User login
The Primary Care Chronic Pain Program (PC-CPP) of the Women’s Primary Care Clinics at the VA Salt Lake City Health Care System (VASLCHCS) in Utah was the first VA primary care clinical service to incorporate patient participation in obtaining chronic opioid medications in the treatment of chronic noncancer pain. In addition, the program used a multimodality approach for chronic pain treatment and veteran education about the relationship between physical and mental health issues.
Treatment Complexity
Chronic, noncancer pain is a complex issue in the primary care setting. Diagnosis is difficult, patient education is time consuming, goals and expectations are often unclear, and the experience can be unsatisfying for the patient and the provider.1 These issues, combined with an estimated prevalence rate of 71% for moderate pain among veterans seen in primary care, present a unique challenge for the primary care provider (PCP), given the limited time available to spend with these complex patients.2 Comorbidity rates with mental health issues (eg, depression, anxiety, substance use disorders, etc), which range from 18% to 44%, add to the management challenges for PCPs.3
Veterans also pose unique challenges in pain care as they have a 2-fold greater risk of death from opioid overdose compared with that of the general population, and Utah has been shown to have the highest rate of veteran overdoses.4 Developing programs to help PCPs efficiently manage patients with chronic noncancer pain and mental health comorbidities was vital at VASLCHCS.
Before VASLCHCS established the PC-CPP, the treatment for chronic noncancer pain and related mental health comorbidities followed a biomedical model that separated physical and mental health with the treatment focus on pharmacologic management of symptoms by separate services. Consistent with the biomedical model, management of chronic noncancer pain commonly included long-term use of opioids.
Over the past 2 decades, the use of opioids for treating chronic noncancer pain has significantly increased, with more than 62 million opioid prescriptions dispensed in 2012.5 There are no longitudinal follow-up studies, however, beyond 16 weeks on the use of opioids.6 Further, patients who are prescribed increased opioids continue to report high levels of pain, poor quality of life, and functional disability.7 High-dose opioids also are associated with overdose deaths.
Likewise, PCPs in the Women’s Primary Care Clinics at the VASLCHCS struggled with decreasing opioid use, often because other interventions for managing pain and related mental health conditions in primary care were not readily available. Although the VASLCHCS has an effective specialty pain service caring for patients with complex pain issues, opioid morphine equivalent doses > 200 mg/d, and palliative care, patients with chronic noncancer pain treated in the primary care setting did not have a consistent treatment approach.
A chart review of women veterans seen in Women’s Primary Care Clinic (N = 122) revealed that the majority of patients lacked timely urine drug screening, state database queries, signed medication management agreements, and documentation consistent with state and national guidelines. Additionally, many patients lacked provider follow-through regarding alternative and adjunctive therapy consults, which were often discontinued after failed contact attempts or no-shows to scheduled appointments.
There also was a general consensus among the Women’s Primary Care Clinic PCPs that caring for patients with chronic noncancer pain was exhausting, time consuming, ineffective, and often straining on the patient-provider relationship, as evidenced in many patients’ request to change providers secondary to pain management. The PC-CPP was developed to help systematically facilitate safe opioid prescribing, manage chronic pain issues, and document evidence-based care among women veterans receiving treatment for chronic noncancer pain at the Women’s Primary Care Clinics at VASLCHCS while coordinating and following through with nonpharmacologic interventions.
Program Development
National, state, VA, and professional licensure guidelines for chronic noncancer pain treatment standards were reviewed with the goal of creating a program that was evidence based, would benefit the patient in terms of opioid prescribing and pain control, and improve function while identifying key elements of care and documentation that adequately covered the prescriber of retribution.1,8-10
Concurrent to a review of the guidelines was a review of the literature with the goal of identifying useful patient education and alternative interventions and chronic pain programs that were already established and might meet the clinic’s needs.10,11 These reviews provided direction for a generalized approach to caring for patients with chronic nonmalignant pain. They also clarified that although pain education programs existed nationally, a program that offered a holistic, reproducible, adherence-driven yet patient-centered approach to the patient prescribed opioids chronically in a primary care setting was lacking.
Guideline recommendations included but were not limited to the following1,8-10:
- Patient education about chronic pain and opioids
- Evaluation of pain, function, opioid misuse risk at least twice yearly
- Patient-centered and driven treatment plans
- A holistic approach to chronic pain interventions
- Review of treatment plan efficacy at least twice yearly
- Enzyme multiplied immunoassay technique urine drug screening (UDS) 2 times per year
- State prescription monitoring program query annually
- Signed iMedConsent for treatment of chronic pain
- Plan for safe discontinuation of opioids
- Documentation that the above has been performed with patient understanding
The literature suggested a multimodality approach to chronic nonmalignant pain by minimizing the use of opioids over time while emphasizing nonpharmacologic therapies, such as cognitive behavioral therapy (CBT), mindfulness, meditation, yoga, and spiritual growth, to name a few.10,11 These findings are based on several studies, which suggested that passive coping strategies (eg, use of medication for immediate relief, depending on others, restricting medications) result in an increase in subjective pain among chronic nonmalignant pain patients.12 Helping patients reduce frequent use of passive coping strategies is believed to decrease pain.12 Active coping strategies (eg, engaging in therapies, staying busy or active, distracting attention from pain) have been found to decrease pain.12 The PC-CPP program shifted health care outcomes and responsibilities away from the hierarchal PCP-patient relationship toward a collaborative relationship that encourages patient-driven, patient-centered care outcomes and shared responsibilities.
Program Overview
The PC-CPP was shaped by the following hypotheses: (1) Transparent expectations and consequences would increase functional scores and decrease chronic opioid doses; (2) Treatment plans consisting of chronic opioid prescriptions linked with interactive nonpharmacologic interventions led to decreased pain and increased functional scores; (3) Transparent expectations combined with a streamlined approach to the chronic nonmalignant pain patient would improve patient and PCP satisfaction scores.
The PC-CPP was developed to provide an efficient, effective, and evidence-based approach to managing chronic nonmalignant pain and opioid therapy issues in the primary care setting. Referred patients attend 1 shared medical appointment (SMA) every 6 months with up to 19 other female veterans also referred to the PC-CPP. The group was composed of only female veterans as the pilot study for this SMA occurred in the Women’s Clinic. At each 6-month SMA, patients received education from the Taking Opioids Responsibly for Your Safety and the Safety of Others (TORYSSO) guide13 and signed the corresponding long-term opioid therapy for pain informed consent form (iMedConsent).
The patient and a staff member developed a treatment plan that was patient driven and included at least 1 nonpharmacologic treatment option. The 1-hour nonpharmacologic sessions were either group or individual and occurred weekly for 6 to 8 weeks. These options included CBT for chronic pain, Living Well With Chronic Conditions, trauma-sensitive yoga, smoking cessation, mindfulness for stress and anxiety, MOVE! weight management, Walk With Ease, and a self-help option (VA-issued Manage Stress Workbook, 2014). The workbook was included as an option for those who lived far away, were limited by work schedules, or were unable to afford the copays for a 6- to 8-session program.
Inclusion and Exclusion Criteria
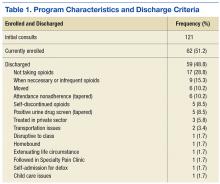
Any female veteran patient enrolled in the VASLCHCS with a chronic nonmalignant pain diagnosis who received daily opioids for 3 or more consecutive months from a PCP was included. Excluded individuals were those with cognitive decline/dementia, serious mental illness, psychosis, active suicidality, disruptive behavior flag, or those excluded by PCP discretion if it was determined that the patient would do better in a one-on-one setting with the PCP (Table 1). Patients taking > 200 MED/d of opioids who were seen in the VASLCHCS specialty pain clinic were also excluded.
