User login
Pulmonary embolism is a master of disguises. It can appear with classic symptoms such as pleuritic chest pain, hemoptysis, and tachycardia—or it can arrive more insidiously, apparent only as a slight elevation in the respiratory rate.
This matters because 40% of all deaths following gynecologic surgery are directly attributable to pulmonary emboli,1 and pulmonary emboli are the most frequent cause of postoperative death in women with uterine or cervical carcinoma.2
Deep venous thrombosis (DVT) is almost as evasive. We know the signs and symptoms of DVT of the lower extremities—pain, edema, erythema, and a prominent vascular pattern of the superficial veins—but 50% to 80% of patients with these symptoms do not have DVT, and 80% of patients with symptomatic pulmonary embolism have no antecedent signs of thrombosis in the lower extremities.2 Morbidity and expense rise dramatically with DVT, especially when postphlebitic syndrome occurs.
How can we minimize these risks?
A good outcome is most likely when we:
- recognize risk factors,
- provide appropriate perioperative prophylaxis, and
- diagnose and treat venous thromboembolism (VTE) quickly.
This article looks in detail at each of these strategies.
3 factors set the stage for thrombogenesis
- Hypercoagulable state
- Venous stasis
- Vessel endothelial injury
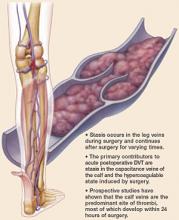
These factors, known as Virchow’s triad, are especially likely at the time of major surgery, or when the patient is advanced in age or has a history of DVT, cancer, lower extremity edema, or venous stasis.
Intraoperative risk factors for postoperative DVT include increased anesthesia time, greater blood loss, and need for transfusion.
Some preventive methods come close to ideal
Being aware of risk factors is vital to provide the appropriate level of prophylaxis (TABLES 1 AND 2).3,4 The first step is identifying high-risk patients and tailoring the regimen to meet their individual needs. The perfect prophylactic method is not yet devised, but would be effective, free of significant side effects, well accepted by the patient and nursing staff, widely applicable to most patient groups, and inexpensive. A number of methods come close.
TABLE 1
Risk factors for thromboembolism
| Major gynecologic surgery |
| Age >40 years |
| Malignancy |
| Previous venous thrombosis (DVT or pulmonary embolism) |
| Obesity |
| Immobility |
| Pregnancy and the postpartum period |
| Oral contraceptives, hormone therapy, or tamoxifen |
| Varicose veins |
| Inherited or acquired thrombophilia (eg, Factor V Leiden) |
| Prolonged surgical procedure |
| Radical vulvectomy, inguinal-femoral lymphadenectomy, or pelvic exenteration |
TABLE 2
Match the preventive strategy to the surgery
| SURGERY | STRATEGY | DURATION OF PROPHYLAXIS* |
|---|---|---|
| Procedures <30 min for benign disease | Prophylaxis not needed | — |
| Laparoscopic gynecologic procedures in women with additional risk factors | Unfractionated heparin, 5,000 bid or | Until hospital discharge |
| LMWH, ≤3,400 U/day or | ||
| External pneumatic compression or | ||
| Graduated compression stockings | ||
| Major surgery for benign disease without additional risk factors | Unfractionated heparin, 5,000 U bid or | Until hospital discharge |
| LMWH, <3,400 U/day or | ||
| External pneumatic compression | ||
| Extensive major surgery in women with cancer or additional risk factors | Unfractionated heparin, 5,000 U tid or | Until hospital discharge |
| LMWH, >3,400 U/day or | ||
| External pneumatic compression | ||
| *For women at particularly high risk (eg, cancer surgery, age >60 years, prior VTE), continue prophylaxis for 2–4 weeks after hospital discharge. | ||
| Modified from Geerts WH, et al20 | ||
Low-dose unfractionated heparin
The most extensively studied prophylactic method is the use of small, subcutaneous doses of heparin. More than 25 controlled trials have shown that, when heparin is given subcutaneously 2 hours before surgery and every 8 to 12 hours afterward, the incidence of DVT diminishes substantially.
The value of low-dose heparin in preventing pulmonary emboli was established by a randomized, controlled, multicenter, international trial, in which fatal postoperative pulmonary emboli declined significantly in general surgery patients given the drug every 8 hours after surgery.5 In gynecologic surgical patients, postoperative DVT also declined significantly.
Increase in minor bleeding complications. Although low-dose heparin is thought to have no measurable effect on coagulation, most large series have noted an increase in minor bleeding complications such as wound hematoma. Up to 10% to 15% of otherwise healthy patients develop transiently prolonged activated partial thromboplastin time (APTT) after 5,000 U of heparin are given subcutaneously.6
Although relatively rare, thrombocytopenia is associated with the use of low-dose heparin. It has been found in 6% of women after gynecologic surgery.6 Therefore, it is reasonable to measure platelets in any patient taking low-dose heparin longer than 4 days to screen for heparin-induced thrombocytopenia.
Fear of major bleeding complications is unsubstantiated. There is ample evidence from placebo-controlled, blinded trials and meta-analysis that the risk of clinically important bleeding does not increase. Moreover, detailed analysis demonstrates that low-dose heparin has a good risk-to-benefit ratio and is cost-effective.
Low-molecular-weight heparins
These drugs are fragments of unfractionated heparin that vary in size from 4,500 to 6,500 daltons. Low-molecular-weight heparin (LMWH) has more anti-Xa and less antithrombin activity than unfractionated heparin and thus has less of an effect on partial thromboplastin time. LMWH may also lead to fewer bleeding complications.7
Once-daily dosing is possible. An increased half-life of 4 hours for LMWH produces greater bioavailability than with low-dose heparin. This allows once-daily dosing.
Pick one: Convenience or cost
Randomized controlled trials have compared LMWH to unfractionated heparin in gynecologic surgical patients. In all studies, DVT occurred in similar, low numbers of women regardless of the heparin used. Bleeding complications also were similar.8
A meta-analysis of general surgery and gynecologic surgery patients from 32 trials likewise found daily LMWH to be as effective as unfractionated heparin in DVT prophylaxis, without any difference in hemorrhagic complications.9
The choice of drugs often boils down to convenience versus cost: Prophylactic LMWH can be given once a day (compared with 2 or 3 times for unfractionated heparin), but is much more expensive.
Mechanical prophylactic methods
External pneumatic compression rivals low-dose heparin. The largest body of literature on mechanical methods to reduce postoperative venous stasis involves intermittent leg compression by pneumatically inflated sleeves placed around the calf or leg during surgery and after. A number of devices and sleeve designs are available, none of which has proven to be superior to the others.
In my experience, calf compression during and after gynecologic surgery lowers the incidence of DVT to a level seen with low-dose heparin. Besides increasing venous flow and pulsatile emptying of the calf veins, pneumatic compression appears to augment endogenous fibrinolysis, which may stimulate lysis of very early thrombi.10
How long is best for external compression? The optimal duration of postoperative external pneumatic compression is unclear. It may be effective when used in the operating room and for the first 24 hours postoperatively in patients with benign conditions who will ambulate on the first day after surgery.11,12
In women undergoing major surgery for gynecologic malignancy, it reduces the incidence of postoperative venous thromboemboli by nearly 3-fold, but only if calf compression is applied intraoperatively and for the first 5 postoperative days.13,14 These women may remain at risk because of stasis and a hypercoagulable state for a longer time than general surgical patients.
External pneumatic leg compression has no serious side effects or risks and is slightly more cost-effective than prophylactic drugs.15 However, to be fully effective, this method must be used consistently, in compliance with the protocol, when the patient is not ambulating.
Stockings can be a help or hazard. Controlled studies of graduated pressure stockings are limited but suggest modest benefit with careful fitting.16 Poorly fitted stockings that roll down the leg may create a tourniquet effect at the knee or mid-thigh. Another disadvantage of the stockings: The limited sizes available do not allow a perfect fit for all patients. This is especially true in obese patients.
The simplicity of elastic stockings and the absence of serious side effects are probably why stockings are often included in routine postoperative care.
Don’t overlook basic precautions. Although they may offer only modest benefit, short preoperative hospital stays and early postoperative ambulation are recommended.
