User login
Clostridium difficile (C difficile) is a gram-positive, toxin-producing bacterium that is of increasing concern among health care providers and patients. Infection with C difficile can have manifestations ranging from mild diarrhea to severe toxic megacolon and can result in prolonged hospitalization with severe cases requiring admission to an intensive care unit.1 In 2014, the US was estimated to have more than 600,000 cases of C difficile infection (CDI), previously known as C difficile–associated diarrhea, and more than 44,000 associated deaths. The annual economic cost of CDI is thought to exceed $5 billion.1 According to studies of health care–associated illness, CDI rates are comparable to or have surpassed rates of methicillin-resistant Staphylococcus aureus infection within the US, including at US Department of Veterans Affairs (VA) acute care centers nationwide.2,3
C difficile has been shown to be the causative agent in 10% to 20% of antibiotic-associated diarrhea episodes.4 Colonization of C difficile is uncommon in healthy adults, but colonization rates are as high as 21% in hospitalized patients, with increasing rates proportional to increasing hospital length of stay.5,6 Although not all colonized patients develop clinically significant CDI, those who do may require multiple treatment courses, over months to years, because of the high risk of disease recurrence. An estimated 25% of patients have a single recurrent episode of CDI within 30 days after treatment completion, and 45% of those patients have additional recurrent infections.7,8 Although probiotics do not have an approved US Food and Drug Administration (FDA) indication, these supplements are often used to try to prevent CDI from developing during concomitant antibiotic use. Probiotics are microorganisms with potential health benefits, but the mechanisms of these benefits are not fully understood. Proposed mechanisms include reduced growth of pathogenic bacteria, modulation of the immune system, and support of the intestinal wall barrier.9 The many probiotic formulations currently marketed include Lactobacillus acidophilus (L acidophilus) capsules and various combinations of L acidophilus, Lactobacillus casei, Bifidobacterium lactis, Bifidobacterium longum, Streptococcus thermophilus, and other bacterial strains.
Dosing and Guidelines
Manufacturers’ suggested dosing for their Lactobacillus capsules, tablets, and packets varies from 1 unit daily to 4 units 4 times daily for dietary supplementation; the products’ labeling does not include any information regarding treatment duration.10-13 In addition, there are no published recommendations or product labeling guiding the dosing of probiotics or their duration of use in the primary prevention of CDI.
In 2017, the Infectious Diseases Society of America (IDSA) and the Society for Healthcare Epidemiology of America (SHEA) updated their CDI treatment guidelines.14 As these guidelines indicate that the data on probiotic use in CDI are inadequate, IDSA and SHEA make no recommendation for or against probiotic use in primary prevention of the disease. The guidelines point to several limitations in the literature, including variability in probiotic formulations studied, duration of probiotic administration, definitions of CDI, and duration of study follow-up.
Given the lack of consensus guidelines that clinicians can use when deciding which probiotic dosing and duration are appropriate for a patient for primary prevention of CDI, we evaluated the literature on the topic and summarize their findings here.
Review of Probiotoc Literature
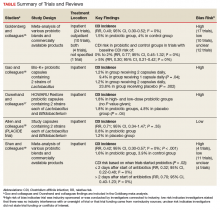
Conflicting data exist about probiotics and their effect on CDI prevention. The literature reviewed was selected based on our assessment of its contribution to the topic and its potential utility to clinicians in determining appropriate probiotic therapies and recommendations. Included in our discussion is a large Cochrane Review of probiotic efficacy, 2 trials of probiotic dosing, the PLACIDE trial, and a systematic review of timely probiotic initiation. All of these studies attempted to determine the effect of probiotics on CDI incidence (Table).
In their 2017 Cochrane Review, Goldenberg and colleagues reviewed 39 trials that investigated the efficacy of probiotics in CDI prevention in 9,955 immunocompetent patients receiving antibiotics.15 The incidence of CDI was significantly lower in patients who received a probiotic than in patients who received placebo or no treatment (1.5% vs 4.0%; relative risk [RR], 0.40; 95% CI, 0.30-0.52; I2 = 0%). It is important to note that trials with a control-group CDI incidence of 0% to 2% (baseline CDI risk) found no statistically significant difference in CDI risk between patients using and not using probiotics (RR, 0.77; 95% CI, 0.45-1.32; I2 = 0%) and that the preceding statistically significant result may have been driven by the inclusion of trials with high baseline CDI risk (> 5%). Trials that enrolled patients who were at this risk level found a statistically significant 70% reduction in CDI risk in those using probiotics (vs no probiotics) while on concomitant antibiotic therapy (RR, 0.30; 95% CI, 0.21-0.42; I2 = 0%).
Probiotic therapy seems to be effective in reducing CDI risk in immunocompetent patients and may be particularly beneficial in patients at higher CDI risk, though Goldenberg and colleagues did not elaborate on what constitutes higher risk and based their conclusion on their control group’s high CDI incidence (> 5%). The most common adverse events (AEs) attributable to probiotics included abdominal cramping, nausea, fever, soft stools, flatulence, and taste disturbance. The review’s findings are limited in that the inclusion of many small trials with high baseline CDI risk likely contributed to a statistically significant result, and 17 of the review’s 39 trials were industry-sponsored or were conducted by investigators with industry associations; another 12 lacked statements about funding or sponsorship.
Two of the trials in the Cochrane Review investigated whether probiotics have a dose effect on CDI prevention. Gao and colleagues randomly assigned 255 hospitalized Asian patients to 3 groups: those receiving placebo, 1 probiotic capsule daily, and 2 probiotic capsules daily.16 Each probiotic capsule contained 50 billion colony-forming units (CFUs) of Lactobacillus. Incidence of CDI was lower in patients taking 2 probiotic capsules daily than in those taking 1 probiotic capsule daily (1.2% vs 9.4%; P = .04) or placebo (1.2% vs 23.8%; P = .002). In the other trial, Ouwehand and colleagues randomly assigned 503 hospitalized Asian patients to 3 groups as well: those receiving placebo, low-dose probiotic (4.17 billion CFUs of Lactobacillus and Bifidobacterium), and high-dose probiotic (17 billion CFUs).17 The incidence of CDI in each probiotic group (low-dose, high-dose) was 1.8%, which was significantly lower than the 4.8% in the placebo group (P = .04).