Patient and PCP Responsibilities
The patient was responsible for timely attendance and full participation in all SMA group classes as determined in the veteran’s Treatment Plan Agreement (TPA). In addition, the patient had to provide UDS when requested (a minimum of twice yearly) and communicate with the PCP if having a procedure requiring additional opioids. This was in line with the current standards set forth by the VA Opioid Safety Initiative (OSI) Taskforce.12
The PC-CPP provided education, evaluation, documentation, and referral and follow-up with the nonpharmacologic treatment options discussed but did not provide prescription medications. The PCP reviewed the medical documents completed in the PC-CPP, and the PCP was strongly encouraged to follow its recommendations. The expectation was that the PCP would support the PC-CPP when the care recommendation was for a pharmacist-guided opioid taper.
Lack of attendance was defined as a no-show or a reschedule. Patients were considered adherent if they missed fewer than 2 SMA appointments and 2 nonpharmacologic treatment appointments every 6 months. The patient was required to attend the SMA and nonpharmacologic treatment on the third appointment to remain adherent with PC-CPP expectations and agreements. Adherence was acknowledged after 12 and 24 months by a reduction in PC-CPP requirements.
Shared Medical Appointment
Patients referred to the program and who met inclusion criteria received letters explaining the importance of SMA attendance and follow-up reminder calls. At least 30 minutes before the SMA, the patient provided an UDS sample at the laboratory. Next, the patient received an individualized program packet that included the TORYSSO guide, iMedConsent, a TPA specific to the program, a brief pain inventory (BPI) and opioid risk tool, a list of medication disposal sites, and short descriptions of available nonpharmacologic therapies.
Each SMA began with a presentation delivered by a pharmacist, a psychologist, and a medical provider, discussing TORYSSO, program expectations, and holistic approaches to pain. Each SMA also included a rotating chronic pain information topic (eg, nutrition and pain, the physiology of addiction, and the value of multiple modalities in pain treatment). Together, the staff and patients reviewed and completed the blank forms enclosed in the individualized
Each visit was entered into a Computerized Patient Record System (CPRS) template, which included a pain diagnosis, Opioid Risk Tool score, pain and functional scores, opioid fill history, last comprehensive metabolic panel, last electrocardiogram if on methadone, dates of signed agreements, patient adherence with SMA and optional therapies, and follow-up (eFigure).
Every patient enrolled in the PC-CPP had to attend a SMA every 6 months. Patients continued this indefinitely while receiving opioids, and requirements were lessened for patients who had a history of meeting program requirements. For those fully adherent after the first year, only 1 nonpharmacologic intervention was needed (instead of 1 every 6 months) yearly. After 2 years of full adherence, nonpharmacologic interventions were no longer necessary as the expectation was that the patient would continue to use the strategies that they had learned over the previous 2 years. Patients left the PC-CPP if they chose to discontinue opioids, met any of the exclusion criteria, or were nonadherent. Tapering opioid medication was recommended for patients who missed a SMA meeting or 2 nonpharmacologic treatment meetings in a 6-month period; received opioids from more than 1 provider; test positive on a UDS for substances that should not be present; consistently testing negative on a UDS for substances that should be present (indicating diversion); or exhibiting other aberrant behavior (frequent requests for early refills, medications often lost/stolen, etc).
Program Barriers
The PC-CPP took about 2 years to set up, and several barriers were encountered. A thorough understanding of the following factors is necessary for establishing a similar program.
Initially, consults were placed by a designee (someone other than the PCP currently caring for the patient). The designee was usually a member of the PC-CPP who placed consults for all patients who had opioids listed on the CPRS profile. Further, patients who had any opioids within the past 3 months were initially included as were patients who wanted pain education but were not taking opioids. After 12 months, it became apparent that the focus of the PC-CPP should center on patients taking opioids for a minimum of 3 months consecutively. Patients who wanted only education could attend other hospital education opportunities, which helped keep the patient load manageable for PC-CPP staff. Further, to lessen patient confusion and improve adherence, the PCP placed the consult and discussed the program with the patient. Class sizes of 5 to 10 patients seemed to be ideal for patient participation and provider workload.
Patient Education
Initially, the SMA did not follow a standard curriculum, but the current format is more consistent, reproducible, streamlined, and organized. This adjustment improved SMA attendance as well as patient satisfaction, as the class started and finished on time. The SMA also started with numerous handouts, including brochures for nonpharmacologic programs offered at this facility. This led to patients feeling overwhelmed, missing the important forms, and wasted paper. Handouts were simplified to 2 color-coded forms (TPA and BPI).
The take-home assessment was streamlined to a single general assessment. This assessment consisted of 2 questions that asked patients to write a summary of what they learned and then write a summary of how they applied what they learned to their pain management. The VA Manage Stress Workbook also was added to the take-home materials. There are currently 5 different take-home options, which are necessary for those who live more than 50 miles from any VA facility or for those who have transportation issues.
Patient Distress
The SMA could be stressful for patients who felt they were being “punished” or who showed up more than 15 minutes late and had to reschedule the SMA. Having a mental health provider available was crucial for these situations.
Therapeutic Option Development
A cornerstone of the program was getting patients to participate in nonpharmacologic treatment options, which required a robust selection of programs. The VASLCHCS was fortunate to have many programs already available (Table 2), but this was not always the case for the VA community-based outpatient clinics (CBOCs).
Stakeholder Support
Before its start, PC-CPP was presented to the Pentad (a group of 5 individuals in the local facility who hold executive leadership positions) for approval. Tapering opioids can lead to feelings of hostility, frustration, or sadness for patients, so having the Pentad support for the program was crucial to address complaints made to patient advocates or senators. Provider support also was important to reinforce program rules. The PC-CPP inclusion criteria included only those patients whose PCP was agreeable to a taper when the patient did not comply with program expectations. This strategy helped to improve patient adherence with the PC-CPP and decrease patient arguments with clinic staff, as all patients are held to the same standards.
Staff
Finding willing staff can be a challenge. It is estimated that each site needed a program leader who can champion the program objectives and drive organization of staff, space, documentation, and consistency for the patients consulted to the PC-CPP. The goal is that the consistent, reproducible expectations for both the PCP and the patient will reduce overall workload for a clinic. Patients may test the firmness and conviction of the staff to the PC-CPP. Having staff who are able and willing to be firm on relaying information for adherence to the patient is vital.
Administrative Support
At a minimum, a medical support assistant was required to help with scheduling, reminder calls and letters, CPRS check-in/check-out, ensuring necessary forms are ready for the SMA, tracking adherence, and following-up on no-shows and rescheduling.
Documentation
The CPRS consult and note template titles required the approval of the template committee. Although the template is helpful, there is still a great deal that needs to be manually entered in the note, such as BPI scores, opioid risk scores, and chosen nonpharmacologic interventions scores of pain, function, and opioid risk as well individual comorbidities, diagnosis, and follow-up dates. Documentation is geared toward easy review for the PCP who should scan the document prior to renewing opioid medications. The PC-CPP consult became a message board. Once the patient attends the SMA, the designated staff will add a comment to the message board, identifying all dates attended, complete history of the patient’s intervention choices and rate of adherence, as well a follow-up SMA date and whether the patient should bring materials such as take-home tests.
