User login
For patients with localized lung cancer, lung resection provides the highest likelihood of a cure. However, only about 20% to 30% of patients are potential candidates for surgical resection because of the stage at which the disease is diagnosed or because of comorbid conditions.1,2 In one study, poor lung function alone ruled out more than 37% of patients who presented with anatomically resectable disease.3 The poor prognosis for patients who do not undergo surgery, the likelihood of early mortality from lung resection, and the potential for loss of lung function following resection are all important considerations in the preoperative pulmonary evaluation of candidates for anatomical lung resection.
PROGNOSIS OF LUNG CANCER POOR WITHOUT SURGICAL RESECTION
Several studies support the poor prognosis of lung cancer patients who do not undergo resection. In one study of 1,297 screen- and symptom-detected patients, the median duration of survival without surgery was 25 months for patients with screen-detected stage I lung cancer (n = 42) and 13 months for those with symptom-detected stage I disease (n = 27).4 Another study of 799 patients with stage I lung cancer who were not treated surgically reported 5- and 10-year survival rates of 16.6% (n = 49) and 7.4% (n = 49), respectively.5 In a study of 251 patients with squamous cell carcinoma on sputum cytology, yet negative chest imaging, the 5-year and 10-year survival rates were 53.2% and 33.5%.6 Another study of 57 patients with potentially resectable disease who did not undergo surgery reported a median survival of 15.6 months, compared with 30.9 months for a group of 346 patients who underwent resection.7
PREDICTORS OF SURGICAL MORTALITY
Several large patient series describe perioperative mortality and the rate of complications for patients undergoing surgical resection for lung cancer. Reported surgical mortality rates in these studies vary from approximately 1% to 5%.2,8–10 The median age of patients in most of these studies was 65 to 70 years, and many patients had significant medical comorbidity. Predictors of increased surgical mortality include pneumonectomy, bilobectomy, American Society of Anesthesiologists (ASA) Physical Status Scale rating, Zubrod performance status score, renal dysfunction, induction chemoradiation therapy, steroid use, older age, urgent procedures, male gender, forced expiratory volume in 1 second (FEV1), and body mass index.11 In France, a thoracic surgery scoring system for in-hospital mortality (Thoracoscore) was developed using data obtained from more than 15,000 patients who were enrolled in a nationally representative thoracic surgery database. Mortality risk factors included in the model were patient age, sex, dyspnea score, ASA score, performance status, priority of surgery, diagnosis, procedure class, and comorbid disease.12 The model was highly accurate for the prediction of mortality, with a C statistic of 0.86. (1.00 corresponds to perfect outcome prediction.) The model was subsequently validated on 1,675 patients from the United States, where a similar accuracy was noted.13 The online version of the Thoracoscore risk assessment tool is available at: http://www.sfar.org/scores2/thoracoscore2.php.
REDUCED PULMONARY FUNCTION AFTER RESECTION
Several outcome measures have been used to assess the impact of resection on pulmonary function and quality of life after surgery. Across various studies, postoperative FEV1 values, diffusing capacity of the lung for carbon monoxide (Dlco) values, and peak oxygen consumption (VO2 peak) were assessed at various time intervals after lobectomy or pneumonectomy. FEV1 varied from 84% to 91% of preoperative values for lobectomy,14–16 and 64% to 66% for pneumonectomy.14–16 The Dlco was 89% to 96% of preoperative values after lobectomy and 72% to 80% after pneumonectomy.14,16 VO2 peak varied from 87% to 100% of preoperative values after lobectomy,14–16 and 71% to 89% after pneumonectomy.14–16
Patients with chronic obstructive pulmonary disease (COPD) typically experience smaller declines in FEV1 after lobectomy (0% to 8%) than those without COPD (16% to 20%). Declines in Dlco and VO2 peak are more variable, with reported decreases of 3% to 20% in those with COPD, and 0% to 21% for those without the disease.17–19
Lobectomy patients continue to recover pulmonary function for approximately 6 months after surgery. In patients who undergo pneumonectomy, improvement is generally limited after 3 months.14–16 Loss of lung function may vary significantly with the location of the resection. For example, resection of an emphysematous portion of the lung will probably result in less loss of function.
Few studies specifically examine quality of life after lung resection in patients with lung cancer. In general, patients who undergo resection have a lower quality of life before surgery than the general population.20 Postsurgical decline in physical measures of health-related quality of life has been reported during the month after surgery, with a return to baseline after 3 months. Mental quality of life scores did not decrease after surgery, and there was little correlation between quality of life outcomes and measures of pulmonary function.20
LUNG FUNCTION TESTING
Lung function testing helps predict the risk of postoperative complications, including mortality. The two most commonly used measures of pulmonary function are FEV1 and Dlco.
Both absolute FEV1 value and percent of predicted FEV1 strongly predict the risk of postoperative complications. It has been difficult to identify one cutoff value below which resection should not be considered. Studies have suggested preoperative absolute FEV1 values of 2 L for pneumonectomy and 1.5 L for lobectomy as cutoffs signifying increased short- and long-term surgical risk.21,22 Percent predicted FEV1, which incorporates patient age, sex, and height, is more commonly used to individualize treatment, since absolute values do not take into consideration other patient-related variables. An FEV1 of 80% predicted or higher has been proposed as a cutoff to proceed with resection without additional testing,23 but this decision must be individualized to each patient.
Similarly, it has been difficult to identify one cutoff value for the Dlco. As one might expect, the lower the value the higher the risk for a given patient. Patients with Dlco values less than 60% predicted normal24 had an increased mortality risk, longer hospital stay, and greater hospital costs in one report.
FEV1 and Dlco are only modestly correlated with one another.25 In one study, 43% of patients with FEV1 greater than 80% of predicted had Dlco less than 80% of predicted.26
According to guidelines developed by the American College of Chest Physicians (ACCP), spirometry is recommended for patients being considered for lung cancer resection.27 Patients with FEV1 that is greater than 80% predicted or greater than 2 L and without evidence of dyspnea or interstitial lung disease are considered suitable candidates for resection, including pneumonectomy, without further testing. Lobectomy without further evaluation may be performed if the FEV1 is greater than 1.5 L and there is no evidence of dyspnea or interstitial lung disease.
Although assessing FEV1 values alone may be adequate in patients being considered for lung cancer resection who have no evidence of either undue dyspnea on exertion or interstitial lung disease, the ACCP recommends also measuring Dlco when these signs are present. Guidelines from the European Respiratory Society (ERS) and the European Society of Thoracic Surgeons (ESTS) recommend routinely measuring Dlco during preoperative evaluation regardless of whether the spirometric evaluation is abnormal.28 Similarly, the British Thoracic Society (BTS) recommends measuring transfer factor of the lung for carbon monoxide (Tlco) in all patients regardless of spirometric values.29
PREDICTING POSTOPERATIVE LUNG FUNCTION
Several methods have been used to predict postoperative lung function.
Segment method
The segment method estimates postoperative lung function by multiplying baseline function by the percentage of lung sections that will remain after resection.30 For example, if preoperative FEV1 is 1 L and surgery will result in the loss of 25% of lung segments, the predicted postoperative FEV1 is 750 mL. In a study using 19 lung segments in the calculation, the predicted postoperative lung function correlated well with actual postoperative lung function for patients undergoing lobectomy, but only modestly for patients undergoing pneumonectomy.30 Another method using 42 subsegments for the calculation, and correcting for segments that were obstructed by tumor, produced very similar results.31
Radionuclide scanning
In other studies, quantitative radionuclide scanning to identify the proportion of lung with poor perfusion produced fair to good correlations between predicted and actual postoperative FEV1.32–35 Techniques that are used less often include quantitative computed tomography (CT) and measurement of airway vibration during respiration.
Studies comparing different methods for predicting postoperative pulmonary function have found that perfusion imaging outperformed other approaches, and that the segment method is not a good predictor of outcome for patients undergoing pneumonectomy.17,36
Additional testing needed
For potential lung resection patients, the ACCP guidelines recommend that if either the FEV1 or Dlco is less than 80% of the predicted value, postoperative lung function should be predicted through additional testing.27 The ERS recommends that predicted postoperative FEV1 should not be used alone to select lung cancer patients for lung resection, especially those with moderate to severe COPD.28 These guidelines also recommend that the first estimate of residual lung function should be calculated based on segment counting, that only segments not totally obstructed should be counted, and that the patency of bronchus and segment structure should be preserved. In addition, patients with borderline function should undergo imaging-based calculation of residual lung function, including ventilation or perfusion scintigraphy before pneumonectomy, or quantitative CT scan before either lobectomy or pneumonectomy.28 The BTS recommends the use of segment counting to estimate postoperative lung function as part of risk assessment for postoperative dyspnea. Ventilation or perfusion scintigraphy should be considered to predict postoperative lung function if a ventilation or perfusion mismatch is suspected. Quantitative CT or MRI may be considered to predict postoperative lung function if the facility is available.29
Predicting mortality and complications: FEV1 and Dlco
The predicted postoperative FEV1 value is an independent predictor of postoperative mortality and other complications. Although there is no absolute cut-off value, studies identify an increased risk of complications below predicted postoperative FEV1 values ranging from 30%37 to 40%.38,39 Predicted postoperative Dlco is another outcome measure that can independently identify increased mortality risk in lung cancer resection patients. Dlco less than 40% has been associated with increased risk of postoperative respiratory complications even in those with predicted postoperative FEV1 values above 40%.26,39 One study stated that a combination of the two values, predicted postoperative FEV1 and predicted postoperative Dlco—called the predicted postoperative product (PPP)—is the best predictor of surgical mortality.40 Another study examined the utility of a prediction rule for pulmonary complications after lung surgery using a point system in which points were assigned based on predicted postoperative Dlco (1 point for each 5% decrement below 100%) plus 2 points for preoperative chemotherapy.41 The risk of complications was 9% for those with scores less than 11, 14% for those with scores of 11 to 13, and 26% for those with scores greater than 13.
