survive without the operation).
Notably, COPD and smoking were only minor risk factors in the ACP analysis.
Procedure-related risk factors. Surgical site was found to be the most important of any of the patient- or procedure-related risk factors. The closer the incision is to the diaphragm, the greater the risk for pulmonary complications. Aortic, thoracic, and abdominal procedures carry the highest risk (Table 1), and among abdominal procedures, upper abdominal surgery (eg, cholecystectomy) is riskier than lower abdominal surgery (eg, gynecologic).
Other procedure-related risk factors identified were emergency surgery, surgery lasting more than 3 hours, use of general anesthesia, and multiple transfusions (Table 1).
Newly identified risk factors
Question: Which of the following has recently been identified as a risk factor for postoperative pulmonary complications?
A. Epidural anesthesia
B. Insulin-treated diabetes
C. Obstructive sleep apnea
D. Immobility
The correct answer is C. There is no evidence that epidural anesthesia or insulin-treated diabetes are risk factors. Immobility seems intuitively correct but has not emerged as a risk factor among high-quality studies in the literature.
Obstructive sleep apnea. The role of obstructive sleep apnea was unclear prior to publication of new data in the last couple of years. Hwang et al enrolled 172 patients who were soon to have elective surgery and had at least two of four clinical features of obstructive sleep apnea (snoring, daytime somnolence, witnessed apnea event, or crowded oropharynx).8 Patients underwent nocturnal oximetry before surgery and were divided into two groups based on number of desaturation episodes per hour. Patients with five or more desaturations had markedly higher rates of postoperative respiratory complications (8 complications among 98 patients) than did patients with fewer than five desaturations (1 complication among 74 patients). The presence of five or more desaturations was also associated with higher rates of cardiac, gastrointestinal, and bleeding complications. Though this was a small study, its results suggest a significant association between obstructive sleep apnea and pulmonary complications.
The issue of whether to screen patients for obstructive sleep apnea before major noncardiac surgery is still unresolved.
Pulmonary hypertension has also been identified as a risk factor in recent years with the publication of two studies that estimated its impact on morbidity and mortality after major noncardiac surgery.9,10 One of the studies, a retrospective database review, found a 28% incidence of respiratory failure among 145 surgical patients with pulmonary hypertension.9 In the other study, a prospective case-control trial, respiratory failure occurred in 21% of patients with pulmonary hypertension compared with only 3% of matched controls.10 In the case-control study, pulmonary hypertension was also associated with significantly elevated rates of heart failure and in-hospital death.
The results of these studies do not support preoperative screening for undiagnosed pulmonary hypertension, but they do underscore the need to recognize established pulmonary hypertension as an important risk factor for postoperative complications.
AN UPDATED INDEX FOR RESPIRATORY FAILURE
Several years ago, investigators from the Veterans Affairs Medical Centers developed a respiratory failure index using a design similar to those of well-established indices for cardiac risk.11 The same group also developed a separate risk index for pneumonia.12
This respiratory failure index was recently updated3 to reflect experience from private and academic hospitals, making the results more generally applicable. The researchers evaluated data from 180,000 patients undergoing major general or vascular surgery (defined according to the NSQIP) over a 3-year period. Respiratory failure was defined as requiring at least 48 hours of ventilation or unplanned reintubation.
Comparison and contrast with the ACP guideline
Question: How does the updated respiratory failure index differ most significantly from the 2006 ACP guideline?
A. New index places greater emphasis on ASA class
B. New index offers a simplified weighted point scheme
C. New index ranks low albumin as a less important risk factor
D. New index attributes low risk to cigarette use
The correct answer is C: low albumin is a minor risk factor in the respiratory failure index, whereas it was one of the single most important predictors in the ACP guideline. As for the other answer choices, the new index places about the same emphasis on ASA class and cigarette use as does the ACP guideline, and it does not offer a simplified approach, as it incorporates 28 different factors.
Overall, most risk factors were similar in the updated respiratory failure index and the ACP guideline, but the index differs in several important ways:
- The index assigns less risk to low albumin, functional dependence, and congestive heart failure
- The index assigns greater risk to orofacial surgery
- The index identifies several new risk factors—high-complexity surgery, preoperative sepsis, ascites, and hypernatremia (serum sodium > 145 mmol/L).
STRATEGIES FOR RISK REDUCTION
Postoperative CPAP: Good option when exercise ability is limited
Among the postoperative lung expansion modalities, continuous positive airway pressure (CPAP) is particularly useful for patients who are unable to perform deep breathing or incentive spirometry exercises. A recent systematic literature review identified nine randomized controlled trials of CPAP vs standard therapy in a total of 654 patients undergoing abdominal surgery.13 Meta-analysis of these studies showed that CPAP was associated with significant reductions in the risk of overall postoperative pulmonary complications (odds ratio [OR] = 0.66; 95% CI, 0.52–0.85), atelectasis (OR = 0.75; 95% CI, 0.58–0.97), and pneumonia (OR = 0.33; 95% CI, 0.14–0.75) relative to standard therapy.