The Cochrane Review’s largest and most rigorous trial was PLACIDE, a 2013 randomized controlled study of the effect of probiotics on CDI.18 Allen and colleagues randomly assigned 2,981 inpatients (aged ≥ 65 years; exposed to antibiotics within preceding 7 days) to 2 groups: those receiving either 1 probiotic capsule daily, or 1 placebo capsule daily, for 21 days. Results showed no difference in CDI incidence between the probiotic and placebo groups (0.8% vs 1.2%; RR, 0.71; 95% CI, 0.34-1.47; P = .35). Of note, this trial is free of industry sponsorship, is the largest probiotic trial to date, has a control-group baseline CDI rate consistent with the rate in hospital and ambulatory settings in the US, and found a negative result regarding probiotic use in CDI prevention. Findings are limited in that the study allowed for initiating probiotic therapy up to 7 days after the start of antibiotics, and patients were given 1 relatively low-dose capsule daily, which may have contributed to lack of an effect on CDI prevention. No serious AEs were attributed to probiotic use.
In a 2017 systematic meta-analysis of 19 studies, Shen and colleagues investigated whether timely use of probiotics prevented CDI in 6,261 hospitalized patients receiving antibiotics.19 The incidence of CDI was significantly lower in patients receiving vs not receiving probiotics (1.6% vs 3.9%; RR, 0.42; 95% CI, 0.30-0.57; I2 = 0%; P < .001).19 A subgroup analysis was performed to compare studies initiating probiotics within 2 days after the start of antibiotics with studies initiating probiotics more than 2 days after the start. CDI risk was reduced by 68% when probiotics were started within 2 days, vs 30% when started after 2 days (RR, 0.32; 95% CI, 0.22-0.48; I2 = 0% vs RR, 0.70; 95% CI, 0.40-1.23; I2 = 0%; P = .02). Of note, no difference was found in efficacy among the various probiotic formulations, and no significant AEs were noted in any study group.
Trials included in the Cochrane Review used many different probiotic regimens over various durations.15 All these trials continued probiotics for at least the duration of antibiotic therapy. The 2 trials that evaluated the effect of probiotic therapy over an extended period required probiotics be started within 48 hours after initiation of antibiotic therapy; one trial continued probiotics for 5 days after completion of antibiotics, and the other for 7 days after completion.16,20 In both trials, CDI was statistically significantly reduced among adults using probiotics compared with adults receiving placebo.
Probiotic Safety
The FDA has not approved probiotics for the prevention or treatment of any health problems. Most probiotics are FDA-regulated as dietary supplements and do not have to meet stringent drug-approval requirements. The FDA has given many strains of common probiotics the Generally Recognized as Safe designation for use in commercially available products and foods.21-23 Probiotic use has not been associated with significant AEs in clinical trials and generally has been considered safe in immunocompetent and otherwise healthy persons.15-19 However, clinical trials have been inadequate in reporting or investigating AEs; the alternative for evaluating the risks of probiotic therapy is case reports.24,25 Theoretical risks associated with probiotics include sepsis, deleterious effects on normal gut digestion, excessive immune stimulation, and possible transfer of antimicrobial resistance genes among microorganisms.26 Boyle and colleagues further described a handful of case reports of sepsis caused by probiotics in immunocompromised individuals; the other theoretical risks have not been reported outside animal studies.26
CDI Risk Factors
Many factors can increase a patient’s CDI risk. Specific antibiotics (eg, ampicillin, amoxicillin, cephalosporins, clindamycin, fluoroquinolones) confer higher risk.27,28 Other factors include inflammatory bowel disease, organ transplantation, chemotherapy, chronic kidney disease, and immunodeficiency. Advanced age increases CDI risk and can increase the severity of infection. The evidence regarding acid suppression and CDI risk is conflicting, though a recent meta-analysis found that use of proton pump inhibitors is associated with a 2-fold higher risk of developing CDI.29 Patient-specific risk factors should be evaluated when the risk–benefit ratio for probiotic use is being considered.
Conclusion
CDIs are becoming increasingly burdensome to the health care system. More research is needed on the role of probiotics in CDI prevention in patients taking antibiotics. Given the limited risk for AEs when probiotics are used in immunocompetent patients and the relatively low cost of these supplements, the risks likely are outweighed by the postulated benefits, and probiotics may be recommended in select patient populations.
The PLACIDE trial found no benefit of probiotics in preventing CDI in a population similar to that of a typical US hospital or ambulatory setting, but its intervention allowed late initiation of relatively low doses of probiotics. Therefore, probiotics may be recommended for CDI prevention in patients taking antibiotics, especially patients at high risk for developing CDI. When clinicians recommend probiotic use in this setting, the probiotic should be initiated within 2 days after the start of antibiotics and should be continued for the duration of antibiotic therapy and for up to 7 days after that therapy is completed. Optimal probiotic dosing, likely dependent on the product used, remains unclear. PLACIDE trial results suggest that a dosage of at least 1 probiotic capsule 2 times daily may confer additional efficacy.
1. Desai K, Gupta SB, Dubberke ER, Prabhu VS, Browne C, Mast TC. Epidemiological and economic burden of Clostridium difficile in the United States: estimates from a modeling approach. BMC Infect Dis. 2016;16:303.
2. Miller BA, Chen LF, Sexton DJ, Anderson DJ. Comparison of the burdens of hospital-onset, healthcare facility-associated Clostridium difficile infection and of healthcare-associated infection due to methicillin-resistant Staphylococcus aureus in community hospitals. Infect Control Hosp Epidemiol. 2011;32(4):387-390.
3. Evans ME, Kralovic SM, Simbartl LA, Jain R, Roselle GA. Effect of a Clostridium difficile infection prevention initiative in Veterans Affairs acute care facilities. Infect Control Hosp Epidemiol. 2016;37(6):720-722.
4. Bartlett JG. Clinical practice. Antibiotic-associated diarrhea. N Engl J Med. 2002;346(5):334-339.
5. Johnson S, Clabots CR, Linn FV, Olson MM, Peterson LR, Gerding DN. Nosocomial Clostridium difficile colonisation and disease. Lancet. 1990;336(8707):97-100.
6. McFarland LV, Mulligan ME, Kwok RY, Stamm WE. Nosocomial acquisition of Clostridium difficile infection. N Engl J Med. 1989;320(4):204-210.