When surgery is considered, ACCP guidelines state an increased risk of perioperative mortality in those lung cancer patients with either a PPP less than 1,650, or a predicted postoperative FEV1 less than 30%.27 These patients should be counseled about nonstandard surgery and nonsurgical treatment options. The ERS guidelines consider a predicted postoperative FEV1 value less than 30% to be a high-risk threshold when assessing pulmonary reserve before surgery.28
EXERCISE TESTING
In general, standardized cardiopulmonary exercise testing using VO2 peak has been shown to predict postoperative complications, including perioperative and long-term morbidity and mortality.42,43 Lower values are associated with a greater risk of poor outcome. Peak VO2 may not add significantly to the risk stratification of patients who have both FEV1 and Dlco values greater than 80%.44
According to ACCP recommendations for exercise testing in patients who are being evaluated for surgery, either an FEV1 or Dlco less than 40% of predicted postoperative (PPO) indicates increased risk for perioperative death and cardiopulmonary complications with standard lung resection. Preoperative exercise testing is recommended for these patients.27 Maximal oxygen consumption (VO2 max) less than 10 mL/kg/min, or the combination of VO2 max less than 15 mL/kg/min with both FEV1 and Dlco less than 40% PPO, also indicates increased risk for death and complications; these patients should be counseled about nonstandard surgery or nonsurgical treatment options. Guidelines from the ERS recommend exercise testing for all patients undergoing lung cancer surgery who have FEV1 or Dlco less than 80% of normal values.28 The VO2 peak measured during incremental exercise on a treadmill or cycle should be regarded as the most important parameter.
Several studies have found that distance traveled during walking tests predicts postoperative complications and can be related to VO2 max (Figure).45 According to ACCP guidelines, lung cancer patients who are potential candidates for standard lung resection are at increased risk for perioperative death and cardiopulmonary complications if they walk less than 25 shuttles on 2 shuttle walk tests or less than 1 flight of stairs. These patients should be counseled about nonstandard surgery and nonsurgical treatment options.27
Conversely, ERS/ESTS guidelines state that the shuttle walk test distance underestimates exercise capacity at the lower range, and does not discriminate between patients with and without complications.28 These guidelines state that shuttle walk test distance should not be used alone to select patients for resection. It may be used as a screening test, since patients walking less than 400 m are likely to also have VO2 peak less than 15 mL/kg/min. A standardized symptom-limited stair climbing test can be a cost-effective screening method to identify those who need more sophisticated exercise tests in order to optimize their perioperative management. The 6-minute walk test is not recommended.
British Thoracic Society guidelines recommend the use of the shuttle walk test as a functional assessment in patients with moderate to high risk of postoperative dyspnea.29 A distance walk of more than 400 m is recommended as a cutoff for acceptable pulmonary function. These guidelines recommend against using pulmonary function and exercise tests as the sole surrogates for a quality of life evaluation.
ALGORITHMS FOR TESTING
The ACCP, ERS/ESTS, and BTS guidelines all include algorithms for the preoperative evaluation of candidates for lung cancer resection.27–29 The guidelines differ from each other in many ways, including when to obtain a Dlco and cardiopulmonary exercise test, and in some of the cutoff values for various pulmonary function measures. ACCP guidelines begin with spirometry testing, supporting lobectomy in patients with spirometry results above the cutoff value of FEV1 greater than 1.5 L and pneumonectomy in those with a cutoff value of FEV1 greater than 2 L, and greater than 80% of predicted, unless the patient has dyspnea or evidence of interstitial lung disease. Measurement of the Dlco is recommended for those who do not meet the FEV1 cutoffs, or in those with unexplained dyspnea or diffuse parenchymal disease on chest radiograph or CT.27
A systematic review and set of treatment recommendations for high-risk patients with stage I lung cancer, developed by the Thoracic Oncology Network of the ACCP and the Workforce on Evidence-Based Surgery of the Society of Thoracic Surgeons, currently under review, will provide additional guidance regarding the use of lung function testing to evaluate risk of morbidity and mortality. These guidelines note that FEV1, Dlco, and peak VO2 all predict morbidity and mortality following major lung resection. Assessment of FEV1 and Dlco, including calculation of the estimated postoperative value, is strongly recommended before resection. The predictive value of peak VO2 is strongest in patients with impaired FEV1 or Dlco, and assessment of peak VO2 before major lung resection is recommended for these patients.
INTERVENTIONS TO DECREASE PERIOPERATIVE RISK
The impact of smoking cessation on perioperative outcome has been a matter of considerable debate. One large study found that the incidence of postoperative complications was actually greater when patients stopped smoking within 8 weeks before cardiac surgery.46 However, a recent meta-analysis including lung resection patients found no relationship between smoking cessation in the weeks before surgery and worse clinical outcomes.47 When a shorter duration of smoking cessation is examined, thoracotomy studies note that patients who continue to smoke within 1 month of pneumonectomy are at increased risk of major pulmonary events.48,49 An examination of perioperative mortality or major complications using data from the Society of Thoracic Surgeons found that smoking cessation within 1 month preceding surgery did not significantly affect perioperative morbidity or mortality, whereas longer abstention from tobacco use was associated with better surgical outcomes.50 The ACCP recommends that all patients with lung cancer be counseled regarding smoking cessation.27 ERS/ESTS guidelines recommend smoking cessation for at least 2 to 4 weeks before surgery, since this may change perioperative smoking behavior and decrease the risk of postoperative complications.28 Pulmonary rehabiliatation in the perioperative period has been shown to improve measures of activity tolerance, allowing resection of marginal candidates, and improving functional outcomes after resection.51 The ERS/ESTS guidelines state that early pre- and postoperative rehabilitation may produce functional benefits in resectable lung cancer patients.28
SUMMARY AND CONCLUSIONS
Lung function testing helps predict the risk of postoperative mortality, perioperative complications, and long-term dyspnea for patients with lung cancer undergoing surgical resection. Predicted postoperative FEV1 and Dlco should be evaluated in most resection candidates. Exercise testing adds to standard lung function testing in those with borderline values, discordance between standard measures, or discordance between subjective and objective lung function. Algorithms for preoperative assessment have been developed by the ACCP, the ERS/ESTS, and the BTS, which differ somewhat in the order of testing and specific testing cutoff values. Smoking cessation and pulmonary rehabilitation can help to reduce perioperative and long-term risks.
- Damhuis RA, Schütte PR. Resection rates and postoperative mortality in 7,899 patients with lung cancer. Eur Respir J 1996; 9:7–10.
- Little AG, Rusch VW, Bonner JA, et al. Patterns of surgical care of lung cancer patients. Ann Thorac Surg 2005; 80:2051–2056.
- Baser S, Shannon VR, Eapen GA, et al. Pulmonary dysfunction as a major cause of inoperability among patients with non-small-cell lung cancer. Clin Lung Cancer 2006; 7:344–349.
- Sobue T, Suzuki T, Matsuda M, Kuroishi T, Ikeda S, Naruke T. Survival for clinical stage I lung cancer not surgically treated: comparison between screen-detected and symptom-detected cases. The Japanese Lung Cancer Screening Research Group. Cancer 1992; 69:685–692.
- Motohiro A, Ueda H, Komatsu H, Yanai N, Mori T; National Chest Hospital Study Group for Lung Cancer. Prognosis of nonsurgically treated, clinical stage I lung cancer patients in Japan. Lung Cancer 2002; 36:65–69.
- Sato M, Saito Y, Endo C, et al. The natural history of radiographically occult bronchogenic squamous cell carcinoma: a retrospective study of overdiagnosis bias. Chest 2004; 126:108–113.
- Loewen GM, Watson D, Kohman L, et al. Preoperative exercise Vo2 measurement for lung resection candidates: results of Cancer and Leukemia Group B Protocol 9238. J Thorac Oncol 2007; 2:619–625.
- Allen MS, Darling GE, Pechet TT, et al. Morbidity and mortality of major pulmonary resections in patients with early-stage lung cancer: initial results of the randomized, prospective ACOSOG Z0030 trial. Ann Thorac Surg 2006; 81:1013–1020.
- Meguid RA, Brooke BS, Chang DC, Sherwood JT, Brock MV, Yang SC. Are surgical outcomes for lung cancer resections improved at teaching hospitals? Ann Thorac Surg 2008; 85:1015–1025.
- Memtsoudis SG, Besculides MC, Zellos L, Patil N, Rogers SO. Trends in lung surgery: United States 1988 to 2002. Chest 2006; 130:1462–1470.
- Kozower BD, Sheng S, O’Brien SM, et al. STS database risk models: predictors of mortality and major morbidity for lung cancer resection. Ann Thorac Surg 2010; 90:875–883.
- Falcoz PE, Conti M, Brouchet L, et al. The Thoracic Surgery Scoring System (Thoracoscore): risk model for in-hospital death in 15,183 patients requiring thoracic surgery [published online ahead of print January 9, 2007]. J Thorac Cardiovasc Surg 2007; 133:325–332. doi: 10.1016/j.jtcvs.2006.09.020
- Chamogeorgakis TP, Connery CP, Bhora F, Nabong A, Toumpoulis IK. Thoracoscore predicts midterm mortality in patients undergoing thoracic surgery. J Thorac Cardiovasc Surg 2007; 134:883–887.
- Bolliger CT, Jordan P, Solèr M, et al. Pulmonary function and exercise capacity after lung resection. Eur Respir J 1996; 9:415–421.
- Nezu K, Kushibe K, Tojo T, Takahama M, Kitamura S. Recovery and limitation of exercise capacity after lung resection for lung cancer. Chest 1998; 113:1511–1516.
- Brunelli A, Xiumé F, Refai M, et al. Evaluation of expiratory volume, diffusion capacity, and exercise tolerance following major lung resection: a prospective follow-up analysis. Chest 2007; 131:141–147.
- Smulders SA, Smeenk FW, Janssen-Heijnen ML, Postmus PE. Actual and predicted postoperative changes in lung function after pneumonectomy: a retrospective analysis. Chest 2004; 125:1735–1741.
- Edwards JG, Duthie DJ, Waller DA. Lobar volume reduction surgery: a method of increasing the lung cancer resection rate in patients with emphysema. Thorax 2001; 56:791–795.
- Bobbio A, Chetta A, Carbognani P, et al. Changes in pulmonary function test and cardiopulmonary exercise capacity in COPD patients after lobar pulmonary resection [published online ahead of print September 6, 2005]. Eur J Cardiothorac Surg 2005; 28:754–758. doi: 10.1016/j.ejcts.2005.08.001
- Brunelli A, Refai M, Salati M, Xiumé F, Sabbatini A. Predicted versus observed FEV1 and Dlco after major lung resection: a prospective evaluation at different postoperative periods. Ann Thorac Surg 2007; 83:1134–1139.