Use nasogastric tubes selectively
Nasogastric tubes can be used either routinely following abdominal surgery or only in select patients—eg, those who have symptomatic abdominal distention or nausea. The difference is important since nasogastric tubes may potentially increase the risk of aspiration and thus lead to a pulmonary complication. Nelson et al conducted a meta-analysis of 24 studies that compared routine nasogastric tube use in abdominal surgery with selective use based on symptoms or abdominal distention.14 They found that routine use was associated with a significant increase in postoperative pulmonary complications (OR = 1.45; 95% CI, 1.08–1.93) relative to selective use, without achieving any of its intended goals.
Laparoscopic vs open surgery: Evidence begins to follow intuition
Intuitively, it seems that laparoscopic procedures should reduce risk for postoperative pulmonary complications compared with open surgical procedures, as they are associated with less postoperative pain, which should facilitate deep breathing and improve postoperative lung volumes. Nevertheless, evidence for whether laparoscopic surgery reduces the risk of pulmonary complications has been mixed until recently.
In 2008, however, Weller and Rosati published an analysis of a nationally representative database of 19,156 patients who underwent bariatric surgery in 2005.15 After adjusting for comorbidities, they found that the rate of postoperative pulmonary complications was nearly double if patients underwent open surgery as opposed to laparoscopic surgery (OR = 1.92; 95% CI, 1.54–2.38). Open surgery was also associated with significantly higher rates of sepsis, cardiovascular events, and reoperation compared with laparoscopic procedures. This study suggests that choosing laparoscopic procedures is another strategy that may reduce pulmonary complication rates, at least in the setting of bariatric surgery.
Postoperative thoracic epidural analgesia
Question: Thoracic epidural analgesia reduces rates of which of the following?
A. Pneumonia following abdominal aortic aneurysm repair
B. Pulmonary complications following coronary bypass surgery
C. Respiratory failure following abdominal surgery
D. All of the above
The correct answer is D. Thoracic epidural analgesia is another important strategy for reducing postoperative pulmonary complications, as demonstrated by a 2007 systematic literature review by Liu and Wu.16 Their analysis showed that rates of pneumonia, respiratory failure, and pulmonary complications overall were reduced by approximately one-third to more than one-half with the use of postoperative thoracic epidural analgesia in patients undergoing aortic aneurysm repair, coronary bypass surgery, and abdominal surgery.
Smoking cessation: The jury is still out
Whether preoperative cigarette cessation reduces pulmonary complication rates has been controversial over the past decade. Early reports showed that among patients who smoke, those who quit shortly before surgery actually had higher complication rates than patients who continued to smoke. The most reasonable explanation seems to be that many patients who stop smoking report increased coughing and sputum production for the first month or two. Selection bias also may have played a role in these findings.
More recently, two randomized trials studied the impact of perioperative smoking intervention programs involving counseling and nicotine replacement.17,18 Unfortunately, both studies primarily studied patients undergoing low-risk procedures and were insufficiently powered to show a difference in pulmonary complication rates. The question of whether smoking cessation is an effective strategy to reduce postoperative pulmonary risk remains unanswered.
Preoperative intensive lung expansion: A promising new intervention
While the effectiveness of postoperative lung expansion techniques is undisputed,7preoperative lung expansion—also known as inspiratory muscle training—has only recently been investigated. Hulzebos et al randomized 279 patients undergoing coronary artery bypass graft surgery who were at high risk for developing pulmonary complications to either usual care or inspiratory muscle training.19 The latter intervention involved 20 minutes per day of incentive spirometry, active breathing, and forced expiration techniques for at least 2 weeks prior to surgery. Rates of high-grade postoperative pulmonary complications were cut in half (OR = 0.52; 95% CI, 0.30–0.92) and rates of pneumonia were reduced by 60% (OR = 0.40; 95% CI, 0.19–0.84) in patients who received inspiratory muscle training relative to the usual-care group.
In clinical practice, preoperative inspiratory muscle training can be done in a chest physical therapy outpatient setting or a pulmonary rehabilitation clinic in the hospital.
SUMMARY
There have been a number of significant recent developments in the perioperative management of pulmonary complications:
- Obstructive sleep apnea has been confirmed as a risk factor, and pulmonary hypertension has emerged as a novel risk factor.
- An updated respiratory failure index has emerged as a useful research tool to identify high-risk patients and to ensure uniform risk stratification in future research.
- Evidence has mounted for the effectiveness of several risk-reduction strategies, including the use of laparoscopic procedures for bariatric surgery; selective use of nasogastric tubes; postoperative thoracic epidural analgesia; and intensive preoperative inspiratory muscle training.
DISCUSSION
Question from the audience: I do preoperative evaluations in an orthopedic ambulatory surgery center. Our surgeons often tell me, “Just order preoperative pulmonary function tests,” or, “Get a blood gas.” How should I respond?
Dr. Smetana: This is an area of some controversy, but in general, spirometry does not add much to a preoperative risk assessment that is based on a history and physical exam. Usually if the spirometry is abnormal, it will not be a surprise after careful clinical assessment. Arterial blood gases have no role in routine preoperative assessment.