7. McFarland LV, Elmer GW, Surawicz CM. Breaking the cycle: treatment strategies for 163 cases of recurrent Clostridium difficile disease. Am J Gastroenterol. 2002;97(7):1769-1775.
8. Kelly CP. Can we identify patients at high risk of recurrent Clostridium difficile infection? Clin Microbiol Infect. 2012;18(suppl 6):21-27.
9. Sartor RB. Probiotics for gastrointestinal diseases. https://www.uptodate.com/contents/probiotics-for-gastrointestinal-diseases. Updated September 4, 2018. Accessed April 4, 2019.
10. VSL#3 (Lactobacillus) [prescribing information]. Covington, LA: Alfasigma USA Inc; July 2017.
11. Culturelle Digestive Health Probiotic Capsules (Lactobacillus) [prescribing information]. Cromwell, CT: I-Health, Inc; 2015.
12. Flora-Q (Lactobacillus) [prescribing information]. Melville, NY: PharmaDerm; May 2012.
13. Lactinex (Lactobacillus) [prescribing information]. Franklin Lakes, NJ: Becton, Dickinson and Company; 2015
14. McDonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66(7):987-994.
15. Goldenberg JZ, Yap C, Lytvyn L, et al. Probiotics for the prevention of Clostridium difficile–associated diarrhea in adults and children. Cochrane Database Syst Rev. 2017;(12):CD006095.
16. Gao XW, Mubasher M, Fang CY, Reifer C, Miller LE. Dose–response efficacy of a proprietary probiotic formula of Lactobacillus acidophilus CL1285 and Lactobacillus casei LBC80R for antibiotic-associated diarrhea and Clostridium difficile–associated diarrhea prophylaxis in adult patients. Am J Gastroenterol. 2010;105(7):1636-1641.
17. Ouwehand AC, DongLian C, Weijian X, et al. Probiotics reduce symptoms of antibiotic use in a hospital setting: a randomized dose response study. Vaccine. 2014;32(4):458-463.
18. Allen SJ, Wareham K, Wang D, et al. Lactobacilli and bifidobacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2013;382(9900):1249-1257.
19. Shen NT, Maw A, Tmanova LL, et al. Timely use of probiotics in hospitalized adults prevents Clostridium difficile infection: a systematic review with meta-regression analysis. Gastroenterology. 2017;152(8):1889-1900.
20. Hickson M, D’Souza AL, Muthu N, et al. Use of probiotic Lactobacillus preparation to prevent diarrhoea associated with antibiotics: randomised double blind placebo controlled trial. BMJ. 2007;335(7610):80.
21. Center for Food Safety and Applied Nutrition. GRAS notice inventory. https://www.fda.gov/Food/IngredientsPackagingLabeling/GRAS/NoticeInventory/default.htm. Updated September 26, 2018. Accessed November 1, 2018.
22. Mattia A, Merker R. Regulation of probiotic substances as ingredients in foods: premarket approval or “generally recognized as safe” notification. Clin Infect Dis. 2008;46(suppl 2):S115-S118.
23. Probiotics: in depth. https://nccih.nih.gov/health/probiotics/introduction.htm. Updated October 2016. Accessed January 15, 2019.
24. Doron S, Snydman DR. Risk and safety of probiotics. Clin Infect Dis. 2015;60(suppl 2):S129-S134.
25. Bafeta A, Koh M, Riveros C, Ravaud P. Harms reporting in randomized controlled trials of interventions aimed at modifying microbiota: a systematic review. Ann Intern Med. 2018;169(4):240-247.
26. Boyle RJ, Robins-Browne RM, Tang ML. Probiotic use in clinical practice: what are the risks? Am J Clin Nutr. 2006;83(6):1256-1264.
27. Leffler DA, Lamont JT. Clostridium difficile infection. N Engl J Med. 2015;372(16):1539-1548.
28. Brown KA, Khanafer N, Daneman N, Fisman DN. Meta-analysis of antibiotics and the risk of community-associated Clostridium difficile infection. Antimicrob Agents Chemoth. 2013;57(5):2326-2332.
29. Oshima T, Wu L, Li M, Fukui H, Watari J, Miwa H. Magnitude and direction of the association between Clostridium difficile infection and proton pump inhibitors in adults and pediatric patients: a systematic review and meta-analysis. J Gastroenterol. 2018;53(1):84-94.
Clostridium difficile (C difficile) is a gram-positive, toxin-producing bacterium that is of increasing concern among health care providers and patients. Infection with C difficile can have manifestations ranging from mild diarrhea to severe toxic megacolon and can result in prolonged hospitalization with severe cases requiring admission to an intensive care unit.1 In 2014, the US was estimated to have more than 600,000 cases of C difficile infection (CDI), previously known as C difficile–associated diarrhea, and more than 44,000 associated deaths. The annual economic cost of CDI is thought to exceed $5 billion.1 According to studies of health care–associated illness, CDI rates are comparable to or have surpassed rates of methicillin-resistant Staphylococcus aureus infection within the US, including at US Department of Veterans Affairs (VA) acute care centers nationwide.2,3
C difficile has been shown to be the causative agent in 10% to 20% of antibiotic-associated diarrhea episodes.4 Colonization of C difficile is uncommon in healthy adults, but colonization rates are as high as 21% in hospitalized patients, with increasing rates proportional to increasing hospital length of stay.5,6 Although not all colonized patients develop clinically significant CDI, those who do may require multiple treatment courses, over months to years, because of the high risk of disease recurrence. An estimated 25% of patients have a single recurrent episode of CDI within 30 days after treatment completion, and 45% of those patients have additional recurrent infections.7,8 Although probiotics do not have an approved US Food and Drug Administration (FDA) indication, these supplements are often used to try to prevent CDI from developing during concomitant antibiotic use. Probiotics are microorganisms with potential health benefits, but the mechanisms of these benefits are not fully understood. Proposed mechanisms include reduced growth of pathogenic bacteria, modulation of the immune system, and support of the intestinal wall barrier.9 The many probiotic formulations currently marketed include Lactobacillus acidophilus (L acidophilus) capsules and various combinations of L acidophilus, Lactobacillus casei, Bifidobacterium lactis, Bifidobacterium longum, Streptococcus thermophilus, and other bacterial strains.
