User login
Proton pump inhibitors (PPIs) have become the mainstay of therapy in the treatment of acid-related disorders since their introduction in 1989. Due to their high potency, excellent tolerability, and generic availability, PPIs have largely replaced histamine-2 receptor antagonists for gastric problems. Since they were first released on the market, the use of PPIs has continued to rise in both the hospital and primary care settings.1 However, this rapid growth has led to the concern of overutilization. A study conducted at the Department of Veterans Affairs (VA) Ann Arbor Health Care System found that out of 946 patients in the ambulatory care setting taking PPIs, only 35% were appropriately prescribed PPIs.2
Although the short-term adverse effects of PPI use seem minimal, chronic PPI use consequences are a growing concern. Chronic PPI use is associated with increased risks of osteoporosis, pneumonia, and Clostridium difficile infections.3 Another long-term risk that has been associated with chronic PPI use is dementia. Dementia is a cognitive syndrome that is characterized by a progressive decline beyond what is expected in normal aging in 1 or more of the cognitive domains of memory, language, orientation, learning capacity, executive function, or social cognition.4 Because it interferes with activities of daily living, dementia is a major cause of disability in the elderly and is an immense burden for caregivers. Currently, about 47 million people globally live with dementia.5 This number is projected to nearly triple by 2050 to 132 million.5 With no cure, identification of risk factors and creation of protective measures are critical in decreasing the prevalence of dementia.
Although the exact pathophysiology behind the link between PPIs and dementia is unknown, several theories exist. One such theory is that PPI-induced vitamin B12 deficiency leads to cognitive decline.6,7 Another theory suggests that PPIs can directly cause dementia by inhibiting enzymes that normally degrade β amyloid.8 This leads to increased levels of β-amyloid plaques, which is a known characteristic of dementia patients. This theory is derived from animal studies that have shown increased amyloid levels in the brains of mice given PPIs.8
Current studies are conflicting regarding the association between PPIs and dementia. Two German prospective, cohort studies found statistically significant increased risks of dementia in patients taking PPIs with hazard ratios (HR) of 1.38 (95% CI, 1.04-1.83) and 1.44 (95% CI, 1.36-1.52), respectively.9,10 A study conducted in Taiwan also found an increased risk of dementia among PPI users with a HR of 1.22 (95% CI, 1.05-1.42).11 On the contrary, other studies have failed to show an increased risk of dementia with PPI use. In fact, Goldstein and colleagues found a decreased risk of dementia in PPI users with a HR of 0.78 (95% CI, 0.76-0.93).12 This study was an observational study conducted in the US using data from the National Alzheimer’s Coordinating Center database.12 Another recent retrospective study conducted in Finland showed that PPI use was not associated with a significantly increased risk of Alzheimer disease.13
Much is unknown about the cause of dementia, and no curative treatment exists. Investigation into potential risk factors for dementia can lead to the development of preventative measures, which can lead to significant improvement in quality of life for both patients and caregivers. Current studies regarding the association between PPIs and dementia are conflicting, and to our knowledge, no study analyzing the effects of PPIs and dementia has been conducted within the veteran population specifically. The objective of the current study is to investigate the association between PPI use and dementia in the veteran population.
Methods
This study is a retrospective, cohort, single-center, chart review study conducted at the Sioux Falls Veteran Affairs Health Care System (SFVAHCS). Data were extracted from the VA electronic health record (EHR) from January 1, 2005 through December 31, 2015. The study included both currently living and deceased veterans who received ≥ 2 documented outpatient visits at the SFVAHCS during the study time frame. Patients also had to be aged ≥ 60 years at the start of the study period. Patients were excluded if they received only a ≤ 30-day PPI prescription. Patients with dementia related to head trauma, acute intoxication, or other known diseases were excluded.
To analyze the primary endpoint of association between PPI use and dementia, the study compared the rate of dementia in a cohort of veterans who had received an outpatient prescription for a PPI within the study time frame vs the rate of dementia in a random, equal number of veterans who had never been prescribed PPIs within the study time frame. In this study, veterans were classified as having dementia if they had a diagnosis of dementia based on ICD-9 or ICD-10 codes (Table 1), or if they had been prescribed medications used to treat dementia (donepezil, ergoloid mesylates, galantamine, memantine, and rivastigmine).