Another basic strategy: elevating the foot of the bed to raise the calf above heart level. This allows gravity to drain the calf veins and should further reduce stasis.
How to detect VTE
DVT has nonspecific signs and symptoms
When DVT occurs in the lower extremities, harbingers such as pain, edema, and erythema are relatively nonspecific; 50% to 80% of patients exhibiting them do not have DVT. Conversely, approximately 80% of patients with symptomatic pulmonary emboli have no signs or symptoms of thrombosis in the lower extremities.
Because of this lack of specificity, additional tests are needed to establish DVT.
Diagnostic studies
A definitive diagnosis of DVT and pulmonary embolism is mandatory because diagnosis based on clinical symptoms and signs alone is frequently wrong. Strategies to reduce the use of ultrasound or spiral CT scanning have been put forward. These studies have evaluated outpatients using algorithms that utilize clinical probability (“clinical decision rule”) and D-dimer levels.
This strategy has been very accurate and avoids the use of ultrasound or spiral CT in low-risk patients. For example, individuals with a low probability score have an incidence of DVT below 5%, so ultrasound is unnecessary. This diagnostic strategy relies on the recognition of elevated D-dimer levels. Unfortunately, D-dimer is increased by a variety of nonthrombotic disorders, including recent surgery, hemorrhage, trauma, pregnancy, and cancer. Therefore, we cannot recommend the use of this strategy for the postoperative gynecologic surgery patient.17,18
Venography no longer the gold standard. Other diagnostic studies may be more useful. Venography has fallen from favor because it is moderately uncomfortable, requires injection of a contrast material that may trigger an allergic reaction or renal injury, and causes phlebitis in approximately 5% of patients.2 Newer, noninvasive diagnostic tests have been developed, fortunately.
Doppler ultrasound. B-mode duplex Doppler imaging is the most common technique to diagnose symptomatic venous thrombosis, especially when it arises in the proximal lower extremity. With duplex Doppler imaging, the femoral vein can be visualized, and clots may be seen directly. Compression of the vein with the tip of the ultrasound probe makes it possible to assess venous collapsibility, which is diminished when a thrombus is present.
Doppler imaging is less accurate when evaluating the calf and pelvic veins.
Magnetic resonance venography (MRV) sensitivity and specificity are comparable to venography. In addition, MRV may detect thrombi in pelvic veins that are not imaged by venography. The primary drawback is the time required to examine the lower extremity and pelvis. Further, MRV rarely identifies calf thrombi (most often not life-threatening, but potentially symptomatic) and is considerably more expensive than ultrasound.
Which prevention strategy works best?
We now consider low-molecular-weight heparin and external pneumatic compression the best choices
Because low-dose unfractionated heparin, low-molecular-weight heparin (LMWH), and external pneumatic compression all reduce the incidence of postoperative venous thromboembolism in high-risk gynecologic surgical patients, the question is: Which strategy is best?
We conducted 2 randomized clinical trials to answer this question.
Trial 1 Low-dose heparin vs pneumatic compression
Women were randomized to receive either low-dose heparin (5,000 U subcutaneously preoperatively and every 8 hours after surgery until hospital discharge) or external pneumatic compression of the calf prior to surgery and until hospital discharge.1
The incidence of DVT was identical in both groups, and no patients developed a pulmonary embolus throughout 30 days of follow-up. However, bleeding complications occurred more often in the group randomized to low-dose heparin. Specifically, nearly 25% had APTT levels in the “therapeutic” range, and significantly more patients required blood transfusions. After this trial, our institution decided to use external pneumatic compression because of its more favorable risk profile.1
Trial 2 LMWH vs pneumatic compression
The question of the best therapy arose again with the advent of LMWH, because of the possibility that these drugs carried a lower risk of bleeding complications. We therefore conducted a second trial to compare LMWH with external pneumatic compression.2
Because higher doses of LMWH had already proven to be more effective in cancer patients, we gave women in the trial 5,000 U dalteparin (Fragmin) preoperatively and 5,000 U daily postoperatively until hospital discharge.
In this trial, external pneumatic compression and LMWH produced similar low frequencies of DVT and no pulmonary emboli throughout 30 days of follow-up. We also found no association between LMWH and bleeding complications or transfusion requirements. Compliance and patient satisfaction were similar for both modalities.2
Bottom line
We now consider LMWH and external pneumatic compression the best choices for prophylaxis in gynecologic surgical patients.
REFERENCES
1. Clarke-Pearson DL, Synan IS, Dodge R, Soper JT, Berchuck A, Coleman RE. A randomized trial of low-dose heparin and intermittent pneumatic calf compression for the prevention of deep venous thrombosis after gynecologic oncology surgery. Am J Obstet Gynecol. 1993;168:1146-1154.
2. Maxwell GL, Synan I, Dodge R, Carroll B, Clarke-Pearson DI. Pneumatic compression versus low molecular weight heparin in gynecologic oncology surgery: a randomized trial. Obstet Gynecol. 2001;98:989-995.
Increasing use of laparoscopic surgery raises an important question: What is the thromboembolic risk of laparoscopy itself? On one hand, many laparoscopic surgeries are prolonged, and intraperitoneal pressure from the pneumoperitoneum reduces venous flow. On the other hand, many patients who have laparoscopy have shorter hospital stays and return sooner to normal activities than those who have open procedures.
Although the risks of venous thromboembolism (VTE) have not been studied as thoroughly as other aspects of laparoscopy, they appear to be low. To date, there are no randomized trials of VTE prophylaxis among women undergoing gynecologic laparoscopy.
The prudent course
Nevertheless, it would seem prudent to consider prophylaxis when women with additional risk factors undergo extensive laparoscopic procedures.
Pulmonary embolism is often stealthy
Many of the typical signs and symptoms of pulmonary embolism are associated with other, more common pulmonary complications following surgery. Classic findings that should alert the physician to the possibility of pulmonary embolism include:
- pleuritic chest pain
- hemoptysis
- shortness of breath
- tachycardia
- tachypnea
Often, however, the signs are subtle and may include only persistent tachycardia or a slight elevation in respiration.
When pulmonary embolism is suspected, a chest x-ray, electrocardiography, and arterial blood gas assessment are warranted. Any abnormality justifies further evaluation by ventilation-perfusion lung scan or a spiral computed tomography scan of the chest. Unfortunately, a high percentage of lung scans are interpreted as “indeterminate.” In such cases, careful clinical evaluation and judgment are needed to determine whether pulmonary arteriography is necessary to document or exclude pulmonary embolism.
Immediate, aggressive therapy is crucial
The treatment of postoperative DVT requires immediate anticoagulant therapy using either unfractionated heparin or LMWH, followed by 6 months of oral anticoagulant therapy with warfarin.
Treatment strategy: Unfractionated heparin
Once VTE is diagnosed, start unfractionated heparin to prevent proximal propagation of the thrombus and allow physiologic thrombolytic pathways to dissolve the clot. After an initial IV bolus of 5,000 U, give the patient a continuous infusion of 30,000 U daily, and adjust the dose to maintain APTT levels at a therapeutic level that is 1.5 to 2.5 times the control value.
Subtherapeutic APTT levels in the first 24 hours mean a risk of recurrent thromboembolism 15 times greater than the risk in patients with appropriate levels. Therefore, aggressive management is warranted to achieve prompt anticoagulation.
Start an oral anticoagulant (warfarin) on the first day of heparin infusion, and monitor the international normalized ratio (INR) daily until a therapeutic level is achieved. The change in the INR after warfarin administration often precedes the anticoagulant effect by about 2 days, during which time low protein C levels are associated with a transient hypercoagulable state. Therefore, it is important to continue the heparin until the INR has been maintained in a therapeutic range for at least 2 days to confirm the proper warfarin dose. Intravenous heparin can be discontinued after 5 days if an adequate INR level has been established.
Alternative strategy: LMWH
A meta-analysis involving more than 1,000 patients from 19 trials suggests that LMWH is more effective, safer, and less costly than unfractionated heparin in preventing recurrent thromboembolism.19 The lower cost derives from the ability to use the drugs in an outpatient setting.