Dosing and Guidelines
Manufacturers’ suggested dosing for their Lactobacillus capsules, tablets, and packets varies from 1 unit daily to 4 units 4 times daily for dietary supplementation; the products’ labeling does not include any information regarding treatment duration.10-13 In addition, there are no published recommendations or product labeling guiding the dosing of probiotics or their duration of use in the primary prevention of CDI.
In 2017, the Infectious Diseases Society of America (IDSA) and the Society for Healthcare Epidemiology of America (SHEA) updated their CDI treatment guidelines.14 As these guidelines indicate that the data on probiotic use in CDI are inadequate, IDSA and SHEA make no recommendation for or against probiotic use in primary prevention of the disease. The guidelines point to several limitations in the literature, including variability in probiotic formulations studied, duration of probiotic administration, definitions of CDI, and duration of study follow-up.
Given the lack of consensus guidelines that clinicians can use when deciding which probiotic dosing and duration are appropriate for a patient for primary prevention of CDI, we evaluated the literature on the topic and summarize their findings here.
Review of Probiotoc Literature
Conflicting data exist about probiotics and their effect on CDI prevention. The literature reviewed was selected based on our assessment of its contribution to the topic and its potential utility to clinicians in determining appropriate probiotic therapies and recommendations. Included in our discussion is a large Cochrane Review of probiotic efficacy, 2 trials of probiotic dosing, the PLACIDE trial, and a systematic review of timely probiotic initiation. All of these studies attempted to determine the effect of probiotics on CDI incidence (Table).
In their 2017 Cochrane Review, Goldenberg and colleagues reviewed 39 trials that investigated the efficacy of probiotics in CDI prevention in 9,955 immunocompetent patients receiving antibiotics.15 The incidence of CDI was significantly lower in patients who received a probiotic than in patients who received placebo or no treatment (1.5% vs 4.0%; relative risk [RR], 0.40; 95% CI, 0.30-0.52; I2 = 0%). It is important to note that trials with a control-group CDI incidence of 0% to 2% (baseline CDI risk) found no statistically significant difference in CDI risk between patients using and not using probiotics (RR, 0.77; 95% CI, 0.45-1.32; I2 = 0%) and that the preceding statistically significant result may have been driven by the inclusion of trials with high baseline CDI risk (> 5%). Trials that enrolled patients who were at this risk level found a statistically significant 70% reduction in CDI risk in those using probiotics (vs no probiotics) while on concomitant antibiotic therapy (RR, 0.30; 95% CI, 0.21-0.42; I2 = 0%).
Probiotic therapy seems to be effective in reducing CDI risk in immunocompetent patients and may be particularly beneficial in patients at higher CDI risk, though Goldenberg and colleagues did not elaborate on what constitutes higher risk and based their conclusion on their control group’s high CDI incidence (> 5%). The most common adverse events (AEs) attributable to probiotics included abdominal cramping, nausea, fever, soft stools, flatulence, and taste disturbance. The review’s findings are limited in that the inclusion of many small trials with high baseline CDI risk likely contributed to a statistically significant result, and 17 of the review’s 39 trials were industry-sponsored or were conducted by investigators with industry associations; another 12 lacked statements about funding or sponsorship.
Two of the trials in the Cochrane Review investigated whether probiotics have a dose effect on CDI prevention. Gao and colleagues randomly assigned 255 hospitalized Asian patients to 3 groups: those receiving placebo, 1 probiotic capsule daily, and 2 probiotic capsules daily.16 Each probiotic capsule contained 50 billion colony-forming units (CFUs) of Lactobacillus. Incidence of CDI was lower in patients taking 2 probiotic capsules daily than in those taking 1 probiotic capsule daily (1.2% vs 9.4%; P = .04) or placebo (1.2% vs 23.8%; P = .002). In the other trial, Ouwehand and colleagues randomly assigned 503 hospitalized Asian patients to 3 groups as well: those receiving placebo, low-dose probiotic (4.17 billion CFUs of Lactobacillus and Bifidobacterium), and high-dose probiotic (17 billion CFUs).17 The incidence of CDI in each probiotic group (low-dose, high-dose) was 1.8%, which was significantly lower than the 4.8% in the placebo group (P = .04).
The Cochrane Review’s largest and most rigorous trial was PLACIDE, a 2013 randomized controlled study of the effect of probiotics on CDI.18 Allen and colleagues randomly assigned 2,981 inpatients (aged ≥ 65 years; exposed to antibiotics within preceding 7 days) to 2 groups: those receiving either 1 probiotic capsule daily, or 1 placebo capsule daily, for 21 days. Results showed no difference in CDI incidence between the probiotic and placebo groups (0.8% vs 1.2%; RR, 0.71; 95% CI, 0.34-1.47; P = .35). Of note, this trial is free of industry sponsorship, is the largest probiotic trial to date, has a control-group baseline CDI rate consistent with the rate in hospital and ambulatory settings in the US, and found a negative result regarding probiotic use in CDI prevention. Findings are limited in that the study allowed for initiating probiotic therapy up to 7 days after the start of antibiotics, and patients were given 1 relatively low-dose capsule daily, which may have contributed to lack of an effect on CDI prevention. No serious AEs were attributed to probiotic use.
In a 2017 systematic meta-analysis of 19 studies, Shen and colleagues investigated whether timely use of probiotics prevented CDI in 6,261 hospitalized patients receiving antibiotics.19 The incidence of CDI was significantly lower in patients receiving vs not receiving probiotics (1.6% vs 3.9%; RR, 0.42; 95% CI, 0.30-0.57; I2 = 0%; P < .001).19 A subgroup analysis was performed to compare studies initiating probiotics within 2 days after the start of antibiotics with studies initiating probiotics more than 2 days after the start. CDI risk was reduced by 68% when probiotics were started within 2 days, vs 30% when started after 2 days (RR, 0.32; 95% CI, 0.22-0.48; I2 = 0% vs RR, 0.70; 95% CI, 0.40-1.23; I2 = 0%; P = .02). Of note, no difference was found in efficacy among the various probiotic formulations, and no significant AEs were noted in any study group.
Trials included in the Cochrane Review used many different probiotic regimens over various durations.15 All these trials continued probiotics for at least the duration of antibiotic therapy. The 2 trials that evaluated the effect of probiotic therapy over an extended period required probiotics be started within 48 hours after initiation of antibiotic therapy; one trial continued probiotics for 5 days after completion of antibiotics, and the other for 7 days after completion.16,20 In both trials, CDI was statistically significantly reduced among adults using probiotics compared with adults receiving placebo.