Time Commitment
Program development carries a heavy time burden. One full-time equivalent clinician for 6 weeks for program development is needed. Time allotment is estimated to be the following:
- Medical provider—30 minutes per patient (chart review, documentation, consult resolution). With training, these duties could be completed by support staff
- Pharmacist—30 minutes per patient (chart review, UDS, Utah Division of Occupational and Professional Licensing, fill history). Additional time is needed for writing opioid tapers for qualifying patients
- Primary care mental health integration—a PhD spent 1 to 2 hours per SMA visit assisting patients who became distressed during the visit. Only once has a patient needed to be escorted to the emergency department for active suicidality. A PhD also spent 10+ hours per week running and managing the CBT for Chronic Pain Group
- Support staff—a registered nurse spent 4 hours each month preparing for the SMA (entering consults, ordering EMITs, purchasing snacks)
Conclusion
In this descriptive report, the authors presented an overview of a newly developed program to manage chronic nonmalignant pain and safe opioid prescribing in a primary care setting. A final report is pending. The intent with this interim report was to describe the PC-CPP at the VASLCHCS, its methods and protocols, and logistic considerations for other providers who are working with patients with chronic pain in a primary care model. Standard operating procedure and inclusion/exclusion criteria were included to help with clinical decision making for patients chronic pain for whom aberrant opioid-related behavior presents a problem.
The authors expect that the PC-CPP will provide more comprehensive assisted care, lending to decreased complications associated with accidental overdose, because since patients have been educated about risks for accidental overdose from chronic opioids and have the responsibility for their outcomes. The authors also anticipated that functional scores (as measured by the BPI) will increase despite lowering opioid doses because patients will use ancillary treatments for pain. The desired outcome is that patients will come to understand that pain control is best approached holistically rather than through opioid monotherapy.
There have been several recent initiatives within the VA to decrease opioid prescribing and increase patient safety. With this in mind, continued expansion of this program to CBOCs and male patients could be useful to providers. Also, this program was conducted in a small setting (Women’s Clinic), and there are many challenges with rolling out such a program in a larger clinic (eg, greater chance for provider disagreement, greater need for administrative staff support). Nonetheless, the benefits of close monitoring of prescription opioids and active encouragement to engage in nonpharmacologic therapies are substantial and deserve further advancement.
1. Federation of State Medical Boards. Model policy on the use of opioid analgesics in the treatment of chronic pain. http://www.fsmb.org/Media/Default /PDF/FSMB/Advocacy/pain_policy_july2013.pdf. Accessed November 4, 2016.
2. Buse D, Loder E, McAlary P. Chronic pain rehabilitation. Pain Management Rounds. 2005;6:355-360.
3. Reid MC, Engles-Horton LL, Weber MB, Kerns RD, Rogers EL, O’Conner PG. Use of opioid medications for chronic noncancer pain syndromes in primary care. J Gen Intern Med. 2002;17(3):173-179.
4. Dart RC, Surratt HL, Cicero TJ, et al. Trends in opioid analgesic abuse and mortality in the United States. N Engl J Med. 2015;372(3):241-248.
5. Bohnert AS, Ilgen MA, Trafton JA, et al. Trends in regional variation in opioid overdose mortality among Veterans Health Administration patients, fiscal year 2001 to 2009. Clin J Pain. 2014;30(7):605-6012.
6. Busse JW, Guyatt GH. Optimizing the use of patient data to improve outcomes for patients: narcotics for chronic noncancer pain. Expert Rev Pharmacoecon Outcomes Res. 2009;9(2):171-179.
7. Eriksen J, Sjøgren P, Bruera E, Ekholm O, Rasmussen NK. Critical issues on opioids in chronic non-cancer pain: an epidemiological study. Pain. 2006;125(1-2):172-179.
8. Agency Medical Directors Group. Interagency guideline on opioid dosing for chronic non-cancer pain: an educational aid to improve care and safety with opioid therapy 2010 update. http://www.agen cymeddirectors.wa.gov/files/opioidgdline.pdf. Accessed November 4, 2016.
9. Utah Department of Health. Utah clinical guidelines on prescribing opioids for treatment of pain. http://health.utah.gov/prescription/pdf/guidelines/final.04.09opioidGuidlines.pdf. Published February 2009. Accessed November 4, 2016.
10. U.S. Department of Veterans Affairs, VA Academic Detailing Service. Pain management, opioid safety. VA educational guide (2014).http://www.va.gov/PAINMANAGEMENT/docs/OSI_1 _Tookit_Provider_AD_Educational_Guide_7_17.pdf. Published July 2014. Accessed November 2016.
11. Dobscha SK, Corson K, Leibowitz RQ, Sullivan MD, Gerrity MS. Rational, design, and baseline findings from a randomized trial of collaborative care for chronic musculoskeletal pain in primary care. Pain Med. 2008;9(8):1050-1064.
12. Mercado AC, Carroll LJ, Cassidy JD, Côté P. Passive coping is a risk factor for disabling neck or low back pain. Pain. 2005;117(1-2):51-57.
13. U.S. Department of Veterans Affairs, VA National Pain Management Program. Taking opioid responsibly for your safety and the safety of others: patient information guide on long-term opioid therapy for pain. http://www.veteranshealthlibrary.org/DiseasesConditions/ChronicPain/142,OpioidsIntro_VA. Updated December 9, 2015. Accessed November 17, 2016.
The Primary Care Chronic Pain Program (PC-CPP) of the Women’s Primary Care Clinics at the VA Salt Lake City Health Care System (VASLCHCS) in Utah was the first VA primary care clinical service to incorporate patient participation in obtaining chronic opioid medications in the treatment of chronic noncancer pain. In addition, the program used a multimodality approach for chronic pain treatment and veteran education about the relationship between physical and mental health issues.
Treatment Complexity
Chronic, noncancer pain is a complex issue in the primary care setting. Diagnosis is difficult, patient education is time consuming, goals and expectations are often unclear, and the experience can be unsatisfying for the patient and the provider.1 These issues, combined with an estimated prevalence rate of 71% for moderate pain among veterans seen in primary care, present a unique challenge for the primary care provider (PCP), given the limited time available to spend with these complex patients.2 Comorbidity rates with mental health issues (eg, depression, anxiety, substance use disorders, etc), which range from 18% to 44%, add to the management challenges for PCPs.3
Veterans also pose unique challenges in pain care as they have a 2-fold greater risk of death from opioid overdose compared with that of the general population, and Utah has been shown to have the highest rate of veteran overdoses.4 Developing programs to help PCPs efficiently manage patients with chronic noncancer pain and mental health comorbidities was vital at VASLCHCS.
Before VASLCHCS established the PC-CPP, the treatment for chronic noncancer pain and related mental health comorbidities followed a biomedical model that separated physical and mental health with the treatment focus on pharmacologic management of symptoms by separate services. Consistent with the biomedical model, management of chronic noncancer pain commonly included long-term use of opioids.
Over the past 2 decades, the use of opioids for treating chronic noncancer pain has significantly increased, with more than 62 million opioid prescriptions dispensed in 2012.5 There are no longitudinal follow-up studies, however, beyond 16 weeks on the use of opioids.6 Further, patients who are prescribed increased opioids continue to report high levels of pain, poor quality of life, and functional disability.7 High-dose opioids also are associated with overdose deaths.
Likewise, PCPs in the Women’s Primary Care Clinics at the VASLCHCS struggled with decreasing opioid use, often because other interventions for managing pain and related mental health conditions in primary care were not readily available. Although the VASLCHCS has an effective specialty pain service caring for patients with complex pain issues, opioid morphine equivalent doses > 200 mg/d, and palliative care, patients with chronic noncancer pain treated in the primary care setting did not have a consistent treatment approach.