Secondary endpoints included analysis of the effects of PPI agent, PPI dose, and PPI duration on the risk of dementia. For the PPI dose analysis, cumulative doses were converted into defined daily doses (DDDs) using the World Health Organization calculation to equalize the different potencies of PPI agents (Table 2).14 In addition, the effect of PPI use on vitamin B12 levels was analyzed as an exploratory endpoint to investigate the hypothesis that PPI may be associated with vitamin B12 deficiency, which in turn may be associated with dementia.6,7
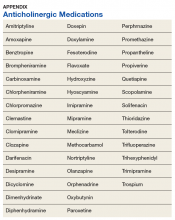
Baseline characteristics were collected to determine the variability between the treatment and control group. Data collected included age, gender, past medical history of diseases that may increase risk of dementia, and anticholinergic drug use. Anticholinergic drugs were included if they were classified as having “definite anticholinergic effects” based on the Aging Brain Care Anticholinergic Burden Scale (Appendix).15
Statistical Analysis
The primary endpoint was analyzed using a χ2 for association test. For the secondary endpoints, a χ2 for association test was used for endpoints with nominal data, and the Mood median test was used for endpoints with continuous data. The exploratory endpoint analyzing vitamin B12 levels was analyzed with the Mood median test. A P value of < .05 was defined as being statistically significant. Power analysis was not performed since all veterans who met the criteria were included in the study.
Results
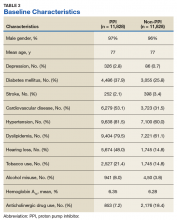
Records of 23,656 veterans were included in the study with 11,828 veterans in both the PPI cohort and the non-PPI cohort (Table 3).
Primary Endpoint
Within the PPI group, 1,119 (9.5%) veterans had dementia compared with only 740 (6.3%) veterans in the non-PPI group. There was a statistically significant association between PPI use and dementia (P < .001). These results yielded an odds ratio of 1.55 for dementia risk in PPI users vs nonusers and a relative risk increase of 51.4% for dementia risk with PPI use compared with no PPI use.
Secondary Endpoints
Users of rabeprazole had the highest rate of dementia (12.8%), followed by lansoprazole (10.9%), omeprazole (9.7%), esomeprazole (7.7%), and pantoprazole (7.0%). The rate of dementia for non-PPI users was 6.3% (P < .001). The median cumulative doses of PPIs were not significant: 597 DDDs (95% CI, 540-630) in the dementia group vs 570 DDDs (95% CI, 540-624) in the nondementia group (P = .79). The median cumulative duration of PPI use in the dementia group was 4.6 years (95% CI, 4.25-4.92) vs 5.3 years (95% CI, 5.08-5.42) in the nondementia group (P < .001).
Exploratory Endpoint
The median B12 level in the PPI group was 521 pg/mL (95% CI, 509-533) compared with 480 pg/mL (95% CI, 465-496) in the non-PPI group (P < .001). However, both groups fell within the normal range for vitamin B12 (200-900 pg/mL).16
Discussion
The aim of this study was to determine whether an association existed between PPI use and dementia. This study showed a statistically significant association between PPI use and dementia within the veteran population. This study also showed a significant association between specific PPI agents and dementia. When analyzing the individual PPI agents, the rabeprazole group yielded the strongest relationship. However, this study was not powered to evaluate and compare risks of dementia between individual PPI agents. More data are needed to determine statistical and clinical significance of associations between individual PPI agents and risk of dementia.
The veterans with dementia had a higher median cumulative PPI dose than did the veterans without dementia; however, the results were not statistically significant. Therefore, the data cannot correlate higher doses of PPI use to increased risk of dementia.
The cumulative duration of PPI use was statistically significant but opposite of the expected outcome. The dementia group had a lower median lifetime duration of PPI use compared with that of the nondementia group. It is difficult to determine the reason for this outcome, but it seems that for this study population, a longer duration of PPI use was not associated with an increased risk of dementia.
Finally, the exploratory endpoint analyzed vitamin B12 levels, since it has been shown that PPI use can lead to vitamin B12 deficiency and that B12 deficiency can lead to dementia.6-8 This study found that the dementia group had significantly higher vitamin B12 levels than the nondementia group. These data suggest that PPI use may not be associated with vitamin B12 deficiency. However, it is important to note that this study was unable to collect data on the use of vitamin B12 supplementation due to the unreliability of over-the-counter (OTC) and non-VA medication use records. Therefore, it is possible that the PPI group had higher rates of B12 deficiency but were effectively treated with B12 supplementation. More research is needed to determine the exact relationship between PPI use, vitamin B12 deficiency, and dementia risk.