Probiotic Safety
The FDA has not approved probiotics for the prevention or treatment of any health problems. Most probiotics are FDA-regulated as dietary supplements and do not have to meet stringent drug-approval requirements. The FDA has given many strains of common probiotics the Generally Recognized as Safe designation for use in commercially available products and foods.21-23 Probiotic use has not been associated with significant AEs in clinical trials and generally has been considered safe in immunocompetent and otherwise healthy persons.15-19 However, clinical trials have been inadequate in reporting or investigating AEs; the alternative for evaluating the risks of probiotic therapy is case reports.24,25 Theoretical risks associated with probiotics include sepsis, deleterious effects on normal gut digestion, excessive immune stimulation, and possible transfer of antimicrobial resistance genes among microorganisms.26 Boyle and colleagues further described a handful of case reports of sepsis caused by probiotics in immunocompromised individuals; the other theoretical risks have not been reported outside animal studies.26
CDI Risk Factors
Many factors can increase a patient’s CDI risk. Specific antibiotics (eg, ampicillin, amoxicillin, cephalosporins, clindamycin, fluoroquinolones) confer higher risk.27,28 Other factors include inflammatory bowel disease, organ transplantation, chemotherapy, chronic kidney disease, and immunodeficiency. Advanced age increases CDI risk and can increase the severity of infection. The evidence regarding acid suppression and CDI risk is conflicting, though a recent meta-analysis found that use of proton pump inhibitors is associated with a 2-fold higher risk of developing CDI.29 Patient-specific risk factors should be evaluated when the risk–benefit ratio for probiotic use is being considered.
Conclusion
CDIs are becoming increasingly burdensome to the health care system. More research is needed on the role of probiotics in CDI prevention in patients taking antibiotics. Given the limited risk for AEs when probiotics are used in immunocompetent patients and the relatively low cost of these supplements, the risks likely are outweighed by the postulated benefits, and probiotics may be recommended in select patient populations.
The PLACIDE trial found no benefit of probiotics in preventing CDI in a population similar to that of a typical US hospital or ambulatory setting, but its intervention allowed late initiation of relatively low doses of probiotics. Therefore, probiotics may be recommended for CDI prevention in patients taking antibiotics, especially patients at high risk for developing CDI. When clinicians recommend probiotic use in this setting, the probiotic should be initiated within 2 days after the start of antibiotics and should be continued for the duration of antibiotic therapy and for up to 7 days after that therapy is completed. Optimal probiotic dosing, likely dependent on the product used, remains unclear. PLACIDE trial results suggest that a dosage of at least 1 probiotic capsule 2 times daily may confer additional efficacy.
Clostridium difficile (C difficile) is a gram-positive, toxin-producing bacterium that is of increasing concern among health care providers and patients. Infection with C difficile can have manifestations ranging from mild diarrhea to severe toxic megacolon and can result in prolonged hospitalization with severe cases requiring admission to an intensive care unit.1 In 2014, the US was estimated to have more than 600,000 cases of C difficile infection (CDI), previously known as C difficile–associated diarrhea, and more than 44,000 associated deaths. The annual economic cost of CDI is thought to exceed $5 billion.1 According to studies of health care–associated illness, CDI rates are comparable to or have surpassed rates of methicillin-resistant Staphylococcus aureus infection within the US, including at US Department of Veterans Affairs (VA) acute care centers nationwide.2,3
C difficile has been shown to be the causative agent in 10% to 20% of antibiotic-associated diarrhea episodes.4 Colonization of C difficile is uncommon in healthy adults, but colonization rates are as high as 21% in hospitalized patients, with increasing rates proportional to increasing hospital length of stay.5,6 Although not all colonized patients develop clinically significant CDI, those who do may require multiple treatment courses, over months to years, because of the high risk of disease recurrence. An estimated 25% of patients have a single recurrent episode of CDI within 30 days after treatment completion, and 45% of those patients have additional recurrent infections.7,8 Although probiotics do not have an approved US Food and Drug Administration (FDA) indication, these supplements are often used to try to prevent CDI from developing during concomitant antibiotic use. Probiotics are microorganisms with potential health benefits, but the mechanisms of these benefits are not fully understood. Proposed mechanisms include reduced growth of pathogenic bacteria, modulation of the immune system, and support of the intestinal wall barrier.9 The many probiotic formulations currently marketed include Lactobacillus acidophilus (L acidophilus) capsules and various combinations of L acidophilus, Lactobacillus casei, Bifidobacterium lactis, Bifidobacterium longum, Streptococcus thermophilus, and other bacterial strains.
Dosing and Guidelines
Manufacturers’ suggested dosing for their Lactobacillus capsules, tablets, and packets varies from 1 unit daily to 4 units 4 times daily for dietary supplementation; the products’ labeling does not include any information regarding treatment duration.10-13 In addition, there are no published recommendations or product labeling guiding the dosing of probiotics or their duration of use in the primary prevention of CDI.
In 2017, the Infectious Diseases Society of America (IDSA) and the Society for Healthcare Epidemiology of America (SHEA) updated their CDI treatment guidelines.14 As these guidelines indicate that the data on probiotic use in CDI are inadequate, IDSA and SHEA make no recommendation for or against probiotic use in primary prevention of the disease. The guidelines point to several limitations in the literature, including variability in probiotic formulations studied, duration of probiotic administration, definitions of CDI, and duration of study follow-up.
Given the lack of consensus guidelines that clinicians can use when deciding which probiotic dosing and duration are appropriate for a patient for primary prevention of CDI, we evaluated the literature on the topic and summarize their findings here.
Review of Probiotoc Literature
Conflicting data exist about probiotics and their effect on CDI prevention. The literature reviewed was selected based on our assessment of its contribution to the topic and its potential utility to clinicians in determining appropriate probiotic therapies and recommendations. Included in our discussion is a large Cochrane Review of probiotic efficacy, 2 trials of probiotic dosing, the PLACIDE trial, and a systematic review of timely probiotic initiation. All of these studies attempted to determine the effect of probiotics on CDI incidence (Table).