A chart review of women veterans seen in Women’s Primary Care Clinic (N = 122) revealed that the majority of patients lacked timely urine drug screening, state database queries, signed medication management agreements, and documentation consistent with state and national guidelines. Additionally, many patients lacked provider follow-through regarding alternative and adjunctive therapy consults, which were often discontinued after failed contact attempts or no-shows to scheduled appointments.
There also was a general consensus among the Women’s Primary Care Clinic PCPs that caring for patients with chronic noncancer pain was exhausting, time consuming, ineffective, and often straining on the patient-provider relationship, as evidenced in many patients’ request to change providers secondary to pain management. The PC-CPP was developed to help systematically facilitate safe opioid prescribing, manage chronic pain issues, and document evidence-based care among women veterans receiving treatment for chronic noncancer pain at the Women’s Primary Care Clinics at VASLCHCS while coordinating and following through with nonpharmacologic interventions.
Program Development
National, state, VA, and professional licensure guidelines for chronic noncancer pain treatment standards were reviewed with the goal of creating a program that was evidence based, would benefit the patient in terms of opioid prescribing and pain control, and improve function while identifying key elements of care and documentation that adequately covered the prescriber of retribution.1,8-10
Concurrent to a review of the guidelines was a review of the literature with the goal of identifying useful patient education and alternative interventions and chronic pain programs that were already established and might meet the clinic’s needs.10,11 These reviews provided direction for a generalized approach to caring for patients with chronic nonmalignant pain. They also clarified that although pain education programs existed nationally, a program that offered a holistic, reproducible, adherence-driven yet patient-centered approach to the patient prescribed opioids chronically in a primary care setting was lacking.
Guideline recommendations included but were not limited to the following1,8-10:
- Patient education about chronic pain and opioids
- Evaluation of pain, function, opioid misuse risk at least twice yearly
- Patient-centered and driven treatment plans
- A holistic approach to chronic pain interventions
- Review of treatment plan efficacy at least twice yearly
- Enzyme multiplied immunoassay technique urine drug screening (UDS) 2 times per year
- State prescription monitoring program query annually
- Signed iMedConsent for treatment of chronic pain
- Plan for safe discontinuation of opioids
- Documentation that the above has been performed with patient understanding
The literature suggested a multimodality approach to chronic nonmalignant pain by minimizing the use of opioids over time while emphasizing nonpharmacologic therapies, such as cognitive behavioral therapy (CBT), mindfulness, meditation, yoga, and spiritual growth, to name a few.10,11 These findings are based on several studies, which suggested that passive coping strategies (eg, use of medication for immediate relief, depending on others, restricting medications) result in an increase in subjective pain among chronic nonmalignant pain patients.12 Helping patients reduce frequent use of passive coping strategies is believed to decrease pain.12 Active coping strategies (eg, engaging in therapies, staying busy or active, distracting attention from pain) have been found to decrease pain.12 The PC-CPP program shifted health care outcomes and responsibilities away from the hierarchal PCP-patient relationship toward a collaborative relationship that encourages patient-driven, patient-centered care outcomes and shared responsibilities.
Program Overview
The PC-CPP was shaped by the following hypotheses: (1) Transparent expectations and consequences would increase functional scores and decrease chronic opioid doses; (2) Treatment plans consisting of chronic opioid prescriptions linked with interactive nonpharmacologic interventions led to decreased pain and increased functional scores; (3) Transparent expectations combined with a streamlined approach to the chronic nonmalignant pain patient would improve patient and PCP satisfaction scores.
The PC-CPP was developed to provide an efficient, effective, and evidence-based approach to managing chronic nonmalignant pain and opioid therapy issues in the primary care setting. Referred patients attend 1 shared medical appointment (SMA) every 6 months with up to 19 other female veterans also referred to the PC-CPP. The group was composed of only female veterans as the pilot study for this SMA occurred in the Women’s Clinic. At each 6-month SMA, patients received education from the Taking Opioids Responsibly for Your Safety and the Safety of Others (TORYSSO) guide13 and signed the corresponding long-term opioid therapy for pain informed consent form (iMedConsent).
The patient and a staff member developed a treatment plan that was patient driven and included at least 1 nonpharmacologic treatment option. The 1-hour nonpharmacologic sessions were either group or individual and occurred weekly for 6 to 8 weeks. These options included CBT for chronic pain, Living Well With Chronic Conditions, trauma-sensitive yoga, smoking cessation, mindfulness for stress and anxiety, MOVE! weight management, Walk With Ease, and a self-help option (VA-issued Manage Stress Workbook, 2014). The workbook was included as an option for those who lived far away, were limited by work schedules, or were unable to afford the copays for a 6- to 8-session program.
Inclusion and Exclusion Criteria
Any female veteran patient enrolled in the VASLCHCS with a chronic nonmalignant pain diagnosis who received daily opioids for 3 or more consecutive months from a PCP was included. Excluded individuals were those with cognitive decline/dementia, serious mental illness, psychosis, active suicidality, disruptive behavior flag, or those excluded by PCP discretion if it was determined that the patient would do better in a one-on-one setting with the PCP (Table 1). Patients taking > 200 MED/d of opioids who were seen in the VASLCHCS specialty pain clinic were also excluded.
Patient and PCP Responsibilities
The patient was responsible for timely attendance and full participation in all SMA group classes as determined in the veteran’s Treatment Plan Agreement (TPA). In addition, the patient had to provide UDS when requested (a minimum of twice yearly) and communicate with the PCP if having a procedure requiring additional opioids. This was in line with the current standards set forth by the VA Opioid Safety Initiative (OSI) Taskforce.12
The PC-CPP provided education, evaluation, documentation, and referral and follow-up with the nonpharmacologic treatment options discussed but did not provide prescription medications. The PCP reviewed the medical documents completed in the PC-CPP, and the PCP was strongly encouraged to follow its recommendations. The expectation was that the PCP would support the PC-CPP when the care recommendation was for a pharmacist-guided opioid taper.
Lack of attendance was defined as a no-show or a reschedule. Patients were considered adherent if they missed fewer than 2 SMA appointments and 2 nonpharmacologic treatment appointments every 6 months. The patient was required to attend the SMA and nonpharmacologic treatment on the third appointment to remain adherent with PC-CPP expectations and agreements. Adherence was acknowledged after 12 and 24 months by a reduction in PC-CPP requirements.
Shared Medical Appointment
Patients referred to the program and who met inclusion criteria received letters explaining the importance of SMA attendance and follow-up reminder calls. At least 30 minutes before the SMA, the patient provided an UDS sample at the laboratory. Next, the patient received an individualized program packet that included the TORYSSO guide, iMedConsent, a TPA specific to the program, a brief pain inventory (BPI) and opioid risk tool, a list of medication disposal sites, and short descriptions of available nonpharmacologic therapies.
Each SMA began with a presentation delivered by a pharmacist, a psychologist, and a medical provider, discussing TORYSSO, program expectations, and holistic approaches to pain. Each SMA also included a rotating chronic pain information topic (eg, nutrition and pain, the physiology of addiction, and the value of multiple modalities in pain treatment). Together, the staff and patients reviewed and completed the blank forms enclosed in the individualized
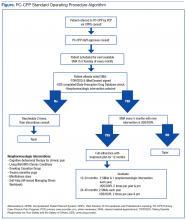
Each visit was entered into a Computerized Patient Record System (CPRS) template, which included a pain diagnosis, Opioid Risk Tool score, pain and functional scores, opioid fill history, last comprehensive metabolic panel, last electrocardiogram if on methadone, dates of signed agreements, patient adherence with SMA and optional therapies, and follow-up (eFigure).