- Boushy SF, Billig DM, North LB, Helgason AH. Clinical course related to preoperative and postoperative pulmonary function in patients with bronchogenic carcinoma. Chest 1971; 59:383–391.
- Wernly JA, DeMeester TR, Kirchner PT, Myerowitz PD, Oxford DE, Golomb HM. Clinical value of quantitative ventilation-perfusion lung scans in the surgical management of bronchogenic carcinoma. J Thorac Cardiovasc Surg 1980; 80:535–543.
- Wyser C, Stulz P, Solèr M, et al. Prospective evaluation of an algorithm for the functional assessment of lung resection candidates. Am J Respir Crit Care Med 1999; 159:1450–1456.
- Bousamra M, Presberg KW, Chammas JH, et al. Early and late morbidity in patients undergoing pulmonary resection with low diffusion capacity. Ann Thorac Surg 1996; 62:968–975.
- Ferguson MK, Little L, Rizzo L, et al. Diffusing capacity predicts morbidity and mortality after pulmonary resection. J Thorac Cardiovasc Surg 1988; 96:894–900.
- Brunelli A, Refai MA, Salati M, Sabbatini A, Morgan-Hughes NJ, Rocco G. Carbon monoxide lung diffusion capacity improves risk stratification in patients without airflow limitation: evidence for systematic measurement before lung resection [published online ahead of print February 14, 2006]. Eur J Cardiothorac Surg 2006; 29:567–570. doi: 10.1016/j.ejcts.2006.01.014
- Colice GL, Shafazand S, Griffin JP, Keenan R, Bolliger CT; American College of Chest Physicians. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: ACCP evidenced-based clinical practice guidelines (2nd edition). Chest 2007; 132( suppl 3):161S–177S.
- Brunelli A, Charloux A, Bolliger CT, et al. ERS/ESTS clinical guidelines on fitness for radical therapy in lung cancer patients (surgery and chemo-radiotherapy). Eur Respir J 2009; 34:17–41.
- Lim E, Baldwin D, Beckles M, et al. Guidelines on the radical management of patients with lung cancer. Thorax 2010; 65( suppl 3):iii1–iii27.
- Zeiher BG, Gross TJ, Kern JA, Lanza LA, Peterson MW. Predicting postoperative pulmonary function in patients undergoing lung resection. Chest 1995; 108:68–72.
- Nakahara K, Monden Y, Ohno K, Miyoshi S, Maeda H, Kawashima Y. A method for predicting postoperative lung function and its relation to postoperative complications in patients with lung cancer. Ann Thorac Surg 1985; 39:260–265.
- Kristersson S, Lindell SE, Svanberg L. Prediction of pulmonary function loss due to pneumonectomy using 133 Xe-radiospirometry. Chest 1972; 62:694–698.
- Bria WF, Kanarek DJ, Kazemi H. Prediction of postoperative pulmonary function following thoracic operations: value of ventilation-perfusion scanning. J Thorac Cardiovasc Surg 1983; 86:186–192.
- Ali MK, Mountain CF, Ewer MS, Johnston D, Haynie TP. Predicting loss of pulmonary function after pulmonary resection for bronchogenic carcinoma. Chest 1980; 77:337–342.
- Corris PA, Ellis DA, Hawkins T, Gibson GJ. Use of radionuclide scanning in the preoperative estimation of pulmonary function after pneumonectomy. Thorax 1987; 42:285–291.
- Bolliger CT, Gückel C, Engel H, et al. Prediction of functional reserves after lung resection: comparison between quantitative computed tomography, scintigraphy, and anatomy. Respiration 2002; 69:482–489.
- Nakahara K, Ohno K, Hashimoto J, et al. Prediction of postoperative respiratory failure in patients undergoing lung resection for lung cancer. Ann Thorac Surg 1988; 46:549–552.
- Markos J, Mullan BP, Hillman DR, et al. Preoperative assessment as a predictor of mortality and morbidity after lung resection. Am Rev Respir Dis 1989; 139:902–910.
- Ribas J, Diaz O, Barberà JA, et al. Invasive exercise testing in the evaluation of patients at high-risk for lung resection. Eur Respir J 1998; 12:1429–1435.
- Pierce RJ, Copland JM, Sharpe K, Barter CE. Preoperative risk evaluation for lung cancer resection: predicted postoperative product as a predictor of surgical mortality. Am J Respir Crit Care Med 1994; 150:947–955.
- Amar D, Munoz D, Shi W, Zhang H, Thaler HT. A clinical prediction rule for pulmonary complications after thoracic surgery for primary lung cancer [published online ahead of print October 27, 2009]. Anesth Analg 2010; 110:1343–1348. doi: 10.1213/ANE.0b013e3181bf5c99
- Benzo R, Kelley GA, Recchi L, Hofman A, Sciurba F. Complications of lung resection and exercise capacity: a meta-analysis [published online ahead of print April 3, 2007]. Respir Med 2007; 101:1790–1797. doi: 10.1016/j.rmed.2007.02.012
- Jones LW, Eves ND, Kraus WE, et al. The lung cancer exercise training study: a randomized trial of aerobic training, resistance training, or both in postsurgical lung cancer patients: rationale and design. BMC Cancer 2010; 10:155.
- Larsen KR, Svendsen UG, Milman N, Brenøe J, Petersen BN. Exercise testing in the preoperative evaluation of patients with bronchogenic carcinoma. Eur Respir J 1997; 10:1559–1565.
- Singh SJ, Morgan MDL, Hardman AE, Rowe C, Bardsley PA. Comparison of oxygen uptake during a conventional treadmill test and the shuttle walking test in chronic airflow limitation. Eur Respir J 1994; 7:2016–2020.
- Warner MA, Divertie MB, Tinker JH. Preoperative cessation of smoking and pulmonary complications in coronary artery bypass patients. Anesthesiology 1984; 60:380–383.
- Myers K, Hajek P, Hinds C, McRobbie H. Stopping smoking shortly before surgery and postoperative complications: a systematic review and meta-analysis [published online ahead of print March 14, 2011]. Arch Intern Med 2011; 171:983–989. doi: 10.1001/archinternmed.2011.97
- Vaporciyan AA, Merriman KW, Ece F, et al. Incidence of major pulmonary morbidity after pneumonectomy: association with timing of smoking cessation. Ann Thorac Surg 2002; 73:420–426.
- Barrera R, Shi W, Amar D, et al. Smoking and timing of cessation: impact on pulmonary complications after thoracotomy. Chest 2005; 127:1977–1983.
- Mason DP, Subramanian S, Nowicki ER, et al. Impact of smoking cessation before resection of lung cancer. A Society of Thoracic Surgeons General Thoracic Surgery Database study. Ann Thorac Surg 2009; 88:362–371.
- Cesario A, Ferri L, Galetta D, et al. Pre-operative pulmonary rehabilitation and surgery for lung cancer. Lung Cancer 2007; 57:118–119.
For patients with localized lung cancer, lung resection provides the highest likelihood of a cure. However, only about 20% to 30% of patients are potential candidates for surgical resection because of the stage at which the disease is diagnosed or because of comorbid conditions.1,2 In one study, poor lung function alone ruled out more than 37% of patients who presented with anatomically resectable disease.3 The poor prognosis for patients who do not undergo surgery, the likelihood of early mortality from lung resection, and the potential for loss of lung function following resection are all important considerations in the preoperative pulmonary evaluation of candidates for anatomical lung resection.
PROGNOSIS OF LUNG CANCER POOR WITHOUT SURGICAL RESECTION
Several studies support the poor prognosis of lung cancer patients who do not undergo resection. In one study of 1,297 screen- and symptom-detected patients, the median duration of survival without surgery was 25 months for patients with screen-detected stage I lung cancer (n = 42) and 13 months for those with symptom-detected stage I disease (n = 27).4 Another study of 799 patients with stage I lung cancer who were not treated surgically reported 5- and 10-year survival rates of 16.6% (n = 49) and 7.4% (n = 49), respectively.5 In a study of 251 patients with squamous cell carcinoma on sputum cytology, yet negative chest imaging, the 5-year and 10-year survival rates were 53.2% and 33.5%.6 Another study of 57 patients with potentially resectable disease who did not undergo surgery reported a median survival of 15.6 months, compared with 30.9 months for a group of 346 patients who underwent resection.7
PREDICTORS OF SURGICAL MORTALITY
Several large patient series describe perioperative mortality and the rate of complications for patients undergoing surgical resection for lung cancer. Reported surgical mortality rates in these studies vary from approximately 1% to 5%.2,8–10 The median age of patients in most of these studies was 65 to 70 years, and many patients had significant medical comorbidity. Predictors of increased surgical mortality include pneumonectomy, bilobectomy, American Society of Anesthesiologists (ASA) Physical Status Scale rating, Zubrod performance status score, renal dysfunction, induction chemoradiation therapy, steroid use, older age, urgent procedures, male gender, forced expiratory volume in 1 second (FEV1), and body mass index.11 In France, a thoracic surgery scoring system for in-hospital mortality (Thoracoscore) was developed using data obtained from more than 15,000 patients who were enrolled in a nationally representative thoracic surgery database. Mortality risk factors included in the model were patient age, sex, dyspnea score, ASA score, performance status, priority of surgery, diagnosis, procedure class, and comorbid disease.12 The model was highly accurate for the prediction of mortality, with a C statistic of 0.86. (1.00 corresponds to perfect outcome prediction.) The model was subsequently validated on 1,675 patients from the United States, where a similar accuracy was noted.13 The online version of the Thoracoscore risk assessment tool is available at: http://www.sfar.org/scores2/thoracoscore2.php.
REDUCED PULMONARY FUNCTION AFTER RESECTION
Several outcome measures have been used to assess the impact of resection on pulmonary function and quality of life after surgery. Across various studies, postoperative FEV1 values, diffusing capacity of the lung for carbon monoxide (Dlco) values, and peak oxygen consumption (VO2 peak) were assessed at various time intervals after lobectomy or pneumonectomy. FEV1 varied from 84% to 91% of preoperative values for lobectomy,14–16 and 64% to 66% for pneumonectomy.14–16 The Dlco was 89% to 96% of preoperative values after lobectomy and 72% to 80% after pneumonectomy.14,16 VO2 peak varied from 87% to 100% of preoperative values after lobectomy,14–16 and 71% to 89% after pneumonectomy.14–16
Patients with chronic obstructive pulmonary disease (COPD) typically experience smaller declines in FEV1 after lobectomy (0% to 8%) than those without COPD (16% to 20%). Declines in Dlco and VO2 peak are more variable, with reported decreases of 3% to 20% in those with COPD, and 0% to 21% for those without the disease.17–19
Lobectomy patients continue to recover pulmonary function for approximately 6 months after surgery. In patients who undergo pneumonectomy, improvement is generally limited after 3 months.14–16 Loss of lung function may vary significantly with the location of the resection. For example, resection of an emphysematous portion of the lung will probably result in less loss of function.