In their 2017 Cochrane Review, Goldenberg and colleagues reviewed 39 trials that investigated the efficacy of probiotics in CDI prevention in 9,955 immunocompetent patients receiving antibiotics.15 The incidence of CDI was significantly lower in patients who received a probiotic than in patients who received placebo or no treatment (1.5% vs 4.0%; relative risk [RR], 0.40; 95% CI, 0.30-0.52; I2 = 0%). It is important to note that trials with a control-group CDI incidence of 0% to 2% (baseline CDI risk) found no statistically significant difference in CDI risk between patients using and not using probiotics (RR, 0.77; 95% CI, 0.45-1.32; I2 = 0%) and that the preceding statistically significant result may have been driven by the inclusion of trials with high baseline CDI risk (> 5%). Trials that enrolled patients who were at this risk level found a statistically significant 70% reduction in CDI risk in those using probiotics (vs no probiotics) while on concomitant antibiotic therapy (RR, 0.30; 95% CI, 0.21-0.42; I2 = 0%).
Probiotic therapy seems to be effective in reducing CDI risk in immunocompetent patients and may be particularly beneficial in patients at higher CDI risk, though Goldenberg and colleagues did not elaborate on what constitutes higher risk and based their conclusion on their control group’s high CDI incidence (> 5%). The most common adverse events (AEs) attributable to probiotics included abdominal cramping, nausea, fever, soft stools, flatulence, and taste disturbance. The review’s findings are limited in that the inclusion of many small trials with high baseline CDI risk likely contributed to a statistically significant result, and 17 of the review’s 39 trials were industry-sponsored or were conducted by investigators with industry associations; another 12 lacked statements about funding or sponsorship.
Two of the trials in the Cochrane Review investigated whether probiotics have a dose effect on CDI prevention. Gao and colleagues randomly assigned 255 hospitalized Asian patients to 3 groups: those receiving placebo, 1 probiotic capsule daily, and 2 probiotic capsules daily.16 Each probiotic capsule contained 50 billion colony-forming units (CFUs) of Lactobacillus. Incidence of CDI was lower in patients taking 2 probiotic capsules daily than in those taking 1 probiotic capsule daily (1.2% vs 9.4%; P = .04) or placebo (1.2% vs 23.8%; P = .002). In the other trial, Ouwehand and colleagues randomly assigned 503 hospitalized Asian patients to 3 groups as well: those receiving placebo, low-dose probiotic (4.17 billion CFUs of Lactobacillus and Bifidobacterium), and high-dose probiotic (17 billion CFUs).17 The incidence of CDI in each probiotic group (low-dose, high-dose) was 1.8%, which was significantly lower than the 4.8% in the placebo group (P = .04).
The Cochrane Review’s largest and most rigorous trial was PLACIDE, a 2013 randomized controlled study of the effect of probiotics on CDI.18 Allen and colleagues randomly assigned 2,981 inpatients (aged ≥ 65 years; exposed to antibiotics within preceding 7 days) to 2 groups: those receiving either 1 probiotic capsule daily, or 1 placebo capsule daily, for 21 days. Results showed no difference in CDI incidence between the probiotic and placebo groups (0.8% vs 1.2%; RR, 0.71; 95% CI, 0.34-1.47; P = .35). Of note, this trial is free of industry sponsorship, is the largest probiotic trial to date, has a control-group baseline CDI rate consistent with the rate in hospital and ambulatory settings in the US, and found a negative result regarding probiotic use in CDI prevention. Findings are limited in that the study allowed for initiating probiotic therapy up to 7 days after the start of antibiotics, and patients were given 1 relatively low-dose capsule daily, which may have contributed to lack of an effect on CDI prevention. No serious AEs were attributed to probiotic use.
In a 2017 systematic meta-analysis of 19 studies, Shen and colleagues investigated whether timely use of probiotics prevented CDI in 6,261 hospitalized patients receiving antibiotics.19 The incidence of CDI was significantly lower in patients receiving vs not receiving probiotics (1.6% vs 3.9%; RR, 0.42; 95% CI, 0.30-0.57; I2 = 0%; P < .001).19 A subgroup analysis was performed to compare studies initiating probiotics within 2 days after the start of antibiotics with studies initiating probiotics more than 2 days after the start. CDI risk was reduced by 68% when probiotics were started within 2 days, vs 30% when started after 2 days (RR, 0.32; 95% CI, 0.22-0.48; I2 = 0% vs RR, 0.70; 95% CI, 0.40-1.23; I2 = 0%; P = .02). Of note, no difference was found in efficacy among the various probiotic formulations, and no significant AEs were noted in any study group.
Trials included in the Cochrane Review used many different probiotic regimens over various durations.15 All these trials continued probiotics for at least the duration of antibiotic therapy. The 2 trials that evaluated the effect of probiotic therapy over an extended period required probiotics be started within 48 hours after initiation of antibiotic therapy; one trial continued probiotics for 5 days after completion of antibiotics, and the other for 7 days after completion.16,20 In both trials, CDI was statistically significantly reduced among adults using probiotics compared with adults receiving placebo.
Probiotic Safety
The FDA has not approved probiotics for the prevention or treatment of any health problems. Most probiotics are FDA-regulated as dietary supplements and do not have to meet stringent drug-approval requirements. The FDA has given many strains of common probiotics the Generally Recognized as Safe designation for use in commercially available products and foods.21-23 Probiotic use has not been associated with significant AEs in clinical trials and generally has been considered safe in immunocompetent and otherwise healthy persons.15-19 However, clinical trials have been inadequate in reporting or investigating AEs; the alternative for evaluating the risks of probiotic therapy is case reports.24,25 Theoretical risks associated with probiotics include sepsis, deleterious effects on normal gut digestion, excessive immune stimulation, and possible transfer of antimicrobial resistance genes among microorganisms.26 Boyle and colleagues further described a handful of case reports of sepsis caused by probiotics in immunocompromised individuals; the other theoretical risks have not been reported outside animal studies.26
CDI Risk Factors
Many factors can increase a patient’s CDI risk. Specific antibiotics (eg, ampicillin, amoxicillin, cephalosporins, clindamycin, fluoroquinolones) confer higher risk.27,28 Other factors include inflammatory bowel disease, organ transplantation, chemotherapy, chronic kidney disease, and immunodeficiency. Advanced age increases CDI risk and can increase the severity of infection. The evidence regarding acid suppression and CDI risk is conflicting, though a recent meta-analysis found that use of proton pump inhibitors is associated with a 2-fold higher risk of developing CDI.29 Patient-specific risk factors should be evaluated when the risk–benefit ratio for probiotic use is being considered.