Every patient enrolled in the PC-CPP had to attend a SMA every 6 months. Patients continued this indefinitely while receiving opioids, and requirements were lessened for patients who had a history of meeting program requirements. For those fully adherent after the first year, only 1 nonpharmacologic intervention was needed (instead of 1 every 6 months) yearly. After 2 years of full adherence, nonpharmacologic interventions were no longer necessary as the expectation was that the patient would continue to use the strategies that they had learned over the previous 2 years. Patients left the PC-CPP if they chose to discontinue opioids, met any of the exclusion criteria, or were nonadherent. Tapering opioid medication was recommended for patients who missed a SMA meeting or 2 nonpharmacologic treatment meetings in a 6-month period; received opioids from more than 1 provider; test positive on a UDS for substances that should not be present; consistently testing negative on a UDS for substances that should be present (indicating diversion); or exhibiting other aberrant behavior (frequent requests for early refills, medications often lost/stolen, etc).
Program Barriers
The PC-CPP took about 2 years to set up, and several barriers were encountered. A thorough understanding of the following factors is necessary for establishing a similar program.
Initially, consults were placed by a designee (someone other than the PCP currently caring for the patient). The designee was usually a member of the PC-CPP who placed consults for all patients who had opioids listed on the CPRS profile. Further, patients who had any opioids within the past 3 months were initially included as were patients who wanted pain education but were not taking opioids. After 12 months, it became apparent that the focus of the PC-CPP should center on patients taking opioids for a minimum of 3 months consecutively. Patients who wanted only education could attend other hospital education opportunities, which helped keep the patient load manageable for PC-CPP staff. Further, to lessen patient confusion and improve adherence, the PCP placed the consult and discussed the program with the patient. Class sizes of 5 to 10 patients seemed to be ideal for patient participation and provider workload.
Patient Education
Initially, the SMA did not follow a standard curriculum, but the current format is more consistent, reproducible, streamlined, and organized. This adjustment improved SMA attendance as well as patient satisfaction, as the class started and finished on time. The SMA also started with numerous handouts, including brochures for nonpharmacologic programs offered at this facility. This led to patients feeling overwhelmed, missing the important forms, and wasted paper. Handouts were simplified to 2 color-coded forms (TPA and BPI).
The take-home assessment was streamlined to a single general assessment. This assessment consisted of 2 questions that asked patients to write a summary of what they learned and then write a summary of how they applied what they learned to their pain management. The VA Manage Stress Workbook also was added to the take-home materials. There are currently 5 different take-home options, which are necessary for those who live more than 50 miles from any VA facility or for those who have transportation issues.
Patient Distress
The SMA could be stressful for patients who felt they were being “punished” or who showed up more than 15 minutes late and had to reschedule the SMA. Having a mental health provider available was crucial for these situations.
Therapeutic Option Development
A cornerstone of the program was getting patients to participate in nonpharmacologic treatment options, which required a robust selection of programs. The VASLCHCS was fortunate to have many programs already available (Table 2), but this was not always the case for the VA community-based outpatient clinics (CBOCs).
Stakeholder Support
Before its start, PC-CPP was presented to the Pentad (a group of 5 individuals in the local facility who hold executive leadership positions) for approval. Tapering opioids can lead to feelings of hostility, frustration, or sadness for patients, so having the Pentad support for the program was crucial to address complaints made to patient advocates or senators. Provider support also was important to reinforce program rules. The PC-CPP inclusion criteria included only those patients whose PCP was agreeable to a taper when the patient did not comply with program expectations. This strategy helped to improve patient adherence with the PC-CPP and decrease patient arguments with clinic staff, as all patients are held to the same standards.
Staff
Finding willing staff can be a challenge. It is estimated that each site needed a program leader who can champion the program objectives and drive organization of staff, space, documentation, and consistency for the patients consulted to the PC-CPP. The goal is that the consistent, reproducible expectations for both the PCP and the patient will reduce overall workload for a clinic. Patients may test the firmness and conviction of the staff to the PC-CPP. Having staff who are able and willing to be firm on relaying information for adherence to the patient is vital.
Administrative Support
At a minimum, a medical support assistant was required to help with scheduling, reminder calls and letters, CPRS check-in/check-out, ensuring necessary forms are ready for the SMA, tracking adherence, and following-up on no-shows and rescheduling.
Documentation
The CPRS consult and note template titles required the approval of the template committee. Although the template is helpful, there is still a great deal that needs to be manually entered in the note, such as BPI scores, opioid risk scores, and chosen nonpharmacologic interventions scores of pain, function, and opioid risk as well individual comorbidities, diagnosis, and follow-up dates. Documentation is geared toward easy review for the PCP who should scan the document prior to renewing opioid medications. The PC-CPP consult became a message board. Once the patient attends the SMA, the designated staff will add a comment to the message board, identifying all dates attended, complete history of the patient’s intervention choices and rate of adherence, as well a follow-up SMA date and whether the patient should bring materials such as take-home tests.
Time Commitment
Program development carries a heavy time burden. One full-time equivalent clinician for 6 weeks for program development is needed. Time allotment is estimated to be the following:
- Medical provider—30 minutes per patient (chart review, documentation, consult resolution). With training, these duties could be completed by support staff
- Pharmacist—30 minutes per patient (chart review, UDS, Utah Division of Occupational and Professional Licensing, fill history). Additional time is needed for writing opioid tapers for qualifying patients
- Primary care mental health integration—a PhD spent 1 to 2 hours per SMA visit assisting patients who became distressed during the visit. Only once has a patient needed to be escorted to the emergency department for active suicidality. A PhD also spent 10+ hours per week running and managing the CBT for Chronic Pain Group
- Support staff—a registered nurse spent 4 hours each month preparing for the SMA (entering consults, ordering EMITs, purchasing snacks)
Conclusion
In this descriptive report, the authors presented an overview of a newly developed program to manage chronic nonmalignant pain and safe opioid prescribing in a primary care setting. A final report is pending. The intent with this interim report was to describe the PC-CPP at the VASLCHCS, its methods and protocols, and logistic considerations for other providers who are working with patients with chronic pain in a primary care model. Standard operating procedure and inclusion/exclusion criteria were included to help with clinical decision making for patients chronic pain for whom aberrant opioid-related behavior presents a problem.
The authors expect that the PC-CPP will provide more comprehensive assisted care, lending to decreased complications associated with accidental overdose, because since patients have been educated about risks for accidental overdose from chronic opioids and have the responsibility for their outcomes. The authors also anticipated that functional scores (as measured by the BPI) will increase despite lowering opioid doses because patients will use ancillary treatments for pain. The desired outcome is that patients will come to understand that pain control is best approached holistically rather than through opioid monotherapy.
There have been several recent initiatives within the VA to decrease opioid prescribing and increase patient safety. With this in mind, continued expansion of this program to CBOCs and male patients could be useful to providers. Also, this program was conducted in a small setting (Women’s Clinic), and there are many challenges with rolling out such a program in a larger clinic (eg, greater chance for provider disagreement, greater need for administrative staff support). Nonetheless, the benefits of close monitoring of prescription opioids and active encouragement to engage in nonpharmacologic therapies are substantial and deserve further advancement.
The Primary Care Chronic Pain Program (PC-CPP) of the Women’s Primary Care Clinics at the VA Salt Lake City Health Care System (VASLCHCS) in Utah was the first VA primary care clinical service to incorporate patient participation in obtaining chronic opioid medications in the treatment of chronic noncancer pain. In addition, the program used a multimodality approach for chronic pain treatment and veteran education about the relationship between physical and mental health issues.