Few studies specifically examine quality of life after lung resection in patients with lung cancer. In general, patients who undergo resection have a lower quality of life before surgery than the general population.20 Postsurgical decline in physical measures of health-related quality of life has been reported during the month after surgery, with a return to baseline after 3 months. Mental quality of life scores did not decrease after surgery, and there was little correlation between quality of life outcomes and measures of pulmonary function.20
LUNG FUNCTION TESTING
Lung function testing helps predict the risk of postoperative complications, including mortality. The two most commonly used measures of pulmonary function are FEV1 and Dlco.
Both absolute FEV1 value and percent of predicted FEV1 strongly predict the risk of postoperative complications. It has been difficult to identify one cutoff value below which resection should not be considered. Studies have suggested preoperative absolute FEV1 values of 2 L for pneumonectomy and 1.5 L for lobectomy as cutoffs signifying increased short- and long-term surgical risk.21,22 Percent predicted FEV1, which incorporates patient age, sex, and height, is more commonly used to individualize treatment, since absolute values do not take into consideration other patient-related variables. An FEV1 of 80% predicted or higher has been proposed as a cutoff to proceed with resection without additional testing,23 but this decision must be individualized to each patient.
Similarly, it has been difficult to identify one cutoff value for the Dlco. As one might expect, the lower the value the higher the risk for a given patient. Patients with Dlco values less than 60% predicted normal24 had an increased mortality risk, longer hospital stay, and greater hospital costs in one report.
FEV1 and Dlco are only modestly correlated with one another.25 In one study, 43% of patients with FEV1 greater than 80% of predicted had Dlco less than 80% of predicted.26
According to guidelines developed by the American College of Chest Physicians (ACCP), spirometry is recommended for patients being considered for lung cancer resection.27 Patients with FEV1 that is greater than 80% predicted or greater than 2 L and without evidence of dyspnea or interstitial lung disease are considered suitable candidates for resection, including pneumonectomy, without further testing. Lobectomy without further evaluation may be performed if the FEV1 is greater than 1.5 L and there is no evidence of dyspnea or interstitial lung disease.
Although assessing FEV1 values alone may be adequate in patients being considered for lung cancer resection who have no evidence of either undue dyspnea on exertion or interstitial lung disease, the ACCP recommends also measuring Dlco when these signs are present. Guidelines from the European Respiratory Society (ERS) and the European Society of Thoracic Surgeons (ESTS) recommend routinely measuring Dlco during preoperative evaluation regardless of whether the spirometric evaluation is abnormal.28 Similarly, the British Thoracic Society (BTS) recommends measuring transfer factor of the lung for carbon monoxide (Tlco) in all patients regardless of spirometric values.29
PREDICTING POSTOPERATIVE LUNG FUNCTION
Several methods have been used to predict postoperative lung function.
Segment method
The segment method estimates postoperative lung function by multiplying baseline function by the percentage of lung sections that will remain after resection.30 For example, if preoperative FEV1 is 1 L and surgery will result in the loss of 25% of lung segments, the predicted postoperative FEV1 is 750 mL. In a study using 19 lung segments in the calculation, the predicted postoperative lung function correlated well with actual postoperative lung function for patients undergoing lobectomy, but only modestly for patients undergoing pneumonectomy.30 Another method using 42 subsegments for the calculation, and correcting for segments that were obstructed by tumor, produced very similar results.31
Radionuclide scanning
In other studies, quantitative radionuclide scanning to identify the proportion of lung with poor perfusion produced fair to good correlations between predicted and actual postoperative FEV1.32–35 Techniques that are used less often include quantitative computed tomography (CT) and measurement of airway vibration during respiration.
Studies comparing different methods for predicting postoperative pulmonary function have found that perfusion imaging outperformed other approaches, and that the segment method is not a good predictor of outcome for patients undergoing pneumonectomy.17,36
Additional testing needed
For potential lung resection patients, the ACCP guidelines recommend that if either the FEV1 or Dlco is less than 80% of the predicted value, postoperative lung function should be predicted through additional testing.27 The ERS recommends that predicted postoperative FEV1 should not be used alone to select lung cancer patients for lung resection, especially those with moderate to severe COPD.28 These guidelines also recommend that the first estimate of residual lung function should be calculated based on segment counting, that only segments not totally obstructed should be counted, and that the patency of bronchus and segment structure should be preserved. In addition, patients with borderline function should undergo imaging-based calculation of residual lung function, including ventilation or perfusion scintigraphy before pneumonectomy, or quantitative CT scan before either lobectomy or pneumonectomy.28 The BTS recommends the use of segment counting to estimate postoperative lung function as part of risk assessment for postoperative dyspnea. Ventilation or perfusion scintigraphy should be considered to predict postoperative lung function if a ventilation or perfusion mismatch is suspected. Quantitative CT or MRI may be considered to predict postoperative lung function if the facility is available.29
Predicting mortality and complications: FEV1 and Dlco
The predicted postoperative FEV1 value is an independent predictor of postoperative mortality and other complications. Although there is no absolute cut-off value, studies identify an increased risk of complications below predicted postoperative FEV1 values ranging from 30%37 to 40%.38,39 Predicted postoperative Dlco is another outcome measure that can independently identify increased mortality risk in lung cancer resection patients. Dlco less than 40% has been associated with increased risk of postoperative respiratory complications even in those with predicted postoperative FEV1 values above 40%.26,39 One study stated that a combination of the two values, predicted postoperative FEV1 and predicted postoperative Dlco—called the predicted postoperative product (PPP)—is the best predictor of surgical mortality.40 Another study examined the utility of a prediction rule for pulmonary complications after lung surgery using a point system in which points were assigned based on predicted postoperative Dlco (1 point for each 5% decrement below 100%) plus 2 points for preoperative chemotherapy.41 The risk of complications was 9% for those with scores less than 11, 14% for those with scores of 11 to 13, and 26% for those with scores greater than 13.
When surgery is considered, ACCP guidelines state an increased risk of perioperative mortality in those lung cancer patients with either a PPP less than 1,650, or a predicted postoperative FEV1 less than 30%.27 These patients should be counseled about nonstandard surgery and nonsurgical treatment options. The ERS guidelines consider a predicted postoperative FEV1 value less than 30% to be a high-risk threshold when assessing pulmonary reserve before surgery.28
EXERCISE TESTING
In general, standardized cardiopulmonary exercise testing using VO2 peak has been shown to predict postoperative complications, including perioperative and long-term morbidity and mortality.42,43 Lower values are associated with a greater risk of poor outcome. Peak VO2 may not add significantly to the risk stratification of patients who have both FEV1 and Dlco values greater than 80%.44
According to ACCP recommendations for exercise testing in patients who are being evaluated for surgery, either an FEV1 or Dlco less than 40% of predicted postoperative (PPO) indicates increased risk for perioperative death and cardiopulmonary complications with standard lung resection. Preoperative exercise testing is recommended for these patients.27 Maximal oxygen consumption (VO2 max) less than 10 mL/kg/min, or the combination of VO2 max less than 15 mL/kg/min with both FEV1 and Dlco less than 40% PPO, also indicates increased risk for death and complications; these patients should be counseled about nonstandard surgery or nonsurgical treatment options. Guidelines from the ERS recommend exercise testing for all patients undergoing lung cancer surgery who have FEV1 or Dlco less than 80% of normal values.28 The VO2 peak measured during incremental exercise on a treadmill or cycle should be regarded as the most important parameter.
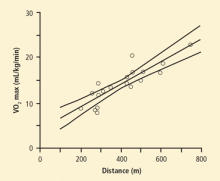
Several studies have found that distance traveled during walking tests predicts postoperative complications and can be related to VO2 max (Figure).45 According to ACCP guidelines, lung cancer patients who are potential candidates for standard lung resection are at increased risk for perioperative death and cardiopulmonary complications if they walk less than 25 shuttles on 2 shuttle walk tests or less than 1 flight of stairs. These patients should be counseled about nonstandard surgery and nonsurgical treatment options.27
Conversely, ERS/ESTS guidelines state that the shuttle walk test distance underestimates exercise capacity at the lower range, and does not discriminate between patients with and without complications.28 These guidelines state that shuttle walk test distance should not be used alone to select patients for resection. It may be used as a screening test, since patients walking less than 400 m are likely to also have VO2 peak less than 15 mL/kg/min. A standardized symptom-limited stair climbing test can be a cost-effective screening method to identify those who need more sophisticated exercise tests in order to optimize their perioperative management. The 6-minute walk test is not recommended.
British Thoracic Society guidelines recommend the use of the shuttle walk test as a functional assessment in patients with moderate to high risk of postoperative dyspnea.29 A distance walk of more than 400 m is recommended as a cutoff for acceptable pulmonary function. These guidelines recommend against using pulmonary function and exercise tests as the sole surrogates for a quality of life evaluation.
ALGORITHMS FOR TESTING
The ACCP, ERS/ESTS, and BTS guidelines all include algorithms for the preoperative evaluation of candidates for lung cancer resection.27–29 The guidelines differ from each other in many ways, including when to obtain a Dlco and cardiopulmonary exercise test, and in some of the cutoff values for various pulmonary function measures. ACCP guidelines begin with spirometry testing, supporting lobectomy in patients with spirometry results above the cutoff value of FEV1 greater than 1.5 L and pneumonectomy in those with a cutoff value of FEV1 greater than 2 L, and greater than 80% of predicted, unless the patient has dyspnea or evidence of interstitial lung disease. Measurement of the Dlco is recommended for those who do not meet the FEV1 cutoffs, or in those with unexplained dyspnea or diffuse parenchymal disease on chest radiograph or CT.27
A systematic review and set of treatment recommendations for high-risk patients with stage I lung cancer, developed by the Thoracic Oncology Network of the ACCP and the Workforce on Evidence-Based Surgery of the Society of Thoracic Surgeons, currently under review, will provide additional guidance regarding the use of lung function testing to evaluate risk of morbidity and mortality. These guidelines note that FEV1, Dlco, and peak VO2 all predict morbidity and mortality following major lung resection. Assessment of FEV1 and Dlco, including calculation of the estimated postoperative value, is strongly recommended before resection. The predictive value of peak VO2 is strongest in patients with impaired FEV1 or Dlco, and assessment of peak VO2 before major lung resection is recommended for these patients.