Question from the audience: A chest x-ray is often requested preoperatively, but is it a necessary study?
Dr. Smetana: The data for preoperative chest x-rays are fairly poor and don’t allow us to assess whether they accurately predict complication rates. Most studies on chest x-rays have looked at how they affect preoperative management—eg, whether they change the anesthesia or even the surgery—and have shown that preoperative management changes in only about 1% to 2% of cases. So the chest x-ray is a fairly low-yield test in this setting.
One could argue that a preoperative chest x-ray might provide a baseline for postoperative comparison, but actually it is not usually helpful in this regard. Having a baseline does not make it easier to correctly diagnose pneumonia postoperatively, for example. Abnormal chest x-rays correlate with higher risk, but most patients with abnormal films would be suspected of being at higher risk anyway based on findings from the clinical assessment.
Question from the audience: Many primary care doctors in my hospital screen patients for pulmonary hypertension, but this raises the question of what to do with any information gained. What do you tell patients? Anesthesiologists?
Dr. Smetana: I don’t recommend preoperative screening for pulmonary hypertension unless there is some specific clinical reason to look for it. We don’t know if the perioperative risks that I described for patients with diagnosed or symptomatic pulmonary hypertension would also apply to patients with unrecognized, asymptomatic pulmonary hypertension that happened to be identified by screening.
Patients with pulmonary hypertension are at very high risk, especially for respiratory failure. But we don’t have any risk-reduction strategies specific to these patients, although I would recommend applying the general risk-reduction strategies that I discussed.
Question from the audience: I saw a man at my high-risk preoperative clinic who scored normally on a 6-minute walk test but then was found sound asleep when I was ready to see him a little while later. I suspected he had undiagnosed sleep apnea, and therefore had an increased risk of postoperative pulmonary complications, but what evidence would I have to delay his surgery to diagnose the sleep apnea and stabilize him on CPAP?
Dr. Smetana: For a patient with clinically suspected but undiagnosed sleep apnea, we have some evidence that the diagnosis should be pursued before surgery is performed.8 If the surgery were elective, it would be appropriate to have the patient evaluated and, if obstructive sleep apnea were diagnosed, treated in the customary way with CPAP. For patients who are hospitalized after surgery, CPAP can be continued as soon as possible in the hospital.
I would not have made this recommendation a few years ago, but now the evidence is more compelling. However, at this point I would not recommend routine preoperative screening of all patients for sleep apnea. Ongoing research is looking at this question.
Follow-up question: How long should surgery be delayed to optimize the patient on CPAP?
Dr. Smetana: Risk for postoperative respiratory failure is reduced very quickly after initiating CPAP therapy. A week would probably be sufficient, but there are no good data to specifically address that question.
Question from the audience: What about patients with asthma who are undergoing surgery—which ones benefit from stress-level steroids and preoperative nebulizer therapy?
Dr. Smetana: Surprisingly, asthma—if well controlled—is not a risk factor for postoperative pulmonary complications. Patients within 80% of their predicted or personal best peak flow appear to have a risk similar to that of patients without asthma. For patients with uncontrolled or poorly controlled asthma, the general rule is the same as for patients with COPD: treat them the same as if they weren’t having surgery. If a patient with asthma has a clinical indication for corticosteroids based on his or her condition, give corticosteroids whether or not surgery is planned. Corticosteroids are safe and do not raise the risk of postoperative wound complications. But we have no evidence to support routine use of steroids for all patients with asthma simply because elective surgery is planned.
Follow-up question: Do you optimize poorly controlled patients with oral prednisone for several days preoperatively, or do you use a stress protocol?
Dr. Smetana: For a patient whom you would normally treat with an outpatient course of prednisone, you should do just that. For a patient with an exacerbation severe enough to require admission for intravenous steroids and inhaled nebulizer therapy, then you should use that strategy. If the surgery is elective, it should be delayed until the patient is at his or her personal best.
Although pulmonary complications are not as well studied as cardiac complications in the postoperative setting, they are just as common following noncardiac surgery and are even more costly. It is worthwhile to identify surgical patients most at risk of postoperative pulmonary complications and take measures known to mitigate risk. This paper discusses important risk factors to identify during a preoperative pulmonary evaluation and then focuses on recent advances in strategies for reducing postoperative pulmonary complications. Teaching questions are included throughout, along with the rationale behind their answers.
POSTOPERATIVE PULMONARY COMPLICATIONS: WHAT ARE WE TRYING TO PREVENT AND WHY?
The definition of postoperative pulmonary complications is more variable and less intuitive than that of cardiac complications. Cardiac complications—postoperative myocardial infarction, cardiac death, and pulmonary edema—are more consistently defined and measured in clinical trials. Studies of postoperative pulmonary complications often group together pneumonia, respiratory failure, atelectasis, bronchospasm, and exacerbation of chronic obstructive pulmonary disease (COPD), making it more difficult to individually evaluate risk factors for different outcomes.