Dosages are unique and weight-adjusted according to each LMWH preparation. Because LMWH has a minimal effect on APTT, serial laboratory monitoring of APTT levels is unnecessary. Nor is monitoring of anti-Xa activity of significant benefit in the dose adjustment of LMWH.
Basic treatment of pulmonary embolism
In most cases, immediate anticoagulant therapy identical to that outlined for DVT is sufficient to prevent repeat thrombosis and embolism and to allow the patient’s endogenous thrombolytic mechanisms to lyse the pulmonary embolus.
Other interventions include:
- Respiratory support, including oxygen, bronchodilators, and intensive care.
- Although massive pulmonary emboli are usually quickly fatal, pulmonary embolectomy has been successful on rare occasions.
- Pulmonary artery catheterization and administration of thrombolytic agents may be important in patients with massive pulmonary embolism.
- Vena cava interruption may be necessary when anticoagulant therapy does not prevent rethrombosis and the formation of emboli from the lower extremities or pelvis. A vena cava umbrella or filter may be inserted percutaneously above the level of the thrombosis and caudad to the renal veins.
Take-home points
- Identify risk factors preoperatively
- VTE prophylaxis is warranted for most gynecologic surgery patients and can reduce the incidence of VTE by at least 60% with appropriate use! Plan prophylaxis in women at moderate, high, and highest risk, and remember that individuals at high and highest risk require more intense prophylaxis to realize a benefit.
- Maintain a high level of suspicion in women with signs and symptoms of DVT or pulmonary embolism in the first postoperative month. It is better to over-evaluate than to miss a potentially fatal complication.
- Treat women with VTE immediately with heparin or LMWH.
1. Jeffcoate TN, Tindall VR. Venous thrombosis and embolism in obstetrics and gynecology. Aust N Z J Obstet Gynecol. 1965;5:119-130.
2. Clarke-Pearson DL, Jelovsek FR, Creasman WT. Thromboembolism complicating surgery for cervical and uterine malignancy: incidence, risk factors, and prophylaxis. Obstet Gynecol. 1983;61:87-94.
3. Clayton JK, Anderson JA, McNicol GP. Preoperative prediction of postoperative deep vein thrombosis. BMJ. 1976;2:910-912.
4. Clarke-Pearson DL, DeLong ER, Synan IS, Coleman RE, Creasman WT. Variables associated with postoperative deep venous thrombosis: a prospective study of 411 gynecology patients and creation of a prognostic model. Obstet Gynecol. 1987;69:146-150.
5. Prevention of fatal postoperative pulmonary embolism by low-dose heparin. An international multicentre trial. Lancet. 1975;2:45-51.
6. Clarke-Pearson DL, DeLong ER, Synan IS, Creasman WT. Complications of low-dose heparin prophylaxis in gynecologic oncology surgery. Obstet Gynecol. 1984;64:689-694.
7. Tapson VF, Hull RD. Management of venous thromboembolic disease. The impact of low-molecular-weight heparin. Clin Chest Med. 1995;16:281-294.
8. Borstad E, Urdal K, Handeland G, Abildgaard U. Comparison of low molecular weight heparin vs. unfractionated heparin in gynecological surgery. II: Reduced dose of low molecular weight heparin. Acta Obstet Gynecol Scand. 1992;71:471-475.
9. Jorgensen LN, Wille-Jorgensen P, Hauch O. Prophylaxis of postoperative thromboembolism with low molecular weight heparins. Br J Surg. 1993;80:689-704.
10. Allenby F, Boardman L, Pflug JJ, Calnan JS. Effects of external pneumatic intermittent compression on fibrinolysis in man. Lancet. 1973;2:1412-1414.
11. Salzman EW, Ploetz J, Bettmann M, Skillman J, Klein L. Intraoperative external pneumatic calf compression to afford long-term prophylaxis against deep vein thrombosis in urological patients. Surgery. 1980;87:239-242.
12. Nicolaides AN, Fernandes e Fernandes J, Pollock AV. Intermittent sequential pneumatic compression of the legs in the prevention of venous stasis and postoperative deep venous thrombosis. Surgery. 1980;87:69-76.
13. Clarke-Pearson DL, Synan IS, Hinshaw WM, Coleman RE, Creasman WT. Prevention of postoperative venous thromboembolism by external pneumatic calf compression in patients with gynecologic malignancy. Obstet Gynecol. 1984;63:92-98.
14. Clarke-Pearson DL, Creasman WT, Coleman RE, Synan IS, Hinshaw WM. Perioperative external pneumatic calf compression as thromboembolism prophylaxis in gynecologic oncology: report of a randomized controlled trial. Gynecol Oncol. 1984;18:226-232.
15. Maxwell GL, Myers ER, Clarke-Pearson DL. Cost-effectiveness of deep venous thrombosis prophylaxis in gynecologic oncology surgery. Obstet Gynecol. 2000;95:206-214.
16. Scurr JH, Ibrahim SZ, Faber RG, Le Quesne LP. The efficacy of graduated compression stockings in the prevention of deep vein thrombosis. Br J Surg. 1977;64:371-373.
17. Wells PS, Owen C, Doucette S, et al. Does this patient have deep vein thrombosis? JAMA. 2006;295:199-207.
18. Writing Group for the Christopher Study Investigators. Effectiveness of managing suspected pulmonary embolism using an algorithm combining clinical D-dimer testing and computed tomography. JAMA. 2006;295:172-179.
19. Buller HR, Kucher N, Kipfmueller F, et al. Antithrombotic therapy for venous thromboembolic disease: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126:401S-428S.
20. Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(suppl):338S-400S.
The author reports no financial relationships relevant to this article.
Pulmonary embolism is a master of disguises. It can appear with classic symptoms such as pleuritic chest pain, hemoptysis, and tachycardia—or it can arrive more insidiously, apparent only as a slight elevation in the respiratory rate.
This matters because 40% of all deaths following gynecologic surgery are directly attributable to pulmonary emboli,1 and pulmonary emboli are the most frequent cause of postoperative death in women with uterine or cervical carcinoma.2
Deep venous thrombosis (DVT) is almost as evasive. We know the signs and symptoms of DVT of the lower extremities—pain, edema, erythema, and a prominent vascular pattern of the superficial veins—but 50% to 80% of patients with these symptoms do not have DVT, and 80% of patients with symptomatic pulmonary embolism have no antecedent signs of thrombosis in the lower extremities.2 Morbidity and expense rise dramatically with DVT, especially when postphlebitic syndrome occurs.
How can we minimize these risks?
A good outcome is most likely when we:
- recognize risk factors,
- provide appropriate perioperative prophylaxis, and
- diagnose and treat venous thromboembolism (VTE) quickly.
This article looks in detail at each of these strategies.
3 factors set the stage for thrombogenesis
- Hypercoagulable state
- Venous stasis
- Vessel endothelial injury
These factors, known as Virchow’s triad, are especially likely at the time of major surgery, or when the patient is advanced in age or has a history of DVT, cancer, lower extremity edema, or venous stasis.
Intraoperative risk factors for postoperative DVT include increased anesthesia time, greater blood loss, and need for transfusion.
Some preventive methods come close to ideal
Being aware of risk factors is vital to provide the appropriate level of prophylaxis (TABLES 1 AND 2).3,4 The first step is identifying high-risk patients and tailoring the regimen to meet their individual needs. The perfect prophylactic method is not yet devised, but would be effective, free of significant side effects, well accepted by the patient and nursing staff, widely applicable to most patient groups, and inexpensive. A number of methods come close.