INTERVENTIONS TO DECREASE PERIOPERATIVE RISK
The impact of smoking cessation on perioperative outcome has been a matter of considerable debate. One large study found that the incidence of postoperative complications was actually greater when patients stopped smoking within 8 weeks before cardiac surgery.46 However, a recent meta-analysis including lung resection patients found no relationship between smoking cessation in the weeks before surgery and worse clinical outcomes.47 When a shorter duration of smoking cessation is examined, thoracotomy studies note that patients who continue to smoke within 1 month of pneumonectomy are at increased risk of major pulmonary events.48,49 An examination of perioperative mortality or major complications using data from the Society of Thoracic Surgeons found that smoking cessation within 1 month preceding surgery did not significantly affect perioperative morbidity or mortality, whereas longer abstention from tobacco use was associated with better surgical outcomes.50 The ACCP recommends that all patients with lung cancer be counseled regarding smoking cessation.27 ERS/ESTS guidelines recommend smoking cessation for at least 2 to 4 weeks before surgery, since this may change perioperative smoking behavior and decrease the risk of postoperative complications.28 Pulmonary rehabiliatation in the perioperative period has been shown to improve measures of activity tolerance, allowing resection of marginal candidates, and improving functional outcomes after resection.51 The ERS/ESTS guidelines state that early pre- and postoperative rehabilitation may produce functional benefits in resectable lung cancer patients.28
SUMMARY AND CONCLUSIONS
Lung function testing helps predict the risk of postoperative mortality, perioperative complications, and long-term dyspnea for patients with lung cancer undergoing surgical resection. Predicted postoperative FEV1 and Dlco should be evaluated in most resection candidates. Exercise testing adds to standard lung function testing in those with borderline values, discordance between standard measures, or discordance between subjective and objective lung function. Algorithms for preoperative assessment have been developed by the ACCP, the ERS/ESTS, and the BTS, which differ somewhat in the order of testing and specific testing cutoff values. Smoking cessation and pulmonary rehabilitation can help to reduce perioperative and long-term risks.
For patients with localized lung cancer, lung resection provides the highest likelihood of a cure. However, only about 20% to 30% of patients are potential candidates for surgical resection because of the stage at which the disease is diagnosed or because of comorbid conditions.1,2 In one study, poor lung function alone ruled out more than 37% of patients who presented with anatomically resectable disease.3 The poor prognosis for patients who do not undergo surgery, the likelihood of early mortality from lung resection, and the potential for loss of lung function following resection are all important considerations in the preoperative pulmonary evaluation of candidates for anatomical lung resection.
PROGNOSIS OF LUNG CANCER POOR WITHOUT SURGICAL RESECTION
Several studies support the poor prognosis of lung cancer patients who do not undergo resection. In one study of 1,297 screen- and symptom-detected patients, the median duration of survival without surgery was 25 months for patients with screen-detected stage I lung cancer (n = 42) and 13 months for those with symptom-detected stage I disease (n = 27).4 Another study of 799 patients with stage I lung cancer who were not treated surgically reported 5- and 10-year survival rates of 16.6% (n = 49) and 7.4% (n = 49), respectively.5 In a study of 251 patients with squamous cell carcinoma on sputum cytology, yet negative chest imaging, the 5-year and 10-year survival rates were 53.2% and 33.5%.6 Another study of 57 patients with potentially resectable disease who did not undergo surgery reported a median survival of 15.6 months, compared with 30.9 months for a group of 346 patients who underwent resection.7
PREDICTORS OF SURGICAL MORTALITY
Several large patient series describe perioperative mortality and the rate of complications for patients undergoing surgical resection for lung cancer. Reported surgical mortality rates in these studies vary from approximately 1% to 5%.2,8–10 The median age of patients in most of these studies was 65 to 70 years, and many patients had significant medical comorbidity. Predictors of increased surgical mortality include pneumonectomy, bilobectomy, American Society of Anesthesiologists (ASA) Physical Status Scale rating, Zubrod performance status score, renal dysfunction, induction chemoradiation therapy, steroid use, older age, urgent procedures, male gender, forced expiratory volume in 1 second (FEV1), and body mass index.11 In France, a thoracic surgery scoring system for in-hospital mortality (Thoracoscore) was developed using data obtained from more than 15,000 patients who were enrolled in a nationally representative thoracic surgery database. Mortality risk factors included in the model were patient age, sex, dyspnea score, ASA score, performance status, priority of surgery, diagnosis, procedure class, and comorbid disease.12 The model was highly accurate for the prediction of mortality, with a C statistic of 0.86. (1.00 corresponds to perfect outcome prediction.) The model was subsequently validated on 1,675 patients from the United States, where a similar accuracy was noted.13 The online version of the Thoracoscore risk assessment tool is available at: http://www.sfar.org/scores2/thoracoscore2.php.
REDUCED PULMONARY FUNCTION AFTER RESECTION
Several outcome measures have been used to assess the impact of resection on pulmonary function and quality of life after surgery. Across various studies, postoperative FEV1 values, diffusing capacity of the lung for carbon monoxide (Dlco) values, and peak oxygen consumption (VO2 peak) were assessed at various time intervals after lobectomy or pneumonectomy. FEV1 varied from 84% to 91% of preoperative values for lobectomy,14–16 and 64% to 66% for pneumonectomy.14–16 The Dlco was 89% to 96% of preoperative values after lobectomy and 72% to 80% after pneumonectomy.14,16 VO2 peak varied from 87% to 100% of preoperative values after lobectomy,14–16 and 71% to 89% after pneumonectomy.14–16
Patients with chronic obstructive pulmonary disease (COPD) typically experience smaller declines in FEV1 after lobectomy (0% to 8%) than those without COPD (16% to 20%). Declines in Dlco and VO2 peak are more variable, with reported decreases of 3% to 20% in those with COPD, and 0% to 21% for those without the disease.17–19
Lobectomy patients continue to recover pulmonary function for approximately 6 months after surgery. In patients who undergo pneumonectomy, improvement is generally limited after 3 months.14–16 Loss of lung function may vary significantly with the location of the resection. For example, resection of an emphysematous portion of the lung will probably result in less loss of function.
Few studies specifically examine quality of life after lung resection in patients with lung cancer. In general, patients who undergo resection have a lower quality of life before surgery than the general population.20 Postsurgical decline in physical measures of health-related quality of life has been reported during the month after surgery, with a return to baseline after 3 months. Mental quality of life scores did not decrease after surgery, and there was little correlation between quality of life outcomes and measures of pulmonary function.20
LUNG FUNCTION TESTING
Lung function testing helps predict the risk of postoperative complications, including mortality. The two most commonly used measures of pulmonary function are FEV1 and Dlco.
Both absolute FEV1 value and percent of predicted FEV1 strongly predict the risk of postoperative complications. It has been difficult to identify one cutoff value below which resection should not be considered. Studies have suggested preoperative absolute FEV1 values of 2 L for pneumonectomy and 1.5 L for lobectomy as cutoffs signifying increased short- and long-term surgical risk.21,22 Percent predicted FEV1, which incorporates patient age, sex, and height, is more commonly used to individualize treatment, since absolute values do not take into consideration other patient-related variables. An FEV1 of 80% predicted or higher has been proposed as a cutoff to proceed with resection without additional testing,23 but this decision must be individualized to each patient.
Similarly, it has been difficult to identify one cutoff value for the Dlco. As one might expect, the lower the value the higher the risk for a given patient. Patients with Dlco values less than 60% predicted normal24 had an increased mortality risk, longer hospital stay, and greater hospital costs in one report.
FEV1 and Dlco are only modestly correlated with one another.25 In one study, 43% of patients with FEV1 greater than 80% of predicted had Dlco less than 80% of predicted.26
According to guidelines developed by the American College of Chest Physicians (ACCP), spirometry is recommended for patients being considered for lung cancer resection.27 Patients with FEV1 that is greater than 80% predicted or greater than 2 L and without evidence of dyspnea or interstitial lung disease are considered suitable candidates for resection, including pneumonectomy, without further testing. Lobectomy without further evaluation may be performed if the FEV1 is greater than 1.5 L and there is no evidence of dyspnea or interstitial lung disease.
Although assessing FEV1 values alone may be adequate in patients being considered for lung cancer resection who have no evidence of either undue dyspnea on exertion or interstitial lung disease, the ACCP recommends also measuring Dlco when these signs are present. Guidelines from the European Respiratory Society (ERS) and the European Society of Thoracic Surgeons (ESTS) recommend routinely measuring Dlco during preoperative evaluation regardless of whether the spirometric evaluation is abnormal.28 Similarly, the British Thoracic Society (BTS) recommends measuring transfer factor of the lung for carbon monoxide (Tlco) in all patients regardless of spirometric values.29
PREDICTING POSTOPERATIVE LUNG FUNCTION
Several methods have been used to predict postoperative lung function.
Segment method
The segment method estimates postoperative lung function by multiplying baseline function by the percentage of lung sections that will remain after resection.30 For example, if preoperative FEV1 is 1 L and surgery will result in the loss of 25% of lung segments, the predicted postoperative FEV1 is 750 mL. In a study using 19 lung segments in the calculation, the predicted postoperative lung function correlated well with actual postoperative lung function for patients undergoing lobectomy, but only modestly for patients undergoing pneumonectomy.30 Another method using 42 subsegments for the calculation, and correcting for segments that were obstructed by tumor, produced very similar results.31
Radionuclide scanning
In other studies, quantitative radionuclide scanning to identify the proportion of lung with poor perfusion produced fair to good correlations between predicted and actual postoperative FEV1.32–35 Techniques that are used less often include quantitative computed tomography (CT) and measurement of airway vibration during respiration.