Conclusion
CDIs are becoming increasingly burdensome to the health care system. More research is needed on the role of probiotics in CDI prevention in patients taking antibiotics. Given the limited risk for AEs when probiotics are used in immunocompetent patients and the relatively low cost of these supplements, the risks likely are outweighed by the postulated benefits, and probiotics may be recommended in select patient populations.
The PLACIDE trial found no benefit of probiotics in preventing CDI in a population similar to that of a typical US hospital or ambulatory setting, but its intervention allowed late initiation of relatively low doses of probiotics. Therefore, probiotics may be recommended for CDI prevention in patients taking antibiotics, especially patients at high risk for developing CDI. When clinicians recommend probiotic use in this setting, the probiotic should be initiated within 2 days after the start of antibiotics and should be continued for the duration of antibiotic therapy and for up to 7 days after that therapy is completed. Optimal probiotic dosing, likely dependent on the product used, remains unclear. PLACIDE trial results suggest that a dosage of at least 1 probiotic capsule 2 times daily may confer additional efficacy.
1. Desai K, Gupta SB, Dubberke ER, Prabhu VS, Browne C, Mast TC. Epidemiological and economic burden of Clostridium difficile in the United States: estimates from a modeling approach. BMC Infect Dis. 2016;16:303.
2. Miller BA, Chen LF, Sexton DJ, Anderson DJ. Comparison of the burdens of hospital-onset, healthcare facility-associated Clostridium difficile infection and of healthcare-associated infection due to methicillin-resistant Staphylococcus aureus in community hospitals. Infect Control Hosp Epidemiol. 2011;32(4):387-390.
3. Evans ME, Kralovic SM, Simbartl LA, Jain R, Roselle GA. Effect of a Clostridium difficile infection prevention initiative in Veterans Affairs acute care facilities. Infect Control Hosp Epidemiol. 2016;37(6):720-722.
4. Bartlett JG. Clinical practice. Antibiotic-associated diarrhea. N Engl J Med. 2002;346(5):334-339.
5. Johnson S, Clabots CR, Linn FV, Olson MM, Peterson LR, Gerding DN. Nosocomial Clostridium difficile colonisation and disease. Lancet. 1990;336(8707):97-100.
6. McFarland LV, Mulligan ME, Kwok RY, Stamm WE. Nosocomial acquisition of Clostridium difficile infection. N Engl J Med. 1989;320(4):204-210.
7. McFarland LV, Elmer GW, Surawicz CM. Breaking the cycle: treatment strategies for 163 cases of recurrent Clostridium difficile disease. Am J Gastroenterol. 2002;97(7):1769-1775.
8. Kelly CP. Can we identify patients at high risk of recurrent Clostridium difficile infection? Clin Microbiol Infect. 2012;18(suppl 6):21-27.
9. Sartor RB. Probiotics for gastrointestinal diseases. https://www.uptodate.com/contents/probiotics-for-gastrointestinal-diseases. Updated September 4, 2018. Accessed April 4, 2019.
10. VSL#3 (Lactobacillus) [prescribing information]. Covington, LA: Alfasigma USA Inc; July 2017.
11. Culturelle Digestive Health Probiotic Capsules (Lactobacillus) [prescribing information]. Cromwell, CT: I-Health, Inc; 2015.
12. Flora-Q (Lactobacillus) [prescribing information]. Melville, NY: PharmaDerm; May 2012.
13. Lactinex (Lactobacillus) [prescribing information]. Franklin Lakes, NJ: Becton, Dickinson and Company; 2015
14. McDonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66(7):987-994.
15. Goldenberg JZ, Yap C, Lytvyn L, et al. Probiotics for the prevention of Clostridium difficile–associated diarrhea in adults and children. Cochrane Database Syst Rev. 2017;(12):CD006095.
16. Gao XW, Mubasher M, Fang CY, Reifer C, Miller LE. Dose–response efficacy of a proprietary probiotic formula of Lactobacillus acidophilus CL1285 and Lactobacillus casei LBC80R for antibiotic-associated diarrhea and Clostridium difficile–associated diarrhea prophylaxis in adult patients. Am J Gastroenterol. 2010;105(7):1636-1641.
17. Ouwehand AC, DongLian C, Weijian X, et al. Probiotics reduce symptoms of antibiotic use in a hospital setting: a randomized dose response study. Vaccine. 2014;32(4):458-463.
18. Allen SJ, Wareham K, Wang D, et al. Lactobacilli and bifidobacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2013;382(9900):1249-1257.
19. Shen NT, Maw A, Tmanova LL, et al. Timely use of probiotics in hospitalized adults prevents Clostridium difficile infection: a systematic review with meta-regression analysis. Gastroenterology. 2017;152(8):1889-1900.
20. Hickson M, D’Souza AL, Muthu N, et al. Use of probiotic Lactobacillus preparation to prevent diarrhoea associated with antibiotics: randomised double blind placebo controlled trial. BMJ. 2007;335(7610):80.
21. Center for Food Safety and Applied Nutrition. GRAS notice inventory. https://www.fda.gov/Food/IngredientsPackagingLabeling/GRAS/NoticeInventory/default.htm. Updated September 26, 2018. Accessed November 1, 2018.
22. Mattia A, Merker R. Regulation of probiotic substances as ingredients in foods: premarket approval or “generally recognized as safe” notification. Clin Infect Dis. 2008;46(suppl 2):S115-S118.
23. Probiotics: in depth. https://nccih.nih.gov/health/probiotics/introduction.htm. Updated October 2016. Accessed January 15, 2019.
24. Doron S, Snydman DR. Risk and safety of probiotics. Clin Infect Dis. 2015;60(suppl 2):S129-S134.
25. Bafeta A, Koh M, Riveros C, Ravaud P. Harms reporting in randomized controlled trials of interventions aimed at modifying microbiota: a systematic review. Ann Intern Med. 2018;169(4):240-247.
26. Boyle RJ, Robins-Browne RM, Tang ML. Probiotic use in clinical practice: what are the risks? Am J Clin Nutr. 2006;83(6):1256-1264.