TABLE 1
Risk factors for thromboembolism
| Major gynecologic surgery |
| Age >40 years |
| Malignancy |
| Previous venous thrombosis (DVT or pulmonary embolism) |
| Obesity |
| Immobility |
| Pregnancy and the postpartum period |
| Oral contraceptives, hormone therapy, or tamoxifen |
| Varicose veins |
| Inherited or acquired thrombophilia (eg, Factor V Leiden) |
| Prolonged surgical procedure |
| Radical vulvectomy, inguinal-femoral lymphadenectomy, or pelvic exenteration |
TABLE 2
Match the preventive strategy to the surgery
| SURGERY | STRATEGY | DURATION OF PROPHYLAXIS* |
|---|---|---|
| Procedures <30 min for benign disease | Prophylaxis not needed | — |
| Laparoscopic gynecologic procedures in women with additional risk factors | Unfractionated heparin, 5,000 bid or | Until hospital discharge |
| LMWH, ≤3,400 U/day or | ||
| External pneumatic compression or | ||
| Graduated compression stockings | ||
| Major surgery for benign disease without additional risk factors | Unfractionated heparin, 5,000 U bid or | Until hospital discharge |
| LMWH, <3,400 U/day or | ||
| External pneumatic compression | ||
| Extensive major surgery in women with cancer or additional risk factors | Unfractionated heparin, 5,000 U tid or | Until hospital discharge |
| LMWH, >3,400 U/day or | ||
| External pneumatic compression | ||
| *For women at particularly high risk (eg, cancer surgery, age >60 years, prior VTE), continue prophylaxis for 2–4 weeks after hospital discharge. | ||
| Modified from Geerts WH, et al20 | ||
Low-dose unfractionated heparin
The most extensively studied prophylactic method is the use of small, subcutaneous doses of heparin. More than 25 controlled trials have shown that, when heparin is given subcutaneously 2 hours before surgery and every 8 to 12 hours afterward, the incidence of DVT diminishes substantially.
The value of low-dose heparin in preventing pulmonary emboli was established by a randomized, controlled, multicenter, international trial, in which fatal postoperative pulmonary emboli declined significantly in general surgery patients given the drug every 8 hours after surgery.5 In gynecologic surgical patients, postoperative DVT also declined significantly.
Increase in minor bleeding complications. Although low-dose heparin is thought to have no measurable effect on coagulation, most large series have noted an increase in minor bleeding complications such as wound hematoma. Up to 10% to 15% of otherwise healthy patients develop transiently prolonged activated partial thromboplastin time (APTT) after 5,000 U of heparin are given subcutaneously.6
Although relatively rare, thrombocytopenia is associated with the use of low-dose heparin. It has been found in 6% of women after gynecologic surgery.6 Therefore, it is reasonable to measure platelets in any patient taking low-dose heparin longer than 4 days to screen for heparin-induced thrombocytopenia.
Fear of major bleeding complications is unsubstantiated. There is ample evidence from placebo-controlled, blinded trials and meta-analysis that the risk of clinically important bleeding does not increase. Moreover, detailed analysis demonstrates that low-dose heparin has a good risk-to-benefit ratio and is cost-effective.
Low-molecular-weight heparins
These drugs are fragments of unfractionated heparin that vary in size from 4,500 to 6,500 daltons. Low-molecular-weight heparin (LMWH) has more anti-Xa and less antithrombin activity than unfractionated heparin and thus has less of an effect on partial thromboplastin time. LMWH may also lead to fewer bleeding complications.7
Once-daily dosing is possible. An increased half-life of 4 hours for LMWH produces greater bioavailability than with low-dose heparin. This allows once-daily dosing.
Pick one: Convenience or cost
Randomized controlled trials have compared LMWH to unfractionated heparin in gynecologic surgical patients. In all studies, DVT occurred in similar, low numbers of women regardless of the heparin used. Bleeding complications also were similar.8
A meta-analysis of general surgery and gynecologic surgery patients from 32 trials likewise found daily LMWH to be as effective as unfractionated heparin in DVT prophylaxis, without any difference in hemorrhagic complications.9
The choice of drugs often boils down to convenience versus cost: Prophylactic LMWH can be given once a day (compared with 2 or 3 times for unfractionated heparin), but is much more expensive.
Mechanical prophylactic methods
External pneumatic compression rivals low-dose heparin. The largest body of literature on mechanical methods to reduce postoperative venous stasis involves intermittent leg compression by pneumatically inflated sleeves placed around the calf or leg during surgery and after. A number of devices and sleeve designs are available, none of which has proven to be superior to the others.
In my experience, calf compression during and after gynecologic surgery lowers the incidence of DVT to a level seen with low-dose heparin. Besides increasing venous flow and pulsatile emptying of the calf veins, pneumatic compression appears to augment endogenous fibrinolysis, which may stimulate lysis of very early thrombi.10
How long is best for external compression? The optimal duration of postoperative external pneumatic compression is unclear. It may be effective when used in the operating room and for the first 24 hours postoperatively in patients with benign conditions who will ambulate on the first day after surgery.11,12
In women undergoing major surgery for gynecologic malignancy, it reduces the incidence of postoperative venous thromboemboli by nearly 3-fold, but only if calf compression is applied intraoperatively and for the first 5 postoperative days.13,14 These women may remain at risk because of stasis and a hypercoagulable state for a longer time than general surgical patients.
External pneumatic leg compression has no serious side effects or risks and is slightly more cost-effective than prophylactic drugs.15 However, to be fully effective, this method must be used consistently, in compliance with the protocol, when the patient is not ambulating.
Stockings can be a help or hazard. Controlled studies of graduated pressure stockings are limited but suggest modest benefit with careful fitting.16 Poorly fitted stockings that roll down the leg may create a tourniquet effect at the knee or mid-thigh. Another disadvantage of the stockings: The limited sizes available do not allow a perfect fit for all patients. This is especially true in obese patients.
The simplicity of elastic stockings and the absence of serious side effects are probably why stockings are often included in routine postoperative care.
Don’t overlook basic precautions. Although they may offer only modest benefit, short preoperative hospital stays and early postoperative ambulation are recommended.
Another basic strategy: elevating the foot of the bed to raise the calf above heart level. This allows gravity to drain the calf veins and should further reduce stasis.
How to detect VTE
DVT has nonspecific signs and symptoms
When DVT occurs in the lower extremities, harbingers such as pain, edema, and erythema are relatively nonspecific; 50% to 80% of patients exhibiting them do not have DVT. Conversely, approximately 80% of patients with symptomatic pulmonary emboli have no signs or symptoms of thrombosis in the lower extremities.
Because of this lack of specificity, additional tests are needed to establish DVT.
Diagnostic studies
A definitive diagnosis of DVT and pulmonary embolism is mandatory because diagnosis based on clinical symptoms and signs alone is frequently wrong. Strategies to reduce the use of ultrasound or spiral CT scanning have been put forward. These studies have evaluated outpatients using algorithms that utilize clinical probability (“clinical decision rule”) and D-dimer levels.
This strategy has been very accurate and avoids the use of ultrasound or spiral CT in low-risk patients. For example, individuals with a low probability score have an incidence of DVT below 5%, so ultrasound is unnecessary. This diagnostic strategy relies on the recognition of elevated D-dimer levels. Unfortunately, D-dimer is increased by a variety of nonthrombotic disorders, including recent surgery, hemorrhage, trauma, pregnancy, and cancer. Therefore, we cannot recommend the use of this strategy for the postoperative gynecologic surgery patient.17,18
Venography no longer the gold standard. Other diagnostic studies may be more useful. Venography has fallen from favor because it is moderately uncomfortable, requires injection of a contrast material that may trigger an allergic reaction or renal injury, and causes phlebitis in approximately 5% of patients.2 Newer, noninvasive diagnostic tests have been developed, fortunately.
Doppler ultrasound. B-mode duplex Doppler imaging is the most common technique to diagnose symptomatic venous thrombosis, especially when it arises in the proximal lower extremity. With duplex Doppler imaging, the femoral vein can be visualized, and clots may be seen directly. Compression of the vein with the tip of the ultrasound probe makes it possible to assess venous collapsibility, which is diminished when a thrombus is present.
Doppler imaging is less accurate when evaluating the calf and pelvic veins.
Magnetic resonance venography (MRV) sensitivity and specificity are comparable to venography. In addition, MRV may detect thrombi in pelvic veins that are not imaged by venography. The primary drawback is the time required to examine the lower extremity and pelvis. Further, MRV rarely identifies calf thrombi (most often not life-threatening, but potentially symptomatic) and is considerably more expensive than ultrasound.
Which prevention strategy works best?
We now consider low-molecular-weight heparin and external pneumatic compression the best choices
Because low-dose unfractionated heparin, low-molecular-weight heparin (LMWH), and external pneumatic compression all reduce the incidence of postoperative venous thromboembolism in high-risk gynecologic surgical patients, the question is: Which strategy is best?