Treatment Complexity
Chronic, noncancer pain is a complex issue in the primary care setting. Diagnosis is difficult, patient education is time consuming, goals and expectations are often unclear, and the experience can be unsatisfying for the patient and the provider.1 These issues, combined with an estimated prevalence rate of 71% for moderate pain among veterans seen in primary care, present a unique challenge for the primary care provider (PCP), given the limited time available to spend with these complex patients.2 Comorbidity rates with mental health issues (eg, depression, anxiety, substance use disorders, etc), which range from 18% to 44%, add to the management challenges for PCPs.3
Veterans also pose unique challenges in pain care as they have a 2-fold greater risk of death from opioid overdose compared with that of the general population, and Utah has been shown to have the highest rate of veteran overdoses.4 Developing programs to help PCPs efficiently manage patients with chronic noncancer pain and mental health comorbidities was vital at VASLCHCS.
Before VASLCHCS established the PC-CPP, the treatment for chronic noncancer pain and related mental health comorbidities followed a biomedical model that separated physical and mental health with the treatment focus on pharmacologic management of symptoms by separate services. Consistent with the biomedical model, management of chronic noncancer pain commonly included long-term use of opioids.
Over the past 2 decades, the use of opioids for treating chronic noncancer pain has significantly increased, with more than 62 million opioid prescriptions dispensed in 2012.5 There are no longitudinal follow-up studies, however, beyond 16 weeks on the use of opioids.6 Further, patients who are prescribed increased opioids continue to report high levels of pain, poor quality of life, and functional disability.7 High-dose opioids also are associated with overdose deaths.
Likewise, PCPs in the Women’s Primary Care Clinics at the VASLCHCS struggled with decreasing opioid use, often because other interventions for managing pain and related mental health conditions in primary care were not readily available. Although the VASLCHCS has an effective specialty pain service caring for patients with complex pain issues, opioid morphine equivalent doses > 200 mg/d, and palliative care, patients with chronic noncancer pain treated in the primary care setting did not have a consistent treatment approach.
A chart review of women veterans seen in Women’s Primary Care Clinic (N = 122) revealed that the majority of patients lacked timely urine drug screening, state database queries, signed medication management agreements, and documentation consistent with state and national guidelines. Additionally, many patients lacked provider follow-through regarding alternative and adjunctive therapy consults, which were often discontinued after failed contact attempts or no-shows to scheduled appointments.
There also was a general consensus among the Women’s Primary Care Clinic PCPs that caring for patients with chronic noncancer pain was exhausting, time consuming, ineffective, and often straining on the patient-provider relationship, as evidenced in many patients’ request to change providers secondary to pain management. The PC-CPP was developed to help systematically facilitate safe opioid prescribing, manage chronic pain issues, and document evidence-based care among women veterans receiving treatment for chronic noncancer pain at the Women’s Primary Care Clinics at VASLCHCS while coordinating and following through with nonpharmacologic interventions.
Program Development
National, state, VA, and professional licensure guidelines for chronic noncancer pain treatment standards were reviewed with the goal of creating a program that was evidence based, would benefit the patient in terms of opioid prescribing and pain control, and improve function while identifying key elements of care and documentation that adequately covered the prescriber of retribution.1,8-10
Concurrent to a review of the guidelines was a review of the literature with the goal of identifying useful patient education and alternative interventions and chronic pain programs that were already established and might meet the clinic’s needs.10,11 These reviews provided direction for a generalized approach to caring for patients with chronic nonmalignant pain. They also clarified that although pain education programs existed nationally, a program that offered a holistic, reproducible, adherence-driven yet patient-centered approach to the patient prescribed opioids chronically in a primary care setting was lacking.
Guideline recommendations included but were not limited to the following1,8-10:
- Patient education about chronic pain and opioids
- Evaluation of pain, function, opioid misuse risk at least twice yearly
- Patient-centered and driven treatment plans
- A holistic approach to chronic pain interventions
- Review of treatment plan efficacy at least twice yearly
- Enzyme multiplied immunoassay technique urine drug screening (UDS) 2 times per year
- State prescription monitoring program query annually
- Signed iMedConsent for treatment of chronic pain
- Plan for safe discontinuation of opioids
- Documentation that the above has been performed with patient understanding
The literature suggested a multimodality approach to chronic nonmalignant pain by minimizing the use of opioids over time while emphasizing nonpharmacologic therapies, such as cognitive behavioral therapy (CBT), mindfulness, meditation, yoga, and spiritual growth, to name a few.10,11 These findings are based on several studies, which suggested that passive coping strategies (eg, use of medication for immediate relief, depending on others, restricting medications) result in an increase in subjective pain among chronic nonmalignant pain patients.12 Helping patients reduce frequent use of passive coping strategies is believed to decrease pain.12 Active coping strategies (eg, engaging in therapies, staying busy or active, distracting attention from pain) have been found to decrease pain.12 The PC-CPP program shifted health care outcomes and responsibilities away from the hierarchal PCP-patient relationship toward a collaborative relationship that encourages patient-driven, patient-centered care outcomes and shared responsibilities.
Program Overview
The PC-CPP was shaped by the following hypotheses: (1) Transparent expectations and consequences would increase functional scores and decrease chronic opioid doses; (2) Treatment plans consisting of chronic opioid prescriptions linked with interactive nonpharmacologic interventions led to decreased pain and increased functional scores; (3) Transparent expectations combined with a streamlined approach to the chronic nonmalignant pain patient would improve patient and PCP satisfaction scores.
The PC-CPP was developed to provide an efficient, effective, and evidence-based approach to managing chronic nonmalignant pain and opioid therapy issues in the primary care setting. Referred patients attend 1 shared medical appointment (SMA) every 6 months with up to 19 other female veterans also referred to the PC-CPP. The group was composed of only female veterans as the pilot study for this SMA occurred in the Women’s Clinic. At each 6-month SMA, patients received education from the Taking Opioids Responsibly for Your Safety and the Safety of Others (TORYSSO) guide13 and signed the corresponding long-term opioid therapy for pain informed consent form (iMedConsent).
The patient and a staff member developed a treatment plan that was patient driven and included at least 1 nonpharmacologic treatment option. The 1-hour nonpharmacologic sessions were either group or individual and occurred weekly for 6 to 8 weeks. These options included CBT for chronic pain, Living Well With Chronic Conditions, trauma-sensitive yoga, smoking cessation, mindfulness for stress and anxiety, MOVE! weight management, Walk With Ease, and a self-help option (VA-issued Manage Stress Workbook, 2014). The workbook was included as an option for those who lived far away, were limited by work schedules, or were unable to afford the copays for a 6- to 8-session program.
Inclusion and Exclusion Criteria
Any female veteran patient enrolled in the VASLCHCS with a chronic nonmalignant pain diagnosis who received daily opioids for 3 or more consecutive months from a PCP was included. Excluded individuals were those with cognitive decline/dementia, serious mental illness, psychosis, active suicidality, disruptive behavior flag, or those excluded by PCP discretion if it was determined that the patient would do better in a one-on-one setting with the PCP (Table 1). Patients taking > 200 MED/d of opioids who were seen in the VASLCHCS specialty pain clinic were also excluded.
Patient and PCP Responsibilities
The patient was responsible for timely attendance and full participation in all SMA group classes as determined in the veteran’s Treatment Plan Agreement (TPA). In addition, the patient had to provide UDS when requested (a minimum of twice yearly) and communicate with the PCP if having a procedure requiring additional opioids. This was in line with the current standards set forth by the VA Opioid Safety Initiative (OSI) Taskforce.12
The PC-CPP provided education, evaluation, documentation, and referral and follow-up with the nonpharmacologic treatment options discussed but did not provide prescription medications. The PCP reviewed the medical documents completed in the PC-CPP, and the PCP was strongly encouraged to follow its recommendations. The expectation was that the PCP would support the PC-CPP when the care recommendation was for a pharmacist-guided opioid taper.