Strengths/Limitations
Strengths of this study that support its findings include the large population size. Additionally, the use of the VA EHR allowed for a complete drug dispensing history to be collected, which improves reliability of the data.
This study also had some limitations. First, the causal relationship of PPI use and dementia cannot be proven using a retrospective cohort design. This study’s design can show association, but it cannot prove causation. Also, due to the retrospective design, exposure to PPI use could not be randomized; thus, correlation between PPI use and dementia may be explained by confounding variables that are not captured within this study. This is especially true since the baseline characteristics were not equally distributed between the 2 groups. In fact, the PPI group had higher rates of many clinical comorbidities. This imbalance may have skewed the results of the primary endpoint. Lastly, OTC PPI use and non-VA PPI prescriptions were not available. Therefore, some of the patients included in the non-PPI group may have been PPI users if they received PPIs from OTC or non-VA sources, which could skew the results.
Conclusion
This study showed a significant association between PPI use and dementia within the veteran study population. The study also showed a significant association between PPI use and dementia within the secondary endpoint of individual PPI agent. Higher cumulative dose and duration of PPI use did not seem to increase risk of dementia. Finally, PPI use was not associated with significantly low vitamin B12 levels. More studies are needed to determine causation of dementia and its risk factors.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Sioux Falls VA Health Care System.
1. Savarino V, Dulbecco P, de Bortoli N, Ottonello A, Savarino E. The appropriate use of proton pump inhibitors (PPIs): need for a reappraisal. Eur J Intern Med. 2017;37:19-24.
2. Heidelbaugh J, Goldberg K, Inadomi J. Magnitude and economic effect of overuse of antisecretory therapy in the ambulatory care setting. Am J Manag Care. 2010;16(9):e228-e234.
3. Heidelbaugh JJ, Kim AH, Chang R. Walker PC. Overutilization of proton-pump inhibitors: what the clinician needs to know. Therap Adv Gastroenterol. 2012;5(4):219-232.
4. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, (DSM-5). American Psychiatric Association: Washington, DC; 2013.
5. World Health Organization. Dementia. http://www.who.int/mediacentre/factsheets/fs362/en/. Published December 12, 2017. Accessed March 10, 2019.
6. Vogiatzoglou A, Smith AD, Nurk E, et al. Cognitive function in an elderly population: interaction between vitamin B12 status, depression, and apolipoprotein E ε4: the Hordaland Homocysteine Study. Psychosom Med. 2013;75(1):20-29.
7. Lam JR, Schneider JL, Zhao W, Corley DA. Proton pump inhibitor and histamine 2 receptor antagonist use and vitamin B12 deficiency. JAMA. 2013;310(22):2435-2442.
8. Badiola N, Alcalde V, Pujol A, et al. The proton-pump inhibitor lansoprazole enhances amyloid beta production. PLoS One. 2013;8(3):e58837.
9. Haenisch B, von Holt K, Wiese B, et al. Risk of dementia in elderly patients with the use of proton pump inhibitors. Eur Arch Psychiatry Clin Neurosci. 2015;265(5):419-428.
10. Gomm W, von Holt K, Thomé F, et al. Association between proton pump inhibitors with risk of dementia. A pharmacoepidemiological claims data analysis. JAMA Neurol. 2016;73(4):410-416.
11. Tai SY, Chien CY, Wu DC, et al. Risk of dementia from proton pump inhibitor use in Asian population: a nationwide cohort study in Taiwan. PLoS One. 2017;12(2):e0171006.
12. Goldstein FC, Steenland K, Zhao L, Wharton W, Levey AI, Hajjar I. Proton pump inhibitors and risk of mild cognitive impairment and dementia. J Am Geriatr Soc. 2017;65(9):1969-1674.
13. Taipale H, Tolppanen AM, Tiihonen M. Tanskanen A, Tiihonen J, Hartikainen S. No association between proton pump inhibitor use and risk of Alzheimer’s disease. Am J Gastroenterol. 2017;112(12):1801-1808.
14. World Health Organization Collaborating Centre for Drug Statistics Methodology. Definition and general considerations. https://www.whocc.no/ddd/definition_and_general_considera/. Updated February 7, 2018. Accessed March 13, 2019.
15. Indiana University Center for Aging Research, Aging Brain Program. Anticholinergic cognitive burden scale. http://www.idhca.org/wp-content/uploads/2018/02/DESAI_ACB_scale_-_Legal_size_paper.pdf. Updated 2012. Accessed March 10, 2019.