There are several reasons why it is important to consider pulmonary risk when evaluating patients preoperatively:
Pulmonary complications are as common as cardiac complications following noncardiac surgery. For example, in a secondary analysis of the cohort of noncardiac surgical patients used to validate the Revised Cardiac Risk Index,1 Fleischmann et al found that the incidence of pulmonary complications (2.7%) was highly comparable to that of cardiac complications (2.5%).2
Respiratory failure is a marker of ill health and predicts further complications. Postoperative respiratory failure (often defined as the need for ventilation for more than 48 hours after surgery) is an extremely morbid event. Johnson et al compared the outcomes of patients with and without respiratory failure as a complication of surgery.3 Among patients with respiratory failure, 26% died within 30 days, 6% had a myocardial infarction, 35% developed pneumonia, 10% developed acute renal failure, and 3% developed a deep vein thrombosis or pulmonary embolism; in contrast, rates of each of these events were lower than 2% among patients without respiratory failure.
Pulmonary complications are expensive and require lengthy hospitalization. The National Surgical Quality Improvement Program (NSQIP) compared hospitalization costs and length of stay among patients with various postoperative complications.4 Among infectious, cardiovascular, venous thromboembolic, and pulmonary complications, pulmonary complications were by far the most costly and, along with venous thromboembolic complications, required the longest mean hospital stay.
For these reasons, identifying patients at risk for pulmonary complications and developing a strategy to reduce the risk is clearly worthwhile.
IDENTIFYING RISK FOR PULMONARY COMPLICATIONS
Question: Which of the following is the most important risk factor for postoperative pulmonary complications?
A. High-risk surgical site
B. General anesthesia
C. COPD
D. Obesity
The correct answer is A. Pulmonary complications differ from cardiac complications in an important way: procedure-related factors are more predictive of pulmonary complications than are patient-related factors. Even healthy patients undergoing high-risk surgery are at risk for pulmonary complications. As for the other answer choices, general anesthesia and COPD are risk factors but are not as important as surgical site, and obesity has not been shown to be a risk factor at all.
Take-home points from the 2006 ACP guideline
Patient-related risk factors. As noted in Table 1, the most important patient-related risk factors identified in the ACP guideline are increasing age and increasing American Society of Anesthesiologists (ASA) classification of comorbidity.
The effect of advanced age becomes particularly notable around age 60 years and escalates from there. This effect of age differs from that for cardiac complications, for which age drops out as a risk factor after adjustment for other diseases and risk factors. For pulmonary complications, in contrast, even older patients who are healthy are at increased risk.
The ASA classification is a general index of overall morbidity that ranges from class 1 (normal healthy patient) to class 5 (moribund patient who is not expected to survive without the operation).
Notably, COPD and smoking were only minor risk factors in the ACP analysis.
Procedure-related risk factors. Surgical site was found to be the most important of any of the patient- or procedure-related risk factors. The closer the incision is to the diaphragm, the greater the risk for pulmonary complications. Aortic, thoracic, and abdominal procedures carry the highest risk (Table 1), and among abdominal procedures, upper abdominal surgery (eg, cholecystectomy) is riskier than lower abdominal surgery (eg, gynecologic).
Other procedure-related risk factors identified were emergency surgery, surgery lasting more than 3 hours, use of general anesthesia, and multiple transfusions (Table 1).
Newly identified risk factors
Question: Which of the following has recently been identified as a risk factor for postoperative pulmonary complications?
A. Epidural anesthesia
B. Insulin-treated diabetes
C. Obstructive sleep apnea
D. Immobility
The correct answer is C. There is no evidence that epidural anesthesia or insulin-treated diabetes are risk factors. Immobility seems intuitively correct but has not emerged as a risk factor among high-quality studies in the literature.
Obstructive sleep apnea. The role of obstructive sleep apnea was unclear prior to publication of new data in the last couple of years. Hwang et al enrolled 172 patients who were soon to have elective surgery and had at least two of four clinical features of obstructive sleep apnea (snoring, daytime somnolence, witnessed apnea event, or crowded oropharynx).8 Patients underwent nocturnal oximetry before surgery and were divided into two groups based on number of desaturation episodes per hour. Patients with five or more desaturations had markedly higher rates of postoperative respiratory complications (8 complications among 98 patients) than did patients with fewer than five desaturations (1 complication among 74 patients). The presence of five or more desaturations was also associated with higher rates of cardiac, gastrointestinal, and bleeding complications. Though this was a small study, its results suggest a significant association between obstructive sleep apnea and pulmonary complications.
The issue of whether to screen patients for obstructive sleep apnea before major noncardiac surgery is still unresolved.
Pulmonary hypertension has also been identified as a risk factor in recent years with the publication of two studies that estimated its impact on morbidity and mortality after major noncardiac surgery.9,10 One of the studies, a retrospective database review, found a 28% incidence of respiratory failure among 145 surgical patients with pulmonary hypertension.9 In the other study, a prospective case-control trial, respiratory failure occurred in 21% of patients with pulmonary hypertension compared with only 3% of matched controls.10 In the case-control study, pulmonary hypertension was also associated with significantly elevated rates of heart failure and in-hospital death.