We conducted 2 randomized clinical trials to answer this question.
Trial 1 Low-dose heparin vs pneumatic compression
Women were randomized to receive either low-dose heparin (5,000 U subcutaneously preoperatively and every 8 hours after surgery until hospital discharge) or external pneumatic compression of the calf prior to surgery and until hospital discharge.1
The incidence of DVT was identical in both groups, and no patients developed a pulmonary embolus throughout 30 days of follow-up. However, bleeding complications occurred more often in the group randomized to low-dose heparin. Specifically, nearly 25% had APTT levels in the “therapeutic” range, and significantly more patients required blood transfusions. After this trial, our institution decided to use external pneumatic compression because of its more favorable risk profile.1
Trial 2 LMWH vs pneumatic compression
The question of the best therapy arose again with the advent of LMWH, because of the possibility that these drugs carried a lower risk of bleeding complications. We therefore conducted a second trial to compare LMWH with external pneumatic compression.2
Because higher doses of LMWH had already proven to be more effective in cancer patients, we gave women in the trial 5,000 U dalteparin (Fragmin) preoperatively and 5,000 U daily postoperatively until hospital discharge.
In this trial, external pneumatic compression and LMWH produced similar low frequencies of DVT and no pulmonary emboli throughout 30 days of follow-up. We also found no association between LMWH and bleeding complications or transfusion requirements. Compliance and patient satisfaction were similar for both modalities.2
Bottom line
We now consider LMWH and external pneumatic compression the best choices for prophylaxis in gynecologic surgical patients.
REFERENCES
1. Clarke-Pearson DL, Synan IS, Dodge R, Soper JT, Berchuck A, Coleman RE. A randomized trial of low-dose heparin and intermittent pneumatic calf compression for the prevention of deep venous thrombosis after gynecologic oncology surgery. Am J Obstet Gynecol. 1993;168:1146-1154.
2. Maxwell GL, Synan I, Dodge R, Carroll B, Clarke-Pearson DI. Pneumatic compression versus low molecular weight heparin in gynecologic oncology surgery: a randomized trial. Obstet Gynecol. 2001;98:989-995.
Increasing use of laparoscopic surgery raises an important question: What is the thromboembolic risk of laparoscopy itself? On one hand, many laparoscopic surgeries are prolonged, and intraperitoneal pressure from the pneumoperitoneum reduces venous flow. On the other hand, many patients who have laparoscopy have shorter hospital stays and return sooner to normal activities than those who have open procedures.
Although the risks of venous thromboembolism (VTE) have not been studied as thoroughly as other aspects of laparoscopy, they appear to be low. To date, there are no randomized trials of VTE prophylaxis among women undergoing gynecologic laparoscopy.
The prudent course
Nevertheless, it would seem prudent to consider prophylaxis when women with additional risk factors undergo extensive laparoscopic procedures.
Pulmonary embolism is often stealthy
Many of the typical signs and symptoms of pulmonary embolism are associated with other, more common pulmonary complications following surgery. Classic findings that should alert the physician to the possibility of pulmonary embolism include:
- pleuritic chest pain
- hemoptysis
- shortness of breath
- tachycardia
- tachypnea
Often, however, the signs are subtle and may include only persistent tachycardia or a slight elevation in respiration.
When pulmonary embolism is suspected, a chest x-ray, electrocardiography, and arterial blood gas assessment are warranted. Any abnormality justifies further evaluation by ventilation-perfusion lung scan or a spiral computed tomography scan of the chest. Unfortunately, a high percentage of lung scans are interpreted as “indeterminate.” In such cases, careful clinical evaluation and judgment are needed to determine whether pulmonary arteriography is necessary to document or exclude pulmonary embolism.
Immediate, aggressive therapy is crucial
The treatment of postoperative DVT requires immediate anticoagulant therapy using either unfractionated heparin or LMWH, followed by 6 months of oral anticoagulant therapy with warfarin.
Treatment strategy: Unfractionated heparin
Once VTE is diagnosed, start unfractionated heparin to prevent proximal propagation of the thrombus and allow physiologic thrombolytic pathways to dissolve the clot. After an initial IV bolus of 5,000 U, give the patient a continuous infusion of 30,000 U daily, and adjust the dose to maintain APTT levels at a therapeutic level that is 1.5 to 2.5 times the control value.
Subtherapeutic APTT levels in the first 24 hours mean a risk of recurrent thromboembolism 15 times greater than the risk in patients with appropriate levels. Therefore, aggressive management is warranted to achieve prompt anticoagulation.
Start an oral anticoagulant (warfarin) on the first day of heparin infusion, and monitor the international normalized ratio (INR) daily until a therapeutic level is achieved. The change in the INR after warfarin administration often precedes the anticoagulant effect by about 2 days, during which time low protein C levels are associated with a transient hypercoagulable state. Therefore, it is important to continue the heparin until the INR has been maintained in a therapeutic range for at least 2 days to confirm the proper warfarin dose. Intravenous heparin can be discontinued after 5 days if an adequate INR level has been established.
Alternative strategy: LMWH
A meta-analysis involving more than 1,000 patients from 19 trials suggests that LMWH is more effective, safer, and less costly than unfractionated heparin in preventing recurrent thromboembolism.19 The lower cost derives from the ability to use the drugs in an outpatient setting.
Dosages are unique and weight-adjusted according to each LMWH preparation. Because LMWH has a minimal effect on APTT, serial laboratory monitoring of APTT levels is unnecessary. Nor is monitoring of anti-Xa activity of significant benefit in the dose adjustment of LMWH.
Basic treatment of pulmonary embolism
In most cases, immediate anticoagulant therapy identical to that outlined for DVT is sufficient to prevent repeat thrombosis and embolism and to allow the patient’s endogenous thrombolytic mechanisms to lyse the pulmonary embolus.
Other interventions include:
- Respiratory support, including oxygen, bronchodilators, and intensive care.
- Although massive pulmonary emboli are usually quickly fatal, pulmonary embolectomy has been successful on rare occasions.
- Pulmonary artery catheterization and administration of thrombolytic agents may be important in patients with massive pulmonary embolism.
- Vena cava interruption may be necessary when anticoagulant therapy does not prevent rethrombosis and the formation of emboli from the lower extremities or pelvis. A vena cava umbrella or filter may be inserted percutaneously above the level of the thrombosis and caudad to the renal veins.
Take-home points
- Identify risk factors preoperatively
- VTE prophylaxis is warranted for most gynecologic surgery patients and can reduce the incidence of VTE by at least 60% with appropriate use! Plan prophylaxis in women at moderate, high, and highest risk, and remember that individuals at high and highest risk require more intense prophylaxis to realize a benefit.
- Maintain a high level of suspicion in women with signs and symptoms of DVT or pulmonary embolism in the first postoperative month. It is better to over-evaluate than to miss a potentially fatal complication.
- Treat women with VTE immediately with heparin or LMWH.
Pulmonary embolism is a master of disguises. It can appear with classic symptoms such as pleuritic chest pain, hemoptysis, and tachycardia—or it can arrive more insidiously, apparent only as a slight elevation in the respiratory rate.
This matters because 40% of all deaths following gynecologic surgery are directly attributable to pulmonary emboli,1 and pulmonary emboli are the most frequent cause of postoperative death in women with uterine or cervical carcinoma.2
Deep venous thrombosis (DVT) is almost as evasive. We know the signs and symptoms of DVT of the lower extremities—pain, edema, erythema, and a prominent vascular pattern of the superficial veins—but 50% to 80% of patients with these symptoms do not have DVT, and 80% of patients with symptomatic pulmonary embolism have no antecedent signs of thrombosis in the lower extremities.2 Morbidity and expense rise dramatically with DVT, especially when postphlebitic syndrome occurs.
How can we minimize these risks?
A good outcome is most likely when we:
- recognize risk factors,
- provide appropriate perioperative prophylaxis, and
- diagnose and treat venous thromboembolism (VTE) quickly.
This article looks in detail at each of these strategies.