Studies comparing different methods for predicting postoperative pulmonary function have found that perfusion imaging outperformed other approaches, and that the segment method is not a good predictor of outcome for patients undergoing pneumonectomy.17,36
Additional testing needed
For potential lung resection patients, the ACCP guidelines recommend that if either the FEV1 or Dlco is less than 80% of the predicted value, postoperative lung function should be predicted through additional testing.27 The ERS recommends that predicted postoperative FEV1 should not be used alone to select lung cancer patients for lung resection, especially those with moderate to severe COPD.28 These guidelines also recommend that the first estimate of residual lung function should be calculated based on segment counting, that only segments not totally obstructed should be counted, and that the patency of bronchus and segment structure should be preserved. In addition, patients with borderline function should undergo imaging-based calculation of residual lung function, including ventilation or perfusion scintigraphy before pneumonectomy, or quantitative CT scan before either lobectomy or pneumonectomy.28 The BTS recommends the use of segment counting to estimate postoperative lung function as part of risk assessment for postoperative dyspnea. Ventilation or perfusion scintigraphy should be considered to predict postoperative lung function if a ventilation or perfusion mismatch is suspected. Quantitative CT or MRI may be considered to predict postoperative lung function if the facility is available.29
Predicting mortality and complications: FEV1 and Dlco
The predicted postoperative FEV1 value is an independent predictor of postoperative mortality and other complications. Although there is no absolute cut-off value, studies identify an increased risk of complications below predicted postoperative FEV1 values ranging from 30%37 to 40%.38,39 Predicted postoperative Dlco is another outcome measure that can independently identify increased mortality risk in lung cancer resection patients. Dlco less than 40% has been associated with increased risk of postoperative respiratory complications even in those with predicted postoperative FEV1 values above 40%.26,39 One study stated that a combination of the two values, predicted postoperative FEV1 and predicted postoperative Dlco—called the predicted postoperative product (PPP)—is the best predictor of surgical mortality.40 Another study examined the utility of a prediction rule for pulmonary complications after lung surgery using a point system in which points were assigned based on predicted postoperative Dlco (1 point for each 5% decrement below 100%) plus 2 points for preoperative chemotherapy.41 The risk of complications was 9% for those with scores less than 11, 14% for those with scores of 11 to 13, and 26% for those with scores greater than 13.
When surgery is considered, ACCP guidelines state an increased risk of perioperative mortality in those lung cancer patients with either a PPP less than 1,650, or a predicted postoperative FEV1 less than 30%.27 These patients should be counseled about nonstandard surgery and nonsurgical treatment options. The ERS guidelines consider a predicted postoperative FEV1 value less than 30% to be a high-risk threshold when assessing pulmonary reserve before surgery.28
EXERCISE TESTING
In general, standardized cardiopulmonary exercise testing using VO2 peak has been shown to predict postoperative complications, including perioperative and long-term morbidity and mortality.42,43 Lower values are associated with a greater risk of poor outcome. Peak VO2 may not add significantly to the risk stratification of patients who have both FEV1 and Dlco values greater than 80%.44
According to ACCP recommendations for exercise testing in patients who are being evaluated for surgery, either an FEV1 or Dlco less than 40% of predicted postoperative (PPO) indicates increased risk for perioperative death and cardiopulmonary complications with standard lung resection. Preoperative exercise testing is recommended for these patients.27 Maximal oxygen consumption (VO2 max) less than 10 mL/kg/min, or the combination of VO2 max less than 15 mL/kg/min with both FEV1 and Dlco less than 40% PPO, also indicates increased risk for death and complications; these patients should be counseled about nonstandard surgery or nonsurgical treatment options. Guidelines from the ERS recommend exercise testing for all patients undergoing lung cancer surgery who have FEV1 or Dlco less than 80% of normal values.28 The VO2 peak measured during incremental exercise on a treadmill or cycle should be regarded as the most important parameter.
Several studies have found that distance traveled during walking tests predicts postoperative complications and can be related to VO2 max (Figure).45 According to ACCP guidelines, lung cancer patients who are potential candidates for standard lung resection are at increased risk for perioperative death and cardiopulmonary complications if they walk less than 25 shuttles on 2 shuttle walk tests or less than 1 flight of stairs. These patients should be counseled about nonstandard surgery and nonsurgical treatment options.27
Conversely, ERS/ESTS guidelines state that the shuttle walk test distance underestimates exercise capacity at the lower range, and does not discriminate between patients with and without complications.28 These guidelines state that shuttle walk test distance should not be used alone to select patients for resection. It may be used as a screening test, since patients walking less than 400 m are likely to also have VO2 peak less than 15 mL/kg/min. A standardized symptom-limited stair climbing test can be a cost-effective screening method to identify those who need more sophisticated exercise tests in order to optimize their perioperative management. The 6-minute walk test is not recommended.
British Thoracic Society guidelines recommend the use of the shuttle walk test as a functional assessment in patients with moderate to high risk of postoperative dyspnea.29 A distance walk of more than 400 m is recommended as a cutoff for acceptable pulmonary function. These guidelines recommend against using pulmonary function and exercise tests as the sole surrogates for a quality of life evaluation.
ALGORITHMS FOR TESTING
The ACCP, ERS/ESTS, and BTS guidelines all include algorithms for the preoperative evaluation of candidates for lung cancer resection.27–29 The guidelines differ from each other in many ways, including when to obtain a Dlco and cardiopulmonary exercise test, and in some of the cutoff values for various pulmonary function measures. ACCP guidelines begin with spirometry testing, supporting lobectomy in patients with spirometry results above the cutoff value of FEV1 greater than 1.5 L and pneumonectomy in those with a cutoff value of FEV1 greater than 2 L, and greater than 80% of predicted, unless the patient has dyspnea or evidence of interstitial lung disease. Measurement of the Dlco is recommended for those who do not meet the FEV1 cutoffs, or in those with unexplained dyspnea or diffuse parenchymal disease on chest radiograph or CT.27
A systematic review and set of treatment recommendations for high-risk patients with stage I lung cancer, developed by the Thoracic Oncology Network of the ACCP and the Workforce on Evidence-Based Surgery of the Society of Thoracic Surgeons, currently under review, will provide additional guidance regarding the use of lung function testing to evaluate risk of morbidity and mortality. These guidelines note that FEV1, Dlco, and peak VO2 all predict morbidity and mortality following major lung resection. Assessment of FEV1 and Dlco, including calculation of the estimated postoperative value, is strongly recommended before resection. The predictive value of peak VO2 is strongest in patients with impaired FEV1 or Dlco, and assessment of peak VO2 before major lung resection is recommended for these patients.
INTERVENTIONS TO DECREASE PERIOPERATIVE RISK
The impact of smoking cessation on perioperative outcome has been a matter of considerable debate. One large study found that the incidence of postoperative complications was actually greater when patients stopped smoking within 8 weeks before cardiac surgery.46 However, a recent meta-analysis including lung resection patients found no relationship between smoking cessation in the weeks before surgery and worse clinical outcomes.47 When a shorter duration of smoking cessation is examined, thoracotomy studies note that patients who continue to smoke within 1 month of pneumonectomy are at increased risk of major pulmonary events.48,49 An examination of perioperative mortality or major complications using data from the Society of Thoracic Surgeons found that smoking cessation within 1 month preceding surgery did not significantly affect perioperative morbidity or mortality, whereas longer abstention from tobacco use was associated with better surgical outcomes.50 The ACCP recommends that all patients with lung cancer be counseled regarding smoking cessation.27 ERS/ESTS guidelines recommend smoking cessation for at least 2 to 4 weeks before surgery, since this may change perioperative smoking behavior and decrease the risk of postoperative complications.28 Pulmonary rehabiliatation in the perioperative period has been shown to improve measures of activity tolerance, allowing resection of marginal candidates, and improving functional outcomes after resection.51 The ERS/ESTS guidelines state that early pre- and postoperative rehabilitation may produce functional benefits in resectable lung cancer patients.28
SUMMARY AND CONCLUSIONS
Lung function testing helps predict the risk of postoperative mortality, perioperative complications, and long-term dyspnea for patients with lung cancer undergoing surgical resection. Predicted postoperative FEV1 and Dlco should be evaluated in most resection candidates. Exercise testing adds to standard lung function testing in those with borderline values, discordance between standard measures, or discordance between subjective and objective lung function. Algorithms for preoperative assessment have been developed by the ACCP, the ERS/ESTS, and the BTS, which differ somewhat in the order of testing and specific testing cutoff values. Smoking cessation and pulmonary rehabilitation can help to reduce perioperative and long-term risks.
- Damhuis RA, Schütte PR. Resection rates and postoperative mortality in 7,899 patients with lung cancer. Eur Respir J 1996; 9:7–10.
- Little AG, Rusch VW, Bonner JA, et al. Patterns of surgical care of lung cancer patients. Ann Thorac Surg 2005; 80:2051–2056.
- Baser S, Shannon VR, Eapen GA, et al. Pulmonary dysfunction as a major cause of inoperability among patients with non-small-cell lung cancer. Clin Lung Cancer 2006; 7:344–349.
- Sobue T, Suzuki T, Matsuda M, Kuroishi T, Ikeda S, Naruke T. Survival for clinical stage I lung cancer not surgically treated: comparison between screen-detected and symptom-detected cases. The Japanese Lung Cancer Screening Research Group. Cancer 1992; 69:685–692.
- Motohiro A, Ueda H, Komatsu H, Yanai N, Mori T; National Chest Hospital Study Group for Lung Cancer. Prognosis of nonsurgically treated, clinical stage I lung cancer patients in Japan. Lung Cancer 2002; 36:65–69.
- Sato M, Saito Y, Endo C, et al. The natural history of radiographically occult bronchogenic squamous cell carcinoma: a retrospective study of overdiagnosis bias. Chest 2004; 126:108–113.
- Loewen GM, Watson D, Kohman L, et al. Preoperative exercise Vo2 measurement for lung resection candidates: results of Cancer and Leukemia Group B Protocol 9238. J Thorac Oncol 2007; 2:619–625.
- Allen MS, Darling GE, Pechet TT, et al. Morbidity and mortality of major pulmonary resections in patients with early-stage lung cancer: initial results of the randomized, prospective ACOSOG Z0030 trial. Ann Thorac Surg 2006; 81:1013–1020.
- Meguid RA, Brooke BS, Chang DC, Sherwood JT, Brock MV, Yang SC. Are surgical outcomes for lung cancer resections improved at teaching hospitals? Ann Thorac Surg 2008; 85:1015–1025.