The results of these studies do not support preoperative screening for undiagnosed pulmonary hypertension, but they do underscore the need to recognize established pulmonary hypertension as an important risk factor for postoperative complications.
AN UPDATED INDEX FOR RESPIRATORY FAILURE
Several years ago, investigators from the Veterans Affairs Medical Centers developed a respiratory failure index using a design similar to those of well-established indices for cardiac risk.11 The same group also developed a separate risk index for pneumonia.12
This respiratory failure index was recently updated3 to reflect experience from private and academic hospitals, making the results more generally applicable. The researchers evaluated data from 180,000 patients undergoing major general or vascular surgery (defined according to the NSQIP) over a 3-year period. Respiratory failure was defined as requiring at least 48 hours of ventilation or unplanned reintubation.
Comparison and contrast with the ACP guideline
Question: How does the updated respiratory failure index differ most significantly from the 2006 ACP guideline?
A. New index places greater emphasis on ASA class
B. New index offers a simplified weighted point scheme
C. New index ranks low albumin as a less important risk factor
D. New index attributes low risk to cigarette use
The correct answer is C: low albumin is a minor risk factor in the respiratory failure index, whereas it was one of the single most important predictors in the ACP guideline. As for the other answer choices, the new index places about the same emphasis on ASA class and cigarette use as does the ACP guideline, and it does not offer a simplified approach, as it incorporates 28 different factors.
Overall, most risk factors were similar in the updated respiratory failure index and the ACP guideline, but the index differs in several important ways:
- The index assigns less risk to low albumin, functional dependence, and congestive heart failure
- The index assigns greater risk to orofacial surgery
- The index identifies several new risk factors—high-complexity surgery, preoperative sepsis, ascites, and hypernatremia (serum sodium > 145 mmol/L).
STRATEGIES FOR RISK REDUCTION
Postoperative CPAP: Good option when exercise ability is limited
Among the postoperative lung expansion modalities, continuous positive airway pressure (CPAP) is particularly useful for patients who are unable to perform deep breathing or incentive spirometry exercises. A recent systematic literature review identified nine randomized controlled trials of CPAP vs standard therapy in a total of 654 patients undergoing abdominal surgery.13 Meta-analysis of these studies showed that CPAP was associated with significant reductions in the risk of overall postoperative pulmonary complications (odds ratio [OR] = 0.66; 95% CI, 0.52–0.85), atelectasis (OR = 0.75; 95% CI, 0.58–0.97), and pneumonia (OR = 0.33; 95% CI, 0.14–0.75) relative to standard therapy.
Use nasogastric tubes selectively
Nasogastric tubes can be used either routinely following abdominal surgery or only in select patients—eg, those who have symptomatic abdominal distention or nausea. The difference is important since nasogastric tubes may potentially increase the risk of aspiration and thus lead to a pulmonary complication. Nelson et al conducted a meta-analysis of 24 studies that compared routine nasogastric tube use in abdominal surgery with selective use based on symptoms or abdominal distention.14 They found that routine use was associated with a significant increase in postoperative pulmonary complications (OR = 1.45; 95% CI, 1.08–1.93) relative to selective use, without achieving any of its intended goals.
Laparoscopic vs open surgery: Evidence begins to follow intuition
Intuitively, it seems that laparoscopic procedures should reduce risk for postoperative pulmonary complications compared with open surgical procedures, as they are associated with less postoperative pain, which should facilitate deep breathing and improve postoperative lung volumes. Nevertheless, evidence for whether laparoscopic surgery reduces the risk of pulmonary complications has been mixed until recently.
In 2008, however, Weller and Rosati published an analysis of a nationally representative database of 19,156 patients who underwent bariatric surgery in 2005.15 After adjusting for comorbidities, they found that the rate of postoperative pulmonary complications was nearly double if patients underwent open surgery as opposed to laparoscopic surgery (OR = 1.92; 95% CI, 1.54–2.38). Open surgery was also associated with significantly higher rates of sepsis, cardiovascular events, and reoperation compared with laparoscopic procedures. This study suggests that choosing laparoscopic procedures is another strategy that may reduce pulmonary complication rates, at least in the setting of bariatric surgery.
Postoperative thoracic epidural analgesia
Question: Thoracic epidural analgesia reduces rates of which of the following?
A. Pneumonia following abdominal aortic aneurysm repair
B. Pulmonary complications following coronary bypass surgery
C. Respiratory failure following abdominal surgery
D. All of the above
The correct answer is D. Thoracic epidural analgesia is another important strategy for reducing postoperative pulmonary complications, as demonstrated by a 2007 systematic literature review by Liu and Wu.16 Their analysis showed that rates of pneumonia, respiratory failure, and pulmonary complications overall were reduced by approximately one-third to more than one-half with the use of postoperative thoracic epidural analgesia in patients undergoing aortic aneurysm repair, coronary bypass surgery, and abdominal surgery.