3 factors set the stage for thrombogenesis
- Hypercoagulable state
- Venous stasis
- Vessel endothelial injury
These factors, known as Virchow’s triad, are especially likely at the time of major surgery, or when the patient is advanced in age or has a history of DVT, cancer, lower extremity edema, or venous stasis.
Intraoperative risk factors for postoperative DVT include increased anesthesia time, greater blood loss, and need for transfusion.
Some preventive methods come close to ideal
Being aware of risk factors is vital to provide the appropriate level of prophylaxis (TABLES 1 AND 2).3,4 The first step is identifying high-risk patients and tailoring the regimen to meet their individual needs. The perfect prophylactic method is not yet devised, but would be effective, free of significant side effects, well accepted by the patient and nursing staff, widely applicable to most patient groups, and inexpensive. A number of methods come close.
TABLE 1
Risk factors for thromboembolism
| Major gynecologic surgery |
| Age >40 years |
| Malignancy |
| Previous venous thrombosis (DVT or pulmonary embolism) |
| Obesity |
| Immobility |
| Pregnancy and the postpartum period |
| Oral contraceptives, hormone therapy, or tamoxifen |
| Varicose veins |
| Inherited or acquired thrombophilia (eg, Factor V Leiden) |
| Prolonged surgical procedure |
| Radical vulvectomy, inguinal-femoral lymphadenectomy, or pelvic exenteration |
TABLE 2
Match the preventive strategy to the surgery
| SURGERY | STRATEGY | DURATION OF PROPHYLAXIS* |
|---|---|---|
| Procedures <30 min for benign disease | Prophylaxis not needed | — |
| Laparoscopic gynecologic procedures in women with additional risk factors | Unfractionated heparin, 5,000 bid or | Until hospital discharge |
| LMWH, ≤3,400 U/day or | ||
| External pneumatic compression or | ||
| Graduated compression stockings | ||
| Major surgery for benign disease without additional risk factors | Unfractionated heparin, 5,000 U bid or | Until hospital discharge |
| LMWH, <3,400 U/day or | ||
| External pneumatic compression | ||
| Extensive major surgery in women with cancer or additional risk factors | Unfractionated heparin, 5,000 U tid or | Until hospital discharge |
| LMWH, >3,400 U/day or | ||
| External pneumatic compression | ||
| *For women at particularly high risk (eg, cancer surgery, age >60 years, prior VTE), continue prophylaxis for 2–4 weeks after hospital discharge. | ||
| Modified from Geerts WH, et al20 | ||
Low-dose unfractionated heparin
The most extensively studied prophylactic method is the use of small, subcutaneous doses of heparin. More than 25 controlled trials have shown that, when heparin is given subcutaneously 2 hours before surgery and every 8 to 12 hours afterward, the incidence of DVT diminishes substantially.
The value of low-dose heparin in preventing pulmonary emboli was established by a randomized, controlled, multicenter, international trial, in which fatal postoperative pulmonary emboli declined significantly in general surgery patients given the drug every 8 hours after surgery.5 In gynecologic surgical patients, postoperative DVT also declined significantly.
Increase in minor bleeding complications. Although low-dose heparin is thought to have no measurable effect on coagulation, most large series have noted an increase in minor bleeding complications such as wound hematoma. Up to 10% to 15% of otherwise healthy patients develop transiently prolonged activated partial thromboplastin time (APTT) after 5,000 U of heparin are given subcutaneously.6
Although relatively rare, thrombocytopenia is associated with the use of low-dose heparin. It has been found in 6% of women after gynecologic surgery.6 Therefore, it is reasonable to measure platelets in any patient taking low-dose heparin longer than 4 days to screen for heparin-induced thrombocytopenia.
Fear of major bleeding complications is unsubstantiated. There is ample evidence from placebo-controlled, blinded trials and meta-analysis that the risk of clinically important bleeding does not increase. Moreover, detailed analysis demonstrates that low-dose heparin has a good risk-to-benefit ratio and is cost-effective.
Low-molecular-weight heparins
These drugs are fragments of unfractionated heparin that vary in size from 4,500 to 6,500 daltons. Low-molecular-weight heparin (LMWH) has more anti-Xa and less antithrombin activity than unfractionated heparin and thus has less of an effect on partial thromboplastin time. LMWH may also lead to fewer bleeding complications.7
Once-daily dosing is possible. An increased half-life of 4 hours for LMWH produces greater bioavailability than with low-dose heparin. This allows once-daily dosing.
Pick one: Convenience or cost
Randomized controlled trials have compared LMWH to unfractionated heparin in gynecologic surgical patients. In all studies, DVT occurred in similar, low numbers of women regardless of the heparin used. Bleeding complications also were similar.8
A meta-analysis of general surgery and gynecologic surgery patients from 32 trials likewise found daily LMWH to be as effective as unfractionated heparin in DVT prophylaxis, without any difference in hemorrhagic complications.9
The choice of drugs often boils down to convenience versus cost: Prophylactic LMWH can be given once a day (compared with 2 or 3 times for unfractionated heparin), but is much more expensive.
Mechanical prophylactic methods
External pneumatic compression rivals low-dose heparin. The largest body of literature on mechanical methods to reduce postoperative venous stasis involves intermittent leg compression by pneumatically inflated sleeves placed around the calf or leg during surgery and after. A number of devices and sleeve designs are available, none of which has proven to be superior to the others.
In my experience, calf compression during and after gynecologic surgery lowers the incidence of DVT to a level seen with low-dose heparin. Besides increasing venous flow and pulsatile emptying of the calf veins, pneumatic compression appears to augment endogenous fibrinolysis, which may stimulate lysis of very early thrombi.10
How long is best for external compression? The optimal duration of postoperative external pneumatic compression is unclear. It may be effective when used in the operating room and for the first 24 hours postoperatively in patients with benign conditions who will ambulate on the first day after surgery.11,12
In women undergoing major surgery for gynecologic malignancy, it reduces the incidence of postoperative venous thromboemboli by nearly 3-fold, but only if calf compression is applied intraoperatively and for the first 5 postoperative days.13,14 These women may remain at risk because of stasis and a hypercoagulable state for a longer time than general surgical patients.
External pneumatic leg compression has no serious side effects or risks and is slightly more cost-effective than prophylactic drugs.15 However, to be fully effective, this method must be used consistently, in compliance with the protocol, when the patient is not ambulating.
Stockings can be a help or hazard. Controlled studies of graduated pressure stockings are limited but suggest modest benefit with careful fitting.16 Poorly fitted stockings that roll down the leg may create a tourniquet effect at the knee or mid-thigh. Another disadvantage of the stockings: The limited sizes available do not allow a perfect fit for all patients. This is especially true in obese patients.
The simplicity of elastic stockings and the absence of serious side effects are probably why stockings are often included in routine postoperative care.
Don’t overlook basic precautions. Although they may offer only modest benefit, short preoperative hospital stays and early postoperative ambulation are recommended.
Another basic strategy: elevating the foot of the bed to raise the calf above heart level. This allows gravity to drain the calf veins and should further reduce stasis.
How to detect VTE
DVT has nonspecific signs and symptoms
When DVT occurs in the lower extremities, harbingers such as pain, edema, and erythema are relatively nonspecific; 50% to 80% of patients exhibiting them do not have DVT. Conversely, approximately 80% of patients with symptomatic pulmonary emboli have no signs or symptoms of thrombosis in the lower extremities.
Because of this lack of specificity, additional tests are needed to establish DVT.
Diagnostic studies
A definitive diagnosis of DVT and pulmonary embolism is mandatory because diagnosis based on clinical symptoms and signs alone is frequently wrong. Strategies to reduce the use of ultrasound or spiral CT scanning have been put forward. These studies have evaluated outpatients using algorithms that utilize clinical probability (“clinical decision rule”) and D-dimer levels.
This strategy has been very accurate and avoids the use of ultrasound or spiral CT in low-risk patients. For example, individuals with a low probability score have an incidence of DVT below 5%, so ultrasound is unnecessary. This diagnostic strategy relies on the recognition of elevated D-dimer levels. Unfortunately, D-dimer is increased by a variety of nonthrombotic disorders, including recent surgery, hemorrhage, trauma, pregnancy, and cancer. Therefore, we cannot recommend the use of this strategy for the postoperative gynecologic surgery patient.17,18
Venography no longer the gold standard. Other diagnostic studies may be more useful. Venography has fallen from favor because it is moderately uncomfortable, requires injection of a contrast material that may trigger an allergic reaction or renal injury, and causes phlebitis in approximately 5% of patients.2 Newer, noninvasive diagnostic tests have been developed, fortunately.