- Memtsoudis SG, Besculides MC, Zellos L, Patil N, Rogers SO. Trends in lung surgery: United States 1988 to 2002. Chest 2006; 130:1462–1470.
- Kozower BD, Sheng S, O’Brien SM, et al. STS database risk models: predictors of mortality and major morbidity for lung cancer resection. Ann Thorac Surg 2010; 90:875–883.
- Falcoz PE, Conti M, Brouchet L, et al. The Thoracic Surgery Scoring System (Thoracoscore): risk model for in-hospital death in 15,183 patients requiring thoracic surgery [published online ahead of print January 9, 2007]. J Thorac Cardiovasc Surg 2007; 133:325–332. doi: 10.1016/j.jtcvs.2006.09.020
- Chamogeorgakis TP, Connery CP, Bhora F, Nabong A, Toumpoulis IK. Thoracoscore predicts midterm mortality in patients undergoing thoracic surgery. J Thorac Cardiovasc Surg 2007; 134:883–887.
- Bolliger CT, Jordan P, Solèr M, et al. Pulmonary function and exercise capacity after lung resection. Eur Respir J 1996; 9:415–421.
- Nezu K, Kushibe K, Tojo T, Takahama M, Kitamura S. Recovery and limitation of exercise capacity after lung resection for lung cancer. Chest 1998; 113:1511–1516.
- Brunelli A, Xiumé F, Refai M, et al. Evaluation of expiratory volume, diffusion capacity, and exercise tolerance following major lung resection: a prospective follow-up analysis. Chest 2007; 131:141–147.
- Smulders SA, Smeenk FW, Janssen-Heijnen ML, Postmus PE. Actual and predicted postoperative changes in lung function after pneumonectomy: a retrospective analysis. Chest 2004; 125:1735–1741.
- Edwards JG, Duthie DJ, Waller DA. Lobar volume reduction surgery: a method of increasing the lung cancer resection rate in patients with emphysema. Thorax 2001; 56:791–795.
- Bobbio A, Chetta A, Carbognani P, et al. Changes in pulmonary function test and cardiopulmonary exercise capacity in COPD patients after lobar pulmonary resection [published online ahead of print September 6, 2005]. Eur J Cardiothorac Surg 2005; 28:754–758. doi: 10.1016/j.ejcts.2005.08.001
- Brunelli A, Refai M, Salati M, Xiumé F, Sabbatini A. Predicted versus observed FEV1 and Dlco after major lung resection: a prospective evaluation at different postoperative periods. Ann Thorac Surg 2007; 83:1134–1139.
- Boushy SF, Billig DM, North LB, Helgason AH. Clinical course related to preoperative and postoperative pulmonary function in patients with bronchogenic carcinoma. Chest 1971; 59:383–391.
- Wernly JA, DeMeester TR, Kirchner PT, Myerowitz PD, Oxford DE, Golomb HM. Clinical value of quantitative ventilation-perfusion lung scans in the surgical management of bronchogenic carcinoma. J Thorac Cardiovasc Surg 1980; 80:535–543.
- Wyser C, Stulz P, Solèr M, et al. Prospective evaluation of an algorithm for the functional assessment of lung resection candidates. Am J Respir Crit Care Med 1999; 159:1450–1456.
- Bousamra M, Presberg KW, Chammas JH, et al. Early and late morbidity in patients undergoing pulmonary resection with low diffusion capacity. Ann Thorac Surg 1996; 62:968–975.
- Ferguson MK, Little L, Rizzo L, et al. Diffusing capacity predicts morbidity and mortality after pulmonary resection. J Thorac Cardiovasc Surg 1988; 96:894–900.
- Brunelli A, Refai MA, Salati M, Sabbatini A, Morgan-Hughes NJ, Rocco G. Carbon monoxide lung diffusion capacity improves risk stratification in patients without airflow limitation: evidence for systematic measurement before lung resection [published online ahead of print February 14, 2006]. Eur J Cardiothorac Surg 2006; 29:567–570. doi: 10.1016/j.ejcts.2006.01.014
- Colice GL, Shafazand S, Griffin JP, Keenan R, Bolliger CT; American College of Chest Physicians. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: ACCP evidenced-based clinical practice guidelines (2nd edition). Chest 2007; 132( suppl 3):161S–177S.
- Brunelli A, Charloux A, Bolliger CT, et al. ERS/ESTS clinical guidelines on fitness for radical therapy in lung cancer patients (surgery and chemo-radiotherapy). Eur Respir J 2009; 34:17–41.
- Lim E, Baldwin D, Beckles M, et al. Guidelines on the radical management of patients with lung cancer. Thorax 2010; 65( suppl 3):iii1–iii27.
- Zeiher BG, Gross TJ, Kern JA, Lanza LA, Peterson MW. Predicting postoperative pulmonary function in patients undergoing lung resection. Chest 1995; 108:68–72.
- Nakahara K, Monden Y, Ohno K, Miyoshi S, Maeda H, Kawashima Y. A method for predicting postoperative lung function and its relation to postoperative complications in patients with lung cancer. Ann Thorac Surg 1985; 39:260–265.
- Kristersson S, Lindell SE, Svanberg L. Prediction of pulmonary function loss due to pneumonectomy using 133 Xe-radiospirometry. Chest 1972; 62:694–698.
- Bria WF, Kanarek DJ, Kazemi H. Prediction of postoperative pulmonary function following thoracic operations: value of ventilation-perfusion scanning. J Thorac Cardiovasc Surg 1983; 86:186–192.
- Ali MK, Mountain CF, Ewer MS, Johnston D, Haynie TP. Predicting loss of pulmonary function after pulmonary resection for bronchogenic carcinoma. Chest 1980; 77:337–342.
- Corris PA, Ellis DA, Hawkins T, Gibson GJ. Use of radionuclide scanning in the preoperative estimation of pulmonary function after pneumonectomy. Thorax 1987; 42:285–291.
- Bolliger CT, Gückel C, Engel H, et al. Prediction of functional reserves after lung resection: comparison between quantitative computed tomography, scintigraphy, and anatomy. Respiration 2002; 69:482–489.
- Nakahara K, Ohno K, Hashimoto J, et al. Prediction of postoperative respiratory failure in patients undergoing lung resection for lung cancer. Ann Thorac Surg 1988; 46:549–552.
- Markos J, Mullan BP, Hillman DR, et al. Preoperative assessment as a predictor of mortality and morbidity after lung resection. Am Rev Respir Dis 1989; 139:902–910.
- Ribas J, Diaz O, Barberà JA, et al. Invasive exercise testing in the evaluation of patients at high-risk for lung resection. Eur Respir J 1998; 12:1429–1435.
- Pierce RJ, Copland JM, Sharpe K, Barter CE. Preoperative risk evaluation for lung cancer resection: predicted postoperative product as a predictor of surgical mortality. Am J Respir Crit Care Med 1994; 150:947–955.
- Amar D, Munoz D, Shi W, Zhang H, Thaler HT. A clinical prediction rule for pulmonary complications after thoracic surgery for primary lung cancer [published online ahead of print October 27, 2009]. Anesth Analg 2010; 110:1343–1348. doi: 10.1213/ANE.0b013e3181bf5c99
- Benzo R, Kelley GA, Recchi L, Hofman A, Sciurba F. Complications of lung resection and exercise capacity: a meta-analysis [published online ahead of print April 3, 2007]. Respir Med 2007; 101:1790–1797. doi: 10.1016/j.rmed.2007.02.012
- Jones LW, Eves ND, Kraus WE, et al. The lung cancer exercise training study: a randomized trial of aerobic training, resistance training, or both in postsurgical lung cancer patients: rationale and design. BMC Cancer 2010; 10:155.
- Larsen KR, Svendsen UG, Milman N, Brenøe J, Petersen BN. Exercise testing in the preoperative evaluation of patients with bronchogenic carcinoma. Eur Respir J 1997; 10:1559–1565.
- Singh SJ, Morgan MDL, Hardman AE, Rowe C, Bardsley PA. Comparison of oxygen uptake during a conventional treadmill test and the shuttle walking test in chronic airflow limitation. Eur Respir J 1994; 7:2016–2020.
- Warner MA, Divertie MB, Tinker JH. Preoperative cessation of smoking and pulmonary complications in coronary artery bypass patients. Anesthesiology 1984; 60:380–383.
- Myers K, Hajek P, Hinds C, McRobbie H. Stopping smoking shortly before surgery and postoperative complications: a systematic review and meta-analysis [published online ahead of print March 14, 2011]. Arch Intern Med 2011; 171:983–989. doi: 10.1001/archinternmed.2011.97
- Vaporciyan AA, Merriman KW, Ece F, et al. Incidence of major pulmonary morbidity after pneumonectomy: association with timing of smoking cessation. Ann Thorac Surg 2002; 73:420–426.
- Barrera R, Shi W, Amar D, et al. Smoking and timing of cessation: impact on pulmonary complications after thoracotomy. Chest 2005; 127:1977–1983.
- Mason DP, Subramanian S, Nowicki ER, et al. Impact of smoking cessation before resection of lung cancer. A Society of Thoracic Surgeons General Thoracic Surgery Database study. Ann Thorac Surg 2009; 88:362–371.
- Cesario A, Ferri L, Galetta D, et al. Pre-operative pulmonary rehabilitation and surgery for lung cancer. Lung Cancer 2007; 57:118–119.
- Damhuis RA, Schütte PR. Resection rates and postoperative mortality in 7,899 patients with lung cancer. Eur Respir J 1996; 9:7–10.
- Little AG, Rusch VW, Bonner JA, et al. Patterns of surgical care of lung cancer patients. Ann Thorac Surg 2005; 80:2051–2056.
- Baser S, Shannon VR, Eapen GA, et al. Pulmonary dysfunction as a major cause of inoperability among patients with non-small-cell lung cancer. Clin Lung Cancer 2006; 7:344–349.
- Sobue T, Suzuki T, Matsuda M, Kuroishi T, Ikeda S, Naruke T. Survival for clinical stage I lung cancer not surgically treated: comparison between screen-detected and symptom-detected cases. The Japanese Lung Cancer Screening Research Group. Cancer 1992; 69:685–692.
- Motohiro A, Ueda H, Komatsu H, Yanai N, Mori T; National Chest Hospital Study Group for Lung Cancer. Prognosis of nonsurgically treated, clinical stage I lung cancer patients in Japan. Lung Cancer 2002; 36:65–69.