Lack of attendance was defined as a no-show or a reschedule. Patients were considered adherent if they missed fewer than 2 SMA appointments and 2 nonpharmacologic treatment appointments every 6 months. The patient was required to attend the SMA and nonpharmacologic treatment on the third appointment to remain adherent with PC-CPP expectations and agreements. Adherence was acknowledged after 12 and 24 months by a reduction in PC-CPP requirements.
Shared Medical Appointment
Patients referred to the program and who met inclusion criteria received letters explaining the importance of SMA attendance and follow-up reminder calls. At least 30 minutes before the SMA, the patient provided an UDS sample at the laboratory. Next, the patient received an individualized program packet that included the TORYSSO guide, iMedConsent, a TPA specific to the program, a brief pain inventory (BPI) and opioid risk tool, a list of medication disposal sites, and short descriptions of available nonpharmacologic therapies.
Each SMA began with a presentation delivered by a pharmacist, a psychologist, and a medical provider, discussing TORYSSO, program expectations, and holistic approaches to pain. Each SMA also included a rotating chronic pain information topic (eg, nutrition and pain, the physiology of addiction, and the value of multiple modalities in pain treatment). Together, the staff and patients reviewed and completed the blank forms enclosed in the individualized
Each visit was entered into a Computerized Patient Record System (CPRS) template, which included a pain diagnosis, Opioid Risk Tool score, pain and functional scores, opioid fill history, last comprehensive metabolic panel, last electrocardiogram if on methadone, dates of signed agreements, patient adherence with SMA and optional therapies, and follow-up (eFigure).
Every patient enrolled in the PC-CPP had to attend a SMA every 6 months. Patients continued this indefinitely while receiving opioids, and requirements were lessened for patients who had a history of meeting program requirements. For those fully adherent after the first year, only 1 nonpharmacologic intervention was needed (instead of 1 every 6 months) yearly. After 2 years of full adherence, nonpharmacologic interventions were no longer necessary as the expectation was that the patient would continue to use the strategies that they had learned over the previous 2 years. Patients left the PC-CPP if they chose to discontinue opioids, met any of the exclusion criteria, or were nonadherent. Tapering opioid medication was recommended for patients who missed a SMA meeting or 2 nonpharmacologic treatment meetings in a 6-month period; received opioids from more than 1 provider; test positive on a UDS for substances that should not be present; consistently testing negative on a UDS for substances that should be present (indicating diversion); or exhibiting other aberrant behavior (frequent requests for early refills, medications often lost/stolen, etc).
Program Barriers
The PC-CPP took about 2 years to set up, and several barriers were encountered. A thorough understanding of the following factors is necessary for establishing a similar program.
Initially, consults were placed by a designee (someone other than the PCP currently caring for the patient). The designee was usually a member of the PC-CPP who placed consults for all patients who had opioids listed on the CPRS profile. Further, patients who had any opioids within the past 3 months were initially included as were patients who wanted pain education but were not taking opioids. After 12 months, it became apparent that the focus of the PC-CPP should center on patients taking opioids for a minimum of 3 months consecutively. Patients who wanted only education could attend other hospital education opportunities, which helped keep the patient load manageable for PC-CPP staff. Further, to lessen patient confusion and improve adherence, the PCP placed the consult and discussed the program with the patient. Class sizes of 5 to 10 patients seemed to be ideal for patient participation and provider workload.
Patient Education
Initially, the SMA did not follow a standard curriculum, but the current format is more consistent, reproducible, streamlined, and organized. This adjustment improved SMA attendance as well as patient satisfaction, as the class started and finished on time. The SMA also started with numerous handouts, including brochures for nonpharmacologic programs offered at this facility. This led to patients feeling overwhelmed, missing the important forms, and wasted paper. Handouts were simplified to 2 color-coded forms (TPA and BPI).
The take-home assessment was streamlined to a single general assessment. This assessment consisted of 2 questions that asked patients to write a summary of what they learned and then write a summary of how they applied what they learned to their pain management. The VA Manage Stress Workbook also was added to the take-home materials. There are currently 5 different take-home options, which are necessary for those who live more than 50 miles from any VA facility or for those who have transportation issues.
Patient Distress
The SMA could be stressful for patients who felt they were being “punished” or who showed up more than 15 minutes late and had to reschedule the SMA. Having a mental health provider available was crucial for these situations.
Therapeutic Option Development
A cornerstone of the program was getting patients to participate in nonpharmacologic treatment options, which required a robust selection of programs. The VASLCHCS was fortunate to have many programs already available (Table 2), but this was not always the case for the VA community-based outpatient clinics (CBOCs).
Stakeholder Support
Before its start, PC-CPP was presented to the Pentad (a group of 5 individuals in the local facility who hold executive leadership positions) for approval. Tapering opioids can lead to feelings of hostility, frustration, or sadness for patients, so having the Pentad support for the program was crucial to address complaints made to patient advocates or senators. Provider support also was important to reinforce program rules. The PC-CPP inclusion criteria included only those patients whose PCP was agreeable to a taper when the patient did not comply with program expectations. This strategy helped to improve patient adherence with the PC-CPP and decrease patient arguments with clinic staff, as all patients are held to the same standards.
Staff
Finding willing staff can be a challenge. It is estimated that each site needed a program leader who can champion the program objectives and drive organization of staff, space, documentation, and consistency for the patients consulted to the PC-CPP. The goal is that the consistent, reproducible expectations for both the PCP and the patient will reduce overall workload for a clinic. Patients may test the firmness and conviction of the staff to the PC-CPP. Having staff who are able and willing to be firm on relaying information for adherence to the patient is vital.
Administrative Support
At a minimum, a medical support assistant was required to help with scheduling, reminder calls and letters, CPRS check-in/check-out, ensuring necessary forms are ready for the SMA, tracking adherence, and following-up on no-shows and rescheduling.
Documentation
The CPRS consult and note template titles required the approval of the template committee. Although the template is helpful, there is still a great deal that needs to be manually entered in the note, such as BPI scores, opioid risk scores, and chosen nonpharmacologic interventions scores of pain, function, and opioid risk as well individual comorbidities, diagnosis, and follow-up dates. Documentation is geared toward easy review for the PCP who should scan the document prior to renewing opioid medications. The PC-CPP consult became a message board. Once the patient attends the SMA, the designated staff will add a comment to the message board, identifying all dates attended, complete history of the patient’s intervention choices and rate of adherence, as well a follow-up SMA date and whether the patient should bring materials such as take-home tests.
Time Commitment
Program development carries a heavy time burden. One full-time equivalent clinician for 6 weeks for program development is needed. Time allotment is estimated to be the following:
- Medical provider—30 minutes per patient (chart review, documentation, consult resolution). With training, these duties could be completed by support staff
- Pharmacist—30 minutes per patient (chart review, UDS, Utah Division of Occupational and Professional Licensing, fill history). Additional time is needed for writing opioid tapers for qualifying patients
- Primary care mental health integration—a PhD spent 1 to 2 hours per SMA visit assisting patients who became distressed during the visit. Only once has a patient needed to be escorted to the emergency department for active suicidality. A PhD also spent 10+ hours per week running and managing the CBT for Chronic Pain Group
- Support staff—a registered nurse spent 4 hours each month preparing for the SMA (entering consults, ordering EMITs, purchasing snacks)
Conclusion
In this descriptive report, the authors presented an overview of a newly developed program to manage chronic nonmalignant pain and safe opioid prescribing in a primary care setting. A final report is pending. The intent with this interim report was to describe the PC-CPP at the VASLCHCS, its methods and protocols, and logistic considerations for other providers who are working with patients with chronic pain in a primary care model. Standard operating procedure and inclusion/exclusion criteria were included to help with clinical decision making for patients chronic pain for whom aberrant opioid-related behavior presents a problem.