Doppler ultrasound. B-mode duplex Doppler imaging is the most common technique to diagnose symptomatic venous thrombosis, especially when it arises in the proximal lower extremity. With duplex Doppler imaging, the femoral vein can be visualized, and clots may be seen directly. Compression of the vein with the tip of the ultrasound probe makes it possible to assess venous collapsibility, which is diminished when a thrombus is present.
Doppler imaging is less accurate when evaluating the calf and pelvic veins.
Magnetic resonance venography (MRV) sensitivity and specificity are comparable to venography. In addition, MRV may detect thrombi in pelvic veins that are not imaged by venography. The primary drawback is the time required to examine the lower extremity and pelvis. Further, MRV rarely identifies calf thrombi (most often not life-threatening, but potentially symptomatic) and is considerably more expensive than ultrasound.
Which prevention strategy works best?
We now consider low-molecular-weight heparin and external pneumatic compression the best choices
Because low-dose unfractionated heparin, low-molecular-weight heparin (LMWH), and external pneumatic compression all reduce the incidence of postoperative venous thromboembolism in high-risk gynecologic surgical patients, the question is: Which strategy is best?
We conducted 2 randomized clinical trials to answer this question.
Trial 1 Low-dose heparin vs pneumatic compression
Women were randomized to receive either low-dose heparin (5,000 U subcutaneously preoperatively and every 8 hours after surgery until hospital discharge) or external pneumatic compression of the calf prior to surgery and until hospital discharge.1
The incidence of DVT was identical in both groups, and no patients developed a pulmonary embolus throughout 30 days of follow-up. However, bleeding complications occurred more often in the group randomized to low-dose heparin. Specifically, nearly 25% had APTT levels in the “therapeutic” range, and significantly more patients required blood transfusions. After this trial, our institution decided to use external pneumatic compression because of its more favorable risk profile.1
Trial 2 LMWH vs pneumatic compression
The question of the best therapy arose again with the advent of LMWH, because of the possibility that these drugs carried a lower risk of bleeding complications. We therefore conducted a second trial to compare LMWH with external pneumatic compression.2
Because higher doses of LMWH had already proven to be more effective in cancer patients, we gave women in the trial 5,000 U dalteparin (Fragmin) preoperatively and 5,000 U daily postoperatively until hospital discharge.
In this trial, external pneumatic compression and LMWH produced similar low frequencies of DVT and no pulmonary emboli throughout 30 days of follow-up. We also found no association between LMWH and bleeding complications or transfusion requirements. Compliance and patient satisfaction were similar for both modalities.2
Bottom line
We now consider LMWH and external pneumatic compression the best choices for prophylaxis in gynecologic surgical patients.
REFERENCES
1. Clarke-Pearson DL, Synan IS, Dodge R, Soper JT, Berchuck A, Coleman RE. A randomized trial of low-dose heparin and intermittent pneumatic calf compression for the prevention of deep venous thrombosis after gynecologic oncology surgery. Am J Obstet Gynecol. 1993;168:1146-1154.
2. Maxwell GL, Synan I, Dodge R, Carroll B, Clarke-Pearson DI. Pneumatic compression versus low molecular weight heparin in gynecologic oncology surgery: a randomized trial. Obstet Gynecol. 2001;98:989-995.
Increasing use of laparoscopic surgery raises an important question: What is the thromboembolic risk of laparoscopy itself? On one hand, many laparoscopic surgeries are prolonged, and intraperitoneal pressure from the pneumoperitoneum reduces venous flow. On the other hand, many patients who have laparoscopy have shorter hospital stays and return sooner to normal activities than those who have open procedures.
Although the risks of venous thromboembolism (VTE) have not been studied as thoroughly as other aspects of laparoscopy, they appear to be low. To date, there are no randomized trials of VTE prophylaxis among women undergoing gynecologic laparoscopy.
The prudent course
Nevertheless, it would seem prudent to consider prophylaxis when women with additional risk factors undergo extensive laparoscopic procedures.
Pulmonary embolism is often stealthy
Many of the typical signs and symptoms of pulmonary embolism are associated with other, more common pulmonary complications following surgery. Classic findings that should alert the physician to the possibility of pulmonary embolism include:
- pleuritic chest pain
- hemoptysis
- shortness of breath
- tachycardia
- tachypnea
Often, however, the signs are subtle and may include only persistent tachycardia or a slight elevation in respiration.
When pulmonary embolism is suspected, a chest x-ray, electrocardiography, and arterial blood gas assessment are warranted. Any abnormality justifies further evaluation by ventilation-perfusion lung scan or a spiral computed tomography scan of the chest. Unfortunately, a high percentage of lung scans are interpreted as “indeterminate.” In such cases, careful clinical evaluation and judgment are needed to determine whether pulmonary arteriography is necessary to document or exclude pulmonary embolism.
Immediate, aggressive therapy is crucial
The treatment of postoperative DVT requires immediate anticoagulant therapy using either unfractionated heparin or LMWH, followed by 6 months of oral anticoagulant therapy with warfarin.
Treatment strategy: Unfractionated heparin
Once VTE is diagnosed, start unfractionated heparin to prevent proximal propagation of the thrombus and allow physiologic thrombolytic pathways to dissolve the clot. After an initial IV bolus of 5,000 U, give the patient a continuous infusion of 30,000 U daily, and adjust the dose to maintain APTT levels at a therapeutic level that is 1.5 to 2.5 times the control value.
Subtherapeutic APTT levels in the first 24 hours mean a risk of recurrent thromboembolism 15 times greater than the risk in patients with appropriate levels. Therefore, aggressive management is warranted to achieve prompt anticoagulation.
Start an oral anticoagulant (warfarin) on the first day of heparin infusion, and monitor the international normalized ratio (INR) daily until a therapeutic level is achieved. The change in the INR after warfarin administration often precedes the anticoagulant effect by about 2 days, during which time low protein C levels are associated with a transient hypercoagulable state. Therefore, it is important to continue the heparin until the INR has been maintained in a therapeutic range for at least 2 days to confirm the proper warfarin dose. Intravenous heparin can be discontinued after 5 days if an adequate INR level has been established.
Alternative strategy: LMWH
A meta-analysis involving more than 1,000 patients from 19 trials suggests that LMWH is more effective, safer, and less costly than unfractionated heparin in preventing recurrent thromboembolism.19 The lower cost derives from the ability to use the drugs in an outpatient setting.
Dosages are unique and weight-adjusted according to each LMWH preparation. Because LMWH has a minimal effect on APTT, serial laboratory monitoring of APTT levels is unnecessary. Nor is monitoring of anti-Xa activity of significant benefit in the dose adjustment of LMWH.
Basic treatment of pulmonary embolism
In most cases, immediate anticoagulant therapy identical to that outlined for DVT is sufficient to prevent repeat thrombosis and embolism and to allow the patient’s endogenous thrombolytic mechanisms to lyse the pulmonary embolus.
Other interventions include:
- Respiratory support, including oxygen, bronchodilators, and intensive care.
- Although massive pulmonary emboli are usually quickly fatal, pulmonary embolectomy has been successful on rare occasions.
- Pulmonary artery catheterization and administration of thrombolytic agents may be important in patients with massive pulmonary embolism.
- Vena cava interruption may be necessary when anticoagulant therapy does not prevent rethrombosis and the formation of emboli from the lower extremities or pelvis. A vena cava umbrella or filter may be inserted percutaneously above the level of the thrombosis and caudad to the renal veins.
Take-home points
- Identify risk factors preoperatively
- VTE prophylaxis is warranted for most gynecologic surgery patients and can reduce the incidence of VTE by at least 60% with appropriate use! Plan prophylaxis in women at moderate, high, and highest risk, and remember that individuals at high and highest risk require more intense prophylaxis to realize a benefit.