Smoking cessation: The jury is still out
Whether preoperative cigarette cessation reduces pulmonary complication rates has been controversial over the past decade. Early reports showed that among patients who smoke, those who quit shortly before surgery actually had higher complication rates than patients who continued to smoke. The most reasonable explanation seems to be that many patients who stop smoking report increased coughing and sputum production for the first month or two. Selection bias also may have played a role in these findings.
More recently, two randomized trials studied the impact of perioperative smoking intervention programs involving counseling and nicotine replacement.17,18 Unfortunately, both studies primarily studied patients undergoing low-risk procedures and were insufficiently powered to show a difference in pulmonary complication rates. The question of whether smoking cessation is an effective strategy to reduce postoperative pulmonary risk remains unanswered.
Preoperative intensive lung expansion: A promising new intervention
While the effectiveness of postoperative lung expansion techniques is undisputed,7preoperative lung expansion—also known as inspiratory muscle training—has only recently been investigated. Hulzebos et al randomized 279 patients undergoing coronary artery bypass graft surgery who were at high risk for developing pulmonary complications to either usual care or inspiratory muscle training.19 The latter intervention involved 20 minutes per day of incentive spirometry, active breathing, and forced expiration techniques for at least 2 weeks prior to surgery. Rates of high-grade postoperative pulmonary complications were cut in half (OR = 0.52; 95% CI, 0.30–0.92) and rates of pneumonia were reduced by 60% (OR = 0.40; 95% CI, 0.19–0.84) in patients who received inspiratory muscle training relative to the usual-care group.
In clinical practice, preoperative inspiratory muscle training can be done in a chest physical therapy outpatient setting or a pulmonary rehabilitation clinic in the hospital.
SUMMARY
There have been a number of significant recent developments in the perioperative management of pulmonary complications:
- Obstructive sleep apnea has been confirmed as a risk factor, and pulmonary hypertension has emerged as a novel risk factor.
- An updated respiratory failure index has emerged as a useful research tool to identify high-risk patients and to ensure uniform risk stratification in future research.
- Evidence has mounted for the effectiveness of several risk-reduction strategies, including the use of laparoscopic procedures for bariatric surgery; selective use of nasogastric tubes; postoperative thoracic epidural analgesia; and intensive preoperative inspiratory muscle training.
DISCUSSION
Question from the audience: I do preoperative evaluations in an orthopedic ambulatory surgery center. Our surgeons often tell me, “Just order preoperative pulmonary function tests,” or, “Get a blood gas.” How should I respond?
Dr. Smetana: This is an area of some controversy, but in general, spirometry does not add much to a preoperative risk assessment that is based on a history and physical exam. Usually if the spirometry is abnormal, it will not be a surprise after careful clinical assessment. Arterial blood gases have no role in routine preoperative assessment.
Question from the audience: A chest x-ray is often requested preoperatively, but is it a necessary study?
Dr. Smetana: The data for preoperative chest x-rays are fairly poor and don’t allow us to assess whether they accurately predict complication rates. Most studies on chest x-rays have looked at how they affect preoperative management—eg, whether they change the anesthesia or even the surgery—and have shown that preoperative management changes in only about 1% to 2% of cases. So the chest x-ray is a fairly low-yield test in this setting.
One could argue that a preoperative chest x-ray might provide a baseline for postoperative comparison, but actually it is not usually helpful in this regard. Having a baseline does not make it easier to correctly diagnose pneumonia postoperatively, for example. Abnormal chest x-rays correlate with higher risk, but most patients with abnormal films would be suspected of being at higher risk anyway based on findings from the clinical assessment.
Question from the audience: Many primary care doctors in my hospital screen patients for pulmonary hypertension, but this raises the question of what to do with any information gained. What do you tell patients? Anesthesiologists?
Dr. Smetana: I don’t recommend preoperative screening for pulmonary hypertension unless there is some specific clinical reason to look for it. We don’t know if the perioperative risks that I described for patients with diagnosed or symptomatic pulmonary hypertension would also apply to patients with unrecognized, asymptomatic pulmonary hypertension that happened to be identified by screening.
Patients with pulmonary hypertension are at very high risk, especially for respiratory failure. But we don’t have any risk-reduction strategies specific to these patients, although I would recommend applying the general risk-reduction strategies that I discussed.
Question from the audience: I saw a man at my high-risk preoperative clinic who scored normally on a 6-minute walk test but then was found sound asleep when I was ready to see him a little while later. I suspected he had undiagnosed sleep apnea, and therefore had an increased risk of postoperative pulmonary complications, but what evidence would I have to delay his surgery to diagnose the sleep apnea and stabilize him on CPAP?
Dr. Smetana: For a patient with clinically suspected but undiagnosed sleep apnea, we have some evidence that the diagnosis should be pursued before surgery is performed.8 If the surgery were elective, it would be appropriate to have the patient evaluated and, if obstructive sleep apnea were diagnosed, treated in the customary way with CPAP. For patients who are hospitalized after surgery, CPAP can be continued as soon as possible in the hospital.