16. US National Library of Medicine, MedlinePlus. Vitamin B12 level. https://medlineplus.gov/ency/article/003705.htm. Updated March 7, 2019. Accessed March 13, 2019.
Proton pump inhibitors (PPIs) have become the mainstay of therapy in the treatment of acid-related disorders since their introduction in 1989. Due to their high potency, excellent tolerability, and generic availability, PPIs have largely replaced histamine-2 receptor antagonists for gastric problems. Since they were first released on the market, the use of PPIs has continued to rise in both the hospital and primary care settings.1 However, this rapid growth has led to the concern of overutilization. A study conducted at the Department of Veterans Affairs (VA) Ann Arbor Health Care System found that out of 946 patients in the ambulatory care setting taking PPIs, only 35% were appropriately prescribed PPIs.2
Although the short-term adverse effects of PPI use seem minimal, chronic PPI use consequences are a growing concern. Chronic PPI use is associated with increased risks of osteoporosis, pneumonia, and Clostridium difficile infections.3 Another long-term risk that has been associated with chronic PPI use is dementia. Dementia is a cognitive syndrome that is characterized by a progressive decline beyond what is expected in normal aging in 1 or more of the cognitive domains of memory, language, orientation, learning capacity, executive function, or social cognition.4 Because it interferes with activities of daily living, dementia is a major cause of disability in the elderly and is an immense burden for caregivers. Currently, about 47 million people globally live with dementia.5 This number is projected to nearly triple by 2050 to 132 million.5 With no cure, identification of risk factors and creation of protective measures are critical in decreasing the prevalence of dementia.
Although the exact pathophysiology behind the link between PPIs and dementia is unknown, several theories exist. One such theory is that PPI-induced vitamin B12 deficiency leads to cognitive decline.6,7 Another theory suggests that PPIs can directly cause dementia by inhibiting enzymes that normally degrade β amyloid.8 This leads to increased levels of β-amyloid plaques, which is a known characteristic of dementia patients. This theory is derived from animal studies that have shown increased amyloid levels in the brains of mice given PPIs.8
Current studies are conflicting regarding the association between PPIs and dementia. Two German prospective, cohort studies found statistically significant increased risks of dementia in patients taking PPIs with hazard ratios (HR) of 1.38 (95% CI, 1.04-1.83) and 1.44 (95% CI, 1.36-1.52), respectively.9,10 A study conducted in Taiwan also found an increased risk of dementia among PPI users with a HR of 1.22 (95% CI, 1.05-1.42).11 On the contrary, other studies have failed to show an increased risk of dementia with PPI use. In fact, Goldstein and colleagues found a decreased risk of dementia in PPI users with a HR of 0.78 (95% CI, 0.76-0.93).12 This study was an observational study conducted in the US using data from the National Alzheimer’s Coordinating Center database.12 Another recent retrospective study conducted in Finland showed that PPI use was not associated with a significantly increased risk of Alzheimer disease.13
Much is unknown about the cause of dementia, and no curative treatment exists. Investigation into potential risk factors for dementia can lead to the development of preventative measures, which can lead to significant improvement in quality of life for both patients and caregivers. Current studies regarding the association between PPIs and dementia are conflicting, and to our knowledge, no study analyzing the effects of PPIs and dementia has been conducted within the veteran population specifically. The objective of the current study is to investigate the association between PPI use and dementia in the veteran population.
Methods
This study is a retrospective, cohort, single-center, chart review study conducted at the Sioux Falls Veteran Affairs Health Care System (SFVAHCS). Data were extracted from the VA electronic health record (EHR) from January 1, 2005 through December 31, 2015. The study included both currently living and deceased veterans who received ≥ 2 documented outpatient visits at the SFVAHCS during the study time frame. Patients also had to be aged ≥ 60 years at the start of the study period. Patients were excluded if they received only a ≤ 30-day PPI prescription. Patients with dementia related to head trauma, acute intoxication, or other known diseases were excluded.
To analyze the primary endpoint of association between PPI use and dementia, the study compared the rate of dementia in a cohort of veterans who had received an outpatient prescription for a PPI within the study time frame vs the rate of dementia in a random, equal number of veterans who had never been prescribed PPIs within the study time frame. In this study, veterans were classified as having dementia if they had a diagnosis of dementia based on ICD-9 or ICD-10 codes (Table 1), or if they had been prescribed medications used to treat dementia (donepezil, ergoloid mesylates, galantamine, memantine, and rivastigmine).