- Maintain a high level of suspicion in women with signs and symptoms of DVT or pulmonary embolism in the first postoperative month. It is better to over-evaluate than to miss a potentially fatal complication.
- Treat women with VTE immediately with heparin or LMWH.
1. Jeffcoate TN, Tindall VR. Venous thrombosis and embolism in obstetrics and gynecology. Aust N Z J Obstet Gynecol. 1965;5:119-130.
2. Clarke-Pearson DL, Jelovsek FR, Creasman WT. Thromboembolism complicating surgery for cervical and uterine malignancy: incidence, risk factors, and prophylaxis. Obstet Gynecol. 1983;61:87-94.
3. Clayton JK, Anderson JA, McNicol GP. Preoperative prediction of postoperative deep vein thrombosis. BMJ. 1976;2:910-912.
4. Clarke-Pearson DL, DeLong ER, Synan IS, Coleman RE, Creasman WT. Variables associated with postoperative deep venous thrombosis: a prospective study of 411 gynecology patients and creation of a prognostic model. Obstet Gynecol. 1987;69:146-150.
5. Prevention of fatal postoperative pulmonary embolism by low-dose heparin. An international multicentre trial. Lancet. 1975;2:45-51.
6. Clarke-Pearson DL, DeLong ER, Synan IS, Creasman WT. Complications of low-dose heparin prophylaxis in gynecologic oncology surgery. Obstet Gynecol. 1984;64:689-694.
7. Tapson VF, Hull RD. Management of venous thromboembolic disease. The impact of low-molecular-weight heparin. Clin Chest Med. 1995;16:281-294.
8. Borstad E, Urdal K, Handeland G, Abildgaard U. Comparison of low molecular weight heparin vs. unfractionated heparin in gynecological surgery. II: Reduced dose of low molecular weight heparin. Acta Obstet Gynecol Scand. 1992;71:471-475.
9. Jorgensen LN, Wille-Jorgensen P, Hauch O. Prophylaxis of postoperative thromboembolism with low molecular weight heparins. Br J Surg. 1993;80:689-704.
10. Allenby F, Boardman L, Pflug JJ, Calnan JS. Effects of external pneumatic intermittent compression on fibrinolysis in man. Lancet. 1973;2:1412-1414.
11. Salzman EW, Ploetz J, Bettmann M, Skillman J, Klein L. Intraoperative external pneumatic calf compression to afford long-term prophylaxis against deep vein thrombosis in urological patients. Surgery. 1980;87:239-242.
12. Nicolaides AN, Fernandes e Fernandes J, Pollock AV. Intermittent sequential pneumatic compression of the legs in the prevention of venous stasis and postoperative deep venous thrombosis. Surgery. 1980;87:69-76.
13. Clarke-Pearson DL, Synan IS, Hinshaw WM, Coleman RE, Creasman WT. Prevention of postoperative venous thromboembolism by external pneumatic calf compression in patients with gynecologic malignancy. Obstet Gynecol. 1984;63:92-98.
14. Clarke-Pearson DL, Creasman WT, Coleman RE, Synan IS, Hinshaw WM. Perioperative external pneumatic calf compression as thromboembolism prophylaxis in gynecologic oncology: report of a randomized controlled trial. Gynecol Oncol. 1984;18:226-232.
15. Maxwell GL, Myers ER, Clarke-Pearson DL. Cost-effectiveness of deep venous thrombosis prophylaxis in gynecologic oncology surgery. Obstet Gynecol. 2000;95:206-214.
16. Scurr JH, Ibrahim SZ, Faber RG, Le Quesne LP. The efficacy of graduated compression stockings in the prevention of deep vein thrombosis. Br J Surg. 1977;64:371-373.
17. Wells PS, Owen C, Doucette S, et al. Does this patient have deep vein thrombosis? JAMA. 2006;295:199-207.
18. Writing Group for the Christopher Study Investigators. Effectiveness of managing suspected pulmonary embolism using an algorithm combining clinical D-dimer testing and computed tomography. JAMA. 2006;295:172-179.
19. Buller HR, Kucher N, Kipfmueller F, et al. Antithrombotic therapy for venous thromboembolic disease: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126:401S-428S.
20. Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(suppl):338S-400S.
The author reports no financial relationships relevant to this article.
1. Jeffcoate TN, Tindall VR. Venous thrombosis and embolism in obstetrics and gynecology. Aust N Z J Obstet Gynecol. 1965;5:119-130.
2. Clarke-Pearson DL, Jelovsek FR, Creasman WT. Thromboembolism complicating surgery for cervical and uterine malignancy: incidence, risk factors, and prophylaxis. Obstet Gynecol. 1983;61:87-94.
3. Clayton JK, Anderson JA, McNicol GP. Preoperative prediction of postoperative deep vein thrombosis. BMJ. 1976;2:910-912.
4. Clarke-Pearson DL, DeLong ER, Synan IS, Coleman RE, Creasman WT. Variables associated with postoperative deep venous thrombosis: a prospective study of 411 gynecology patients and creation of a prognostic model. Obstet Gynecol. 1987;69:146-150.
5. Prevention of fatal postoperative pulmonary embolism by low-dose heparin. An international multicentre trial. Lancet. 1975;2:45-51.
6. Clarke-Pearson DL, DeLong ER, Synan IS, Creasman WT. Complications of low-dose heparin prophylaxis in gynecologic oncology surgery. Obstet Gynecol. 1984;64:689-694.
7. Tapson VF, Hull RD. Management of venous thromboembolic disease. The impact of low-molecular-weight heparin. Clin Chest Med. 1995;16:281-294.
8. Borstad E, Urdal K, Handeland G, Abildgaard U. Comparison of low molecular weight heparin vs. unfractionated heparin in gynecological surgery. II: Reduced dose of low molecular weight heparin. Acta Obstet Gynecol Scand. 1992;71:471-475.
9. Jorgensen LN, Wille-Jorgensen P, Hauch O. Prophylaxis of postoperative thromboembolism with low molecular weight heparins. Br J Surg. 1993;80:689-704.
10. Allenby F, Boardman L, Pflug JJ, Calnan JS. Effects of external pneumatic intermittent compression on fibrinolysis in man. Lancet. 1973;2:1412-1414.
11. Salzman EW, Ploetz J, Bettmann M, Skillman J, Klein L. Intraoperative external pneumatic calf compression to afford long-term prophylaxis against deep vein thrombosis in urological patients. Surgery. 1980;87:239-242.
12. Nicolaides AN, Fernandes e Fernandes J, Pollock AV. Intermittent sequential pneumatic compression of the legs in the prevention of venous stasis and postoperative deep venous thrombosis. Surgery. 1980;87:69-76.
13. Clarke-Pearson DL, Synan IS, Hinshaw WM, Coleman RE, Creasman WT. Prevention of postoperative venous thromboembolism by external pneumatic calf compression in patients with gynecologic malignancy. Obstet Gynecol. 1984;63:92-98.
14. Clarke-Pearson DL, Creasman WT, Coleman RE, Synan IS, Hinshaw WM. Perioperative external pneumatic calf compression as thromboembolism prophylaxis in gynecologic oncology: report of a randomized controlled trial. Gynecol Oncol. 1984;18:226-232.
15. Maxwell GL, Myers ER, Clarke-Pearson DL. Cost-effectiveness of deep venous thrombosis prophylaxis in gynecologic oncology surgery. Obstet Gynecol. 2000;95:206-214.
16. Scurr JH, Ibrahim SZ, Faber RG, Le Quesne LP. The efficacy of graduated compression stockings in the prevention of deep vein thrombosis. Br J Surg. 1977;64:371-373.
17. Wells PS, Owen C, Doucette S, et al. Does this patient have deep vein thrombosis? JAMA. 2006;295:199-207.
18. Writing Group for the Christopher Study Investigators. Effectiveness of managing suspected pulmonary embolism using an algorithm combining clinical D-dimer testing and computed tomography. JAMA. 2006;295:172-179.
19. Buller HR, Kucher N, Kipfmueller F, et al. Antithrombotic therapy for venous thromboembolic disease: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126:401S-428S.
20. Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(suppl):338S-400S.
The author reports no financial relationships relevant to this article.