I would not have made this recommendation a few years ago, but now the evidence is more compelling. However, at this point I would not recommend routine preoperative screening of all patients for sleep apnea. Ongoing research is looking at this question.
Follow-up question: How long should surgery be delayed to optimize the patient on CPAP?
Dr. Smetana: Risk for postoperative respiratory failure is reduced very quickly after initiating CPAP therapy. A week would probably be sufficient, but there are no good data to specifically address that question.
Question from the audience: What about patients with asthma who are undergoing surgery—which ones benefit from stress-level steroids and preoperative nebulizer therapy?
Dr. Smetana: Surprisingly, asthma—if well controlled—is not a risk factor for postoperative pulmonary complications. Patients within 80% of their predicted or personal best peak flow appear to have a risk similar to that of patients without asthma. For patients with uncontrolled or poorly controlled asthma, the general rule is the same as for patients with COPD: treat them the same as if they weren’t having surgery. If a patient with asthma has a clinical indication for corticosteroids based on his or her condition, give corticosteroids whether or not surgery is planned. Corticosteroids are safe and do not raise the risk of postoperative wound complications. But we have no evidence to support routine use of steroids for all patients with asthma simply because elective surgery is planned.
Follow-up question: Do you optimize poorly controlled patients with oral prednisone for several days preoperatively, or do you use a stress protocol?
Dr. Smetana: For a patient whom you would normally treat with an outpatient course of prednisone, you should do just that. For a patient with an exacerbation severe enough to require admission for intravenous steroids and inhaled nebulizer therapy, then you should use that strategy. If the surgery is elective, it should be delayed until the patient is at his or her personal best.
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Fleischmann KE, Goldman L, Young B, et al. Association between cardiac and noncardiac complications in patients undergoing noncardiac surgery: outcomes and effects on length of stay. Am J Med 2003; 115:515–520.
- Johnson RG, Arozullah AM, Neumayer L, et al. Multivariable predictors of postoperative respiratory failure after general and vascular surgery: results from the Patient Safety in Surgery Study. J Am Coll Surg 2007; 204:1188–1198.
- Dimick JB, Chen SL, Taheri PA, et al. Hospital costs associated with surgical complications: a report from the private-sector National Surgical Quality Improvement Program. J Am Coll Surg 2004; 199:531–537.
- Qaseem A, Snow V, Fitterman N, et al. Risk assessment for and strategies to reduce perioperative pulmonary complications for patients undergoing noncardiothoracic surgery: a guideline from the American College of Physicians. Ann Intern Med 2006; 144:575–580.
- Smetana GW, Lawrence VA, Cornell JE. Preoperative pulmonary risk stratification for noncardiothoracic surgery: systematic review for the American College of Physicians. Ann Intern Med 2006; 144:581–595.
- Lawrence VA, Cornell JE, Smetana GW; American College of Physicians. Strategies to reduce postoperative pulmonary complications after noncardiothoracic surgery: systematic review for the American College of Physicians. Ann Intern Med 2006; 144:596–608.
- Hwang D, Shakir N, Limann B, et al. Association of sleep-disordered breathing with postoperative complications. Chest 2008; 133:1128–1134.
- Ramakrishna G, Sprung J, Ravi BS, et al. Impact of pulmonary hypertension on the outcomes of noncardiac surgery: predictors of perioperative morbidity and mortality. J Am Coll Cardiol 2005; 45:1691–1699.
- Lai HC, Lai HC, Wang KY, Lee WL, Ting CT, Liu TJ. Severe pulmonary hypertension complicates postoperative outcome of noncardiac surgery. Br J Anaesth 2007; 99:184–190.
- Arozullah AM, Daley J, Henderson WG, Khuri SF. Multifactorial risk index for predicting postoperative respiratory failure in men after major noncardiac surgery. The National Veterans Administration Surgical Quality Improvement Program. Ann Surg 2000; 232:242–253.
- Arozullah AM, Khuri SF, Henderson WG, et al. Development and validation of a multifactorial risk index for predicting postoperative pneumonia after major noncardiac surgery. Ann Intern Med 2001; 135:847–857.
- Ferreyra GP, Baussano I, Squadrone V, et al. Continuous positive airway pressure for treatment of respiratory complications after abdominal surgery: a systematic review and meta-analysis. Ann Surg 2008; 247:617–626.
- Nelson R, Edwards S, Tse B. Prophylactic nasogastric decompression after abdominal surgery. Cochrane Database Syst Rev 2007; Jul 18 (3):CD004929.
- Weller WE, Rosati C. Comparing outcomes of laparoscopic versus open bariatric surgery. Ann Surg 2008; 248:10–15.
- Liu SS, Wu CL. Effect of postoperative analgesia on major postoperative complications: a systematic update of the evidence. Anesth Analg 2007; 104:689–702.
- Møller AM, Villebro N, Pedersen T, Tønnesen H. Effect of preoperative smoking intervention on postoperative complications: a randomised clinical trial. Lancet 2002; 359:114–117.