- Sato M, Saito Y, Endo C, et al. The natural history of radiographically occult bronchogenic squamous cell carcinoma: a retrospective study of overdiagnosis bias. Chest 2004; 126:108–113.
- Loewen GM, Watson D, Kohman L, et al. Preoperative exercise Vo2 measurement for lung resection candidates: results of Cancer and Leukemia Group B Protocol 9238. J Thorac Oncol 2007; 2:619–625.
- Allen MS, Darling GE, Pechet TT, et al. Morbidity and mortality of major pulmonary resections in patients with early-stage lung cancer: initial results of the randomized, prospective ACOSOG Z0030 trial. Ann Thorac Surg 2006; 81:1013–1020.
- Meguid RA, Brooke BS, Chang DC, Sherwood JT, Brock MV, Yang SC. Are surgical outcomes for lung cancer resections improved at teaching hospitals? Ann Thorac Surg 2008; 85:1015–1025.
- Memtsoudis SG, Besculides MC, Zellos L, Patil N, Rogers SO. Trends in lung surgery: United States 1988 to 2002. Chest 2006; 130:1462–1470.
- Kozower BD, Sheng S, O’Brien SM, et al. STS database risk models: predictors of mortality and major morbidity for lung cancer resection. Ann Thorac Surg 2010; 90:875–883.
- Falcoz PE, Conti M, Brouchet L, et al. The Thoracic Surgery Scoring System (Thoracoscore): risk model for in-hospital death in 15,183 patients requiring thoracic surgery [published online ahead of print January 9, 2007]. J Thorac Cardiovasc Surg 2007; 133:325–332. doi: 10.1016/j.jtcvs.2006.09.020
- Chamogeorgakis TP, Connery CP, Bhora F, Nabong A, Toumpoulis IK. Thoracoscore predicts midterm mortality in patients undergoing thoracic surgery. J Thorac Cardiovasc Surg 2007; 134:883–887.
- Bolliger CT, Jordan P, Solèr M, et al. Pulmonary function and exercise capacity after lung resection. Eur Respir J 1996; 9:415–421.
- Nezu K, Kushibe K, Tojo T, Takahama M, Kitamura S. Recovery and limitation of exercise capacity after lung resection for lung cancer. Chest 1998; 113:1511–1516.
- Brunelli A, Xiumé F, Refai M, et al. Evaluation of expiratory volume, diffusion capacity, and exercise tolerance following major lung resection: a prospective follow-up analysis. Chest 2007; 131:141–147.
- Smulders SA, Smeenk FW, Janssen-Heijnen ML, Postmus PE. Actual and predicted postoperative changes in lung function after pneumonectomy: a retrospective analysis. Chest 2004; 125:1735–1741.
- Edwards JG, Duthie DJ, Waller DA. Lobar volume reduction surgery: a method of increasing the lung cancer resection rate in patients with emphysema. Thorax 2001; 56:791–795.
- Bobbio A, Chetta A, Carbognani P, et al. Changes in pulmonary function test and cardiopulmonary exercise capacity in COPD patients after lobar pulmonary resection [published online ahead of print September 6, 2005]. Eur J Cardiothorac Surg 2005; 28:754–758. doi: 10.1016/j.ejcts.2005.08.001
- Brunelli A, Refai M, Salati M, Xiumé F, Sabbatini A. Predicted versus observed FEV1 and Dlco after major lung resection: a prospective evaluation at different postoperative periods. Ann Thorac Surg 2007; 83:1134–1139.
- Boushy SF, Billig DM, North LB, Helgason AH. Clinical course related to preoperative and postoperative pulmonary function in patients with bronchogenic carcinoma. Chest 1971; 59:383–391.
- Wernly JA, DeMeester TR, Kirchner PT, Myerowitz PD, Oxford DE, Golomb HM. Clinical value of quantitative ventilation-perfusion lung scans in the surgical management of bronchogenic carcinoma. J Thorac Cardiovasc Surg 1980; 80:535–543.
- Wyser C, Stulz P, Solèr M, et al. Prospective evaluation of an algorithm for the functional assessment of lung resection candidates. Am J Respir Crit Care Med 1999; 159:1450–1456.
- Bousamra M, Presberg KW, Chammas JH, et al. Early and late morbidity in patients undergoing pulmonary resection with low diffusion capacity. Ann Thorac Surg 1996; 62:968–975.
- Ferguson MK, Little L, Rizzo L, et al. Diffusing capacity predicts morbidity and mortality after pulmonary resection. J Thorac Cardiovasc Surg 1988; 96:894–900.
- Brunelli A, Refai MA, Salati M, Sabbatini A, Morgan-Hughes NJ, Rocco G. Carbon monoxide lung diffusion capacity improves risk stratification in patients without airflow limitation: evidence for systematic measurement before lung resection [published online ahead of print February 14, 2006]. Eur J Cardiothorac Surg 2006; 29:567–570. doi: 10.1016/j.ejcts.2006.01.014
- Colice GL, Shafazand S, Griffin JP, Keenan R, Bolliger CT; American College of Chest Physicians. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: ACCP evidenced-based clinical practice guidelines (2nd edition). Chest 2007; 132( suppl 3):161S–177S.
- Brunelli A, Charloux A, Bolliger CT, et al. ERS/ESTS clinical guidelines on fitness for radical therapy in lung cancer patients (surgery and chemo-radiotherapy). Eur Respir J 2009; 34:17–41.
- Lim E, Baldwin D, Beckles M, et al. Guidelines on the radical management of patients with lung cancer. Thorax 2010; 65( suppl 3):iii1–iii27.
- Zeiher BG, Gross TJ, Kern JA, Lanza LA, Peterson MW. Predicting postoperative pulmonary function in patients undergoing lung resection. Chest 1995; 108:68–72.
- Nakahara K, Monden Y, Ohno K, Miyoshi S, Maeda H, Kawashima Y. A method for predicting postoperative lung function and its relation to postoperative complications in patients with lung cancer. Ann Thorac Surg 1985; 39:260–265.
- Kristersson S, Lindell SE, Svanberg L. Prediction of pulmonary function loss due to pneumonectomy using 133 Xe-radiospirometry. Chest 1972; 62:694–698.
- Bria WF, Kanarek DJ, Kazemi H. Prediction of postoperative pulmonary function following thoracic operations: value of ventilation-perfusion scanning. J Thorac Cardiovasc Surg 1983; 86:186–192.
- Ali MK, Mountain CF, Ewer MS, Johnston D, Haynie TP. Predicting loss of pulmonary function after pulmonary resection for bronchogenic carcinoma. Chest 1980; 77:337–342.
- Corris PA, Ellis DA, Hawkins T, Gibson GJ. Use of radionuclide scanning in the preoperative estimation of pulmonary function after pneumonectomy. Thorax 1987; 42:285–291.
- Bolliger CT, Gückel C, Engel H, et al. Prediction of functional reserves after lung resection: comparison between quantitative computed tomography, scintigraphy, and anatomy. Respiration 2002; 69:482–489.
- Nakahara K, Ohno K, Hashimoto J, et al. Prediction of postoperative respiratory failure in patients undergoing lung resection for lung cancer. Ann Thorac Surg 1988; 46:549–552.
- Markos J, Mullan BP, Hillman DR, et al. Preoperative assessment as a predictor of mortality and morbidity after lung resection. Am Rev Respir Dis 1989; 139:902–910.
- Ribas J, Diaz O, Barberà JA, et al. Invasive exercise testing in the evaluation of patients at high-risk for lung resection. Eur Respir J 1998; 12:1429–1435.
- Pierce RJ, Copland JM, Sharpe K, Barter CE. Preoperative risk evaluation for lung cancer resection: predicted postoperative product as a predictor of surgical mortality. Am J Respir Crit Care Med 1994; 150:947–955.
- Amar D, Munoz D, Shi W, Zhang H, Thaler HT. A clinical prediction rule for pulmonary complications after thoracic surgery for primary lung cancer [published online ahead of print October 27, 2009]. Anesth Analg 2010; 110:1343–1348. doi: 10.1213/ANE.0b013e3181bf5c99
- Benzo R, Kelley GA, Recchi L, Hofman A, Sciurba F. Complications of lung resection and exercise capacity: a meta-analysis [published online ahead of print April 3, 2007]. Respir Med 2007; 101:1790–1797. doi: 10.1016/j.rmed.2007.02.012
- Jones LW, Eves ND, Kraus WE, et al. The lung cancer exercise training study: a randomized trial of aerobic training, resistance training, or both in postsurgical lung cancer patients: rationale and design. BMC Cancer 2010; 10:155.
- Larsen KR, Svendsen UG, Milman N, Brenøe J, Petersen BN. Exercise testing in the preoperative evaluation of patients with bronchogenic carcinoma. Eur Respir J 1997; 10:1559–1565.
- Singh SJ, Morgan MDL, Hardman AE, Rowe C, Bardsley PA. Comparison of oxygen uptake during a conventional treadmill test and the shuttle walking test in chronic airflow limitation. Eur Respir J 1994; 7:2016–2020.
- Warner MA, Divertie MB, Tinker JH. Preoperative cessation of smoking and pulmonary complications in coronary artery bypass patients. Anesthesiology 1984; 60:380–383.
- Myers K, Hajek P, Hinds C, McRobbie H. Stopping smoking shortly before surgery and postoperative complications: a systematic review and meta-analysis [published online ahead of print March 14, 2011]. Arch Intern Med 2011; 171:983–989. doi: 10.1001/archinternmed.2011.97
- Vaporciyan AA, Merriman KW, Ece F, et al. Incidence of major pulmonary morbidity after pneumonectomy: association with timing of smoking cessation. Ann Thorac Surg 2002; 73:420–426.
- Barrera R, Shi W, Amar D, et al. Smoking and timing of cessation: impact on pulmonary complications after thoracotomy. Chest 2005; 127:1977–1983.
- Mason DP, Subramanian S, Nowicki ER, et al. Impact of smoking cessation before resection of lung cancer. A Society of Thoracic Surgeons General Thoracic Surgery Database study. Ann Thorac Surg 2009; 88:362–371.
- Cesario A, Ferri L, Galetta D, et al. Pre-operative pulmonary rehabilitation and surgery for lung cancer. Lung Cancer 2007; 57:118–119.