The authors expect that the PC-CPP will provide more comprehensive assisted care, lending to decreased complications associated with accidental overdose, because since patients have been educated about risks for accidental overdose from chronic opioids and have the responsibility for their outcomes. The authors also anticipated that functional scores (as measured by the BPI) will increase despite lowering opioid doses because patients will use ancillary treatments for pain. The desired outcome is that patients will come to understand that pain control is best approached holistically rather than through opioid monotherapy.
There have been several recent initiatives within the VA to decrease opioid prescribing and increase patient safety. With this in mind, continued expansion of this program to CBOCs and male patients could be useful to providers. Also, this program was conducted in a small setting (Women’s Clinic), and there are many challenges with rolling out such a program in a larger clinic (eg, greater chance for provider disagreement, greater need for administrative staff support). Nonetheless, the benefits of close monitoring of prescription opioids and active encouragement to engage in nonpharmacologic therapies are substantial and deserve further advancement.
1. Federation of State Medical Boards. Model policy on the use of opioid analgesics in the treatment of chronic pain. http://www.fsmb.org/Media/Default /PDF/FSMB/Advocacy/pain_policy_july2013.pdf. Accessed November 4, 2016.
2. Buse D, Loder E, McAlary P. Chronic pain rehabilitation. Pain Management Rounds. 2005;6:355-360.
3. Reid MC, Engles-Horton LL, Weber MB, Kerns RD, Rogers EL, O’Conner PG. Use of opioid medications for chronic noncancer pain syndromes in primary care. J Gen Intern Med. 2002;17(3):173-179.
4. Dart RC, Surratt HL, Cicero TJ, et al. Trends in opioid analgesic abuse and mortality in the United States. N Engl J Med. 2015;372(3):241-248.
5. Bohnert AS, Ilgen MA, Trafton JA, et al. Trends in regional variation in opioid overdose mortality among Veterans Health Administration patients, fiscal year 2001 to 2009. Clin J Pain. 2014;30(7):605-6012.
6. Busse JW, Guyatt GH. Optimizing the use of patient data to improve outcomes for patients: narcotics for chronic noncancer pain. Expert Rev Pharmacoecon Outcomes Res. 2009;9(2):171-179.
7. Eriksen J, Sjøgren P, Bruera E, Ekholm O, Rasmussen NK. Critical issues on opioids in chronic non-cancer pain: an epidemiological study. Pain. 2006;125(1-2):172-179.
8. Agency Medical Directors Group. Interagency guideline on opioid dosing for chronic non-cancer pain: an educational aid to improve care and safety with opioid therapy 2010 update. http://www.agen cymeddirectors.wa.gov/files/opioidgdline.pdf. Accessed November 4, 2016.
9. Utah Department of Health. Utah clinical guidelines on prescribing opioids for treatment of pain. http://health.utah.gov/prescription/pdf/guidelines/final.04.09opioidGuidlines.pdf. Published February 2009. Accessed November 4, 2016.
10. U.S. Department of Veterans Affairs, VA Academic Detailing Service. Pain management, opioid safety. VA educational guide (2014).http://www.va.gov/PAINMANAGEMENT/docs/OSI_1 _Tookit_Provider_AD_Educational_Guide_7_17.pdf. Published July 2014. Accessed November 2016.
11. Dobscha SK, Corson K, Leibowitz RQ, Sullivan MD, Gerrity MS. Rational, design, and baseline findings from a randomized trial of collaborative care for chronic musculoskeletal pain in primary care. Pain Med. 2008;9(8):1050-1064.
12. Mercado AC, Carroll LJ, Cassidy JD, Côté P. Passive coping is a risk factor for disabling neck or low back pain. Pain. 2005;117(1-2):51-57.
13. U.S. Department of Veterans Affairs, VA National Pain Management Program. Taking opioid responsibly for your safety and the safety of others: patient information guide on long-term opioid therapy for pain. http://www.veteranshealthlibrary.org/DiseasesConditions/ChronicPain/142,OpioidsIntro_VA. Updated December 9, 2015. Accessed November 17, 2016.
1. Federation of State Medical Boards. Model policy on the use of opioid analgesics in the treatment of chronic pain. http://www.fsmb.org/Media/Default /PDF/FSMB/Advocacy/pain_policy_july2013.pdf. Accessed November 4, 2016.
2. Buse D, Loder E, McAlary P. Chronic pain rehabilitation. Pain Management Rounds. 2005;6:355-360.
3. Reid MC, Engles-Horton LL, Weber MB, Kerns RD, Rogers EL, O’Conner PG. Use of opioid medications for chronic noncancer pain syndromes in primary care. J Gen Intern Med. 2002;17(3):173-179.
4. Dart RC, Surratt HL, Cicero TJ, et al. Trends in opioid analgesic abuse and mortality in the United States. N Engl J Med. 2015;372(3):241-248.
5. Bohnert AS, Ilgen MA, Trafton JA, et al. Trends in regional variation in opioid overdose mortality among Veterans Health Administration patients, fiscal year 2001 to 2009. Clin J Pain. 2014;30(7):605-6012.
6. Busse JW, Guyatt GH. Optimizing the use of patient data to improve outcomes for patients: narcotics for chronic noncancer pain. Expert Rev Pharmacoecon Outcomes Res. 2009;9(2):171-179.
7. Eriksen J, Sjøgren P, Bruera E, Ekholm O, Rasmussen NK. Critical issues on opioids in chronic non-cancer pain: an epidemiological study. Pain. 2006;125(1-2):172-179.
8. Agency Medical Directors Group. Interagency guideline on opioid dosing for chronic non-cancer pain: an educational aid to improve care and safety with opioid therapy 2010 update. http://www.agen cymeddirectors.wa.gov/files/opioidgdline.pdf. Accessed November 4, 2016.
9. Utah Department of Health. Utah clinical guidelines on prescribing opioids for treatment of pain. http://health.utah.gov/prescription/pdf/guidelines/final.04.09opioidGuidlines.pdf. Published February 2009. Accessed November 4, 2016.
10. U.S. Department of Veterans Affairs, VA Academic Detailing Service. Pain management, opioid safety. VA educational guide (2014).http://www.va.gov/PAINMANAGEMENT/docs/OSI_1 _Tookit_Provider_AD_Educational_Guide_7_17.pdf. Published July 2014. Accessed November 2016.
11. Dobscha SK, Corson K, Leibowitz RQ, Sullivan MD, Gerrity MS. Rational, design, and baseline findings from a randomized trial of collaborative care for chronic musculoskeletal pain in primary care. Pain Med. 2008;9(8):1050-1064.
12. Mercado AC, Carroll LJ, Cassidy JD, Côté P. Passive coping is a risk factor for disabling neck or low back pain. Pain. 2005;117(1-2):51-57.
13. U.S. Department of Veterans Affairs, VA National Pain Management Program. Taking opioid responsibly for your safety and the safety of others: patient information guide on long-term opioid therapy for pain. http://www.veteranshealthlibrary.org/DiseasesConditions/ChronicPain/142,OpioidsIntro_VA. Updated December 9, 2015. Accessed November 17, 2016.