27. Leffler DA, Lamont JT. Clostridium difficile infection. N Engl J Med. 2015;372(16):1539-1548.
28. Brown KA, Khanafer N, Daneman N, Fisman DN. Meta-analysis of antibiotics and the risk of community-associated Clostridium difficile infection. Antimicrob Agents Chemoth. 2013;57(5):2326-2332.
29. Oshima T, Wu L, Li M, Fukui H, Watari J, Miwa H. Magnitude and direction of the association between Clostridium difficile infection and proton pump inhibitors in adults and pediatric patients: a systematic review and meta-analysis. J Gastroenterol. 2018;53(1):84-94.
1. Desai K, Gupta SB, Dubberke ER, Prabhu VS, Browne C, Mast TC. Epidemiological and economic burden of Clostridium difficile in the United States: estimates from a modeling approach. BMC Infect Dis. 2016;16:303.
2. Miller BA, Chen LF, Sexton DJ, Anderson DJ. Comparison of the burdens of hospital-onset, healthcare facility-associated Clostridium difficile infection and of healthcare-associated infection due to methicillin-resistant Staphylococcus aureus in community hospitals. Infect Control Hosp Epidemiol. 2011;32(4):387-390.
3. Evans ME, Kralovic SM, Simbartl LA, Jain R, Roselle GA. Effect of a Clostridium difficile infection prevention initiative in Veterans Affairs acute care facilities. Infect Control Hosp Epidemiol. 2016;37(6):720-722.
4. Bartlett JG. Clinical practice. Antibiotic-associated diarrhea. N Engl J Med. 2002;346(5):334-339.
5. Johnson S, Clabots CR, Linn FV, Olson MM, Peterson LR, Gerding DN. Nosocomial Clostridium difficile colonisation and disease. Lancet. 1990;336(8707):97-100.
6. McFarland LV, Mulligan ME, Kwok RY, Stamm WE. Nosocomial acquisition of Clostridium difficile infection. N Engl J Med. 1989;320(4):204-210.
7. McFarland LV, Elmer GW, Surawicz CM. Breaking the cycle: treatment strategies for 163 cases of recurrent Clostridium difficile disease. Am J Gastroenterol. 2002;97(7):1769-1775.
8. Kelly CP. Can we identify patients at high risk of recurrent Clostridium difficile infection? Clin Microbiol Infect. 2012;18(suppl 6):21-27.
9. Sartor RB. Probiotics for gastrointestinal diseases. https://www.uptodate.com/contents/probiotics-for-gastrointestinal-diseases. Updated September 4, 2018. Accessed April 4, 2019.
10. VSL#3 (Lactobacillus) [prescribing information]. Covington, LA: Alfasigma USA Inc; July 2017.
11. Culturelle Digestive Health Probiotic Capsules (Lactobacillus) [prescribing information]. Cromwell, CT: I-Health, Inc; 2015.
12. Flora-Q (Lactobacillus) [prescribing information]. Melville, NY: PharmaDerm; May 2012.
13. Lactinex (Lactobacillus) [prescribing information]. Franklin Lakes, NJ: Becton, Dickinson and Company; 2015
14. McDonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66(7):987-994.
15. Goldenberg JZ, Yap C, Lytvyn L, et al. Probiotics for the prevention of Clostridium difficile–associated diarrhea in adults and children. Cochrane Database Syst Rev. 2017;(12):CD006095.
16. Gao XW, Mubasher M, Fang CY, Reifer C, Miller LE. Dose–response efficacy of a proprietary probiotic formula of Lactobacillus acidophilus CL1285 and Lactobacillus casei LBC80R for antibiotic-associated diarrhea and Clostridium difficile–associated diarrhea prophylaxis in adult patients. Am J Gastroenterol. 2010;105(7):1636-1641.
17. Ouwehand AC, DongLian C, Weijian X, et al. Probiotics reduce symptoms of antibiotic use in a hospital setting: a randomized dose response study. Vaccine. 2014;32(4):458-463.
18. Allen SJ, Wareham K, Wang D, et al. Lactobacilli and bifidobacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2013;382(9900):1249-1257.
19. Shen NT, Maw A, Tmanova LL, et al. Timely use of probiotics in hospitalized adults prevents Clostridium difficile infection: a systematic review with meta-regression analysis. Gastroenterology. 2017;152(8):1889-1900.
20. Hickson M, D’Souza AL, Muthu N, et al. Use of probiotic Lactobacillus preparation to prevent diarrhoea associated with antibiotics: randomised double blind placebo controlled trial. BMJ. 2007;335(7610):80.
21. Center for Food Safety and Applied Nutrition. GRAS notice inventory. https://www.fda.gov/Food/IngredientsPackagingLabeling/GRAS/NoticeInventory/default.htm. Updated September 26, 2018. Accessed November 1, 2018.
22. Mattia A, Merker R. Regulation of probiotic substances as ingredients in foods: premarket approval or “generally recognized as safe” notification. Clin Infect Dis. 2008;46(suppl 2):S115-S118.
23. Probiotics: in depth. https://nccih.nih.gov/health/probiotics/introduction.htm. Updated October 2016. Accessed January 15, 2019.
24. Doron S, Snydman DR. Risk and safety of probiotics. Clin Infect Dis. 2015;60(suppl 2):S129-S134.
25. Bafeta A, Koh M, Riveros C, Ravaud P. Harms reporting in randomized controlled trials of interventions aimed at modifying microbiota: a systematic review. Ann Intern Med. 2018;169(4):240-247.
26. Boyle RJ, Robins-Browne RM, Tang ML. Probiotic use in clinical practice: what are the risks? Am J Clin Nutr. 2006;83(6):1256-1264.
27. Leffler DA, Lamont JT. Clostridium difficile infection. N Engl J Med. 2015;372(16):1539-1548.
28. Brown KA, Khanafer N, Daneman N, Fisman DN. Meta-analysis of antibiotics and the risk of community-associated Clostridium difficile infection. Antimicrob Agents Chemoth. 2013;57(5):2326-2332.
29. Oshima T, Wu L, Li M, Fukui H, Watari J, Miwa H. Magnitude and direction of the association between Clostridium difficile infection and proton pump inhibitors in adults and pediatric patients: a systematic review and meta-analysis. J Gastroenterol. 2018;53(1):84-94.