- Lindström D, Sadr Azodi O, Wladis A, et al. Effects of a perioperative smoking cessation intervention on postoperative complications: a randomized trial. Ann Surg 2008; 248:739–745.
- Hulzebos EH, Helders PJ, Favié NJ, et al. Preoperative intensive inspiratory muscle training to prevent postoperative pulmonary complications in high-risk patients undergoing CABG surgery: a randomized clinical trial. JAMA 2006; 296:1851–1857.
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Fleischmann KE, Goldman L, Young B, et al. Association between cardiac and noncardiac complications in patients undergoing noncardiac surgery: outcomes and effects on length of stay. Am J Med 2003; 115:515–520.
- Johnson RG, Arozullah AM, Neumayer L, et al. Multivariable predictors of postoperative respiratory failure after general and vascular surgery: results from the Patient Safety in Surgery Study. J Am Coll Surg 2007; 204:1188–1198.
- Dimick JB, Chen SL, Taheri PA, et al. Hospital costs associated with surgical complications: a report from the private-sector National Surgical Quality Improvement Program. J Am Coll Surg 2004; 199:531–537.
- Qaseem A, Snow V, Fitterman N, et al. Risk assessment for and strategies to reduce perioperative pulmonary complications for patients undergoing noncardiothoracic surgery: a guideline from the American College of Physicians. Ann Intern Med 2006; 144:575–580.
- Smetana GW, Lawrence VA, Cornell JE. Preoperative pulmonary risk stratification for noncardiothoracic surgery: systematic review for the American College of Physicians. Ann Intern Med 2006; 144:581–595.
- Lawrence VA, Cornell JE, Smetana GW; American College of Physicians. Strategies to reduce postoperative pulmonary complications after noncardiothoracic surgery: systematic review for the American College of Physicians. Ann Intern Med 2006; 144:596–608.
- Hwang D, Shakir N, Limann B, et al. Association of sleep-disordered breathing with postoperative complications. Chest 2008; 133:1128–1134.
- Ramakrishna G, Sprung J, Ravi BS, et al. Impact of pulmonary hypertension on the outcomes of noncardiac surgery: predictors of perioperative morbidity and mortality. J Am Coll Cardiol 2005; 45:1691–1699.
- Lai HC, Lai HC, Wang KY, Lee WL, Ting CT, Liu TJ. Severe pulmonary hypertension complicates postoperative outcome of noncardiac surgery. Br J Anaesth 2007; 99:184–190.
- Arozullah AM, Daley J, Henderson WG, Khuri SF. Multifactorial risk index for predicting postoperative respiratory failure in men after major noncardiac surgery. The National Veterans Administration Surgical Quality Improvement Program. Ann Surg 2000; 232:242–253.
- Arozullah AM, Khuri SF, Henderson WG, et al. Development and validation of a multifactorial risk index for predicting postoperative pneumonia after major noncardiac surgery. Ann Intern Med 2001; 135:847–857.
- Ferreyra GP, Baussano I, Squadrone V, et al. Continuous positive airway pressure for treatment of respiratory complications after abdominal surgery: a systematic review and meta-analysis. Ann Surg 2008; 247:617–626.
- Nelson R, Edwards S, Tse B. Prophylactic nasogastric decompression after abdominal surgery. Cochrane Database Syst Rev 2007; Jul 18 (3):CD004929.
- Weller WE, Rosati C. Comparing outcomes of laparoscopic versus open bariatric surgery. Ann Surg 2008; 248:10–15.
- Liu SS, Wu CL. Effect of postoperative analgesia on major postoperative complications: a systematic update of the evidence. Anesth Analg 2007; 104:689–702.
- Møller AM, Villebro N, Pedersen T, Tønnesen H. Effect of preoperative smoking intervention on postoperative complications: a randomised clinical trial. Lancet 2002; 359:114–117.
- Lindström D, Sadr Azodi O, Wladis A, et al. Effects of a perioperative smoking cessation intervention on postoperative complications: a randomized trial. Ann Surg 2008; 248:739–745.
- Hulzebos EH, Helders PJ, Favié NJ, et al. Preoperative intensive inspiratory muscle training to prevent postoperative pulmonary complications in high-risk patients undergoing CABG surgery: a randomized clinical trial. JAMA 2006; 296:1851–1857.
KEY POINTS
- Pulmonary complications are as common as cardiac complications following noncardiac surgery.
- Surgical site is the most important predictor of risk for postoperative pulmonary complications: aortic, thoracic, and upper abdominal surgeries are high-risk procedures, even in healthy patients.
- Obstructive sleep apnea and pulmonary hypertension have recently been identified as risk factors, but the limited available evidence does not support preoperative screening for these conditions in patients without symptoms.
- Postoperative continuous positive airway pressure therapy is effective for reducing pulmonary complications in patients who are unable to perform deep breathing or incentive spirometry exercises.
- The jury is out on whether smoking cessation shortly before surgery lowers risk for postoperative pulmonary complications.