Secondary endpoints included analysis of the effects of PPI agent, PPI dose, and PPI duration on the risk of dementia. For the PPI dose analysis, cumulative doses were converted into defined daily doses (DDDs) using the World Health Organization calculation to equalize the different potencies of PPI agents (Table 2).14 In addition, the effect of PPI use on vitamin B12 levels was analyzed as an exploratory endpoint to investigate the hypothesis that PPI may be associated with vitamin B12 deficiency, which in turn may be associated with dementia.6,7
Baseline characteristics were collected to determine the variability between the treatment and control group. Data collected included age, gender, past medical history of diseases that may increase risk of dementia, and anticholinergic drug use. Anticholinergic drugs were included if they were classified as having “definite anticholinergic effects” based on the Aging Brain Care Anticholinergic Burden Scale (Appendix).15
Statistical Analysis
The primary endpoint was analyzed using a χ2 for association test. For the secondary endpoints, a χ2 for association test was used for endpoints with nominal data, and the Mood median test was used for endpoints with continuous data. The exploratory endpoint analyzing vitamin B12 levels was analyzed with the Mood median test. A P value of < .05 was defined as being statistically significant. Power analysis was not performed since all veterans who met the criteria were included in the study.
Results
Records of 23,656 veterans were included in the study with 11,828 veterans in both the PPI cohort and the non-PPI cohort (Table 3).
Primary Endpoint
Within the PPI group, 1,119 (9.5%) veterans had dementia compared with only 740 (6.3%) veterans in the non-PPI group. There was a statistically significant association between PPI use and dementia (P < .001). These results yielded an odds ratio of 1.55 for dementia risk in PPI users vs nonusers and a relative risk increase of 51.4% for dementia risk with PPI use compared with no PPI use.
Secondary Endpoints
Users of rabeprazole had the highest rate of dementia (12.8%), followed by lansoprazole (10.9%), omeprazole (9.7%), esomeprazole (7.7%), and pantoprazole (7.0%). The rate of dementia for non-PPI users was 6.3% (P < .001). The median cumulative doses of PPIs were not significant: 597 DDDs (95% CI, 540-630) in the dementia group vs 570 DDDs (95% CI, 540-624) in the nondementia group (P = .79). The median cumulative duration of PPI use in the dementia group was 4.6 years (95% CI, 4.25-4.92) vs 5.3 years (95% CI, 5.08-5.42) in the nondementia group (P < .001).
Exploratory Endpoint
The median B12 level in the PPI group was 521 pg/mL (95% CI, 509-533) compared with 480 pg/mL (95% CI, 465-496) in the non-PPI group (P < .001). However, both groups fell within the normal range for vitamin B12 (200-900 pg/mL).16
Discussion
The aim of this study was to determine whether an association existed between PPI use and dementia. This study showed a statistically significant association between PPI use and dementia within the veteran population. This study also showed a significant association between specific PPI agents and dementia. When analyzing the individual PPI agents, the rabeprazole group yielded the strongest relationship. However, this study was not powered to evaluate and compare risks of dementia between individual PPI agents. More data are needed to determine statistical and clinical significance of associations between individual PPI agents and risk of dementia.
The veterans with dementia had a higher median cumulative PPI dose than did the veterans without dementia; however, the results were not statistically significant. Therefore, the data cannot correlate higher doses of PPI use to increased risk of dementia.
The cumulative duration of PPI use was statistically significant but opposite of the expected outcome. The dementia group had a lower median lifetime duration of PPI use compared with that of the nondementia group. It is difficult to determine the reason for this outcome, but it seems that for this study population, a longer duration of PPI use was not associated with an increased risk of dementia.
Finally, the exploratory endpoint analyzed vitamin B12 levels, since it has been shown that PPI use can lead to vitamin B12 deficiency and that B12 deficiency can lead to dementia.6-8 This study found that the dementia group had significantly higher vitamin B12 levels than the nondementia group. These data suggest that PPI use may not be associated with vitamin B12 deficiency. However, it is important to note that this study was unable to collect data on the use of vitamin B12 supplementation due to the unreliability of over-the-counter (OTC) and non-VA medication use records. Therefore, it is possible that the PPI group had higher rates of B12 deficiency but were effectively treated with B12 supplementation. More research is needed to determine the exact relationship between PPI use, vitamin B12 deficiency, and dementia risk.
Strengths/Limitations
Strengths of this study that support its findings include the large population size. Additionally, the use of the VA EHR allowed for a complete drug dispensing history to be collected, which improves reliability of the data.
This study also had some limitations. First, the causal relationship of PPI use and dementia cannot be proven using a retrospective cohort design. This study’s design can show association, but it cannot prove causation. Also, due to the retrospective design, exposure to PPI use could not be randomized; thus, correlation between PPI use and dementia may be explained by confounding variables that are not captured within this study. This is especially true since the baseline characteristics were not equally distributed between the 2 groups. In fact, the PPI group had higher rates of many clinical comorbidities. This imbalance may have skewed the results of the primary endpoint. Lastly, OTC PPI use and non-VA PPI prescriptions were not available. Therefore, some of the patients included in the non-PPI group may have been PPI users if they received PPIs from OTC or non-VA sources, which could skew the results.
Conclusion
This study showed a significant association between PPI use and dementia within the veteran study population. The study also showed a significant association between PPI use and dementia within the secondary endpoint of individual PPI agent. Higher cumulative dose and duration of PPI use did not seem to increase risk of dementia. Finally, PPI use was not associated with significantly low vitamin B12 levels. More studies are needed to determine causation of dementia and its risk factors.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Sioux Falls VA Health Care System.
Proton pump inhibitors (PPIs) have become the mainstay of therapy in the treatment of acid-related disorders since their introduction in 1989. Due to their high potency, excellent tolerability, and generic availability, PPIs have largely replaced histamine-2 receptor antagonists for gastric problems. Since they were first released on the market, the use of PPIs has continued to rise in both the hospital and primary care settings.1 However, this rapid growth has led to the concern of overutilization. A study conducted at the Department of Veterans Affairs (VA) Ann Arbor Health Care System found that out of 946 patients in the ambulatory care setting taking PPIs, only 35% were appropriately prescribed PPIs.2
Although the short-term adverse effects of PPI use seem minimal, chronic PPI use consequences are a growing concern. Chronic PPI use is associated with increased risks of osteoporosis, pneumonia, and Clostridium difficile infections.3 Another long-term risk that has been associated with chronic PPI use is dementia. Dementia is a cognitive syndrome that is characterized by a progressive decline beyond what is expected in normal aging in 1 or more of the cognitive domains of memory, language, orientation, learning capacity, executive function, or social cognition.4 Because it interferes with activities of daily living, dementia is a major cause of disability in the elderly and is an immense burden for caregivers. Currently, about 47 million people globally live with dementia.5 This number is projected to nearly triple by 2050 to 132 million.5 With no cure, identification of risk factors and creation of protective measures are critical in decreasing the prevalence of dementia.
Although the exact pathophysiology behind the link between PPIs and dementia is unknown, several theories exist. One such theory is that PPI-induced vitamin B12 deficiency leads to cognitive decline.6,7 Another theory suggests that PPIs can directly cause dementia by inhibiting enzymes that normally degrade β amyloid.8 This leads to increased levels of β-amyloid plaques, which is a known characteristic of dementia patients. This theory is derived from animal studies that have shown increased amyloid levels in the brains of mice given PPIs.8
Current studies are conflicting regarding the association between PPIs and dementia. Two German prospective, cohort studies found statistically significant increased risks of dementia in patients taking PPIs with hazard ratios (HR) of 1.38 (95% CI, 1.04-1.83) and 1.44 (95% CI, 1.36-1.52), respectively.9,10 A study conducted in Taiwan also found an increased risk of dementia among PPI users with a HR of 1.22 (95% CI, 1.05-1.42).11 On the contrary, other studies have failed to show an increased risk of dementia with PPI use. In fact, Goldstein and colleagues found a decreased risk of dementia in PPI users with a HR of 0.78 (95% CI, 0.76-0.93).12 This study was an observational study conducted in the US using data from the National Alzheimer’s Coordinating Center database.12 Another recent retrospective study conducted in Finland showed that PPI use was not associated with a significantly increased risk of Alzheimer disease.13
Much is unknown about the cause of dementia, and no curative treatment exists. Investigation into potential risk factors for dementia can lead to the development of preventative measures, which can lead to significant improvement in quality of life for both patients and caregivers. Current studies regarding the association between PPIs and dementia are conflicting, and to our knowledge, no study analyzing the effects of PPIs and dementia has been conducted within the veteran population specifically. The objective of the current study is to investigate the association between PPI use and dementia in the veteran population.
Methods
This study is a retrospective, cohort, single-center, chart review study conducted at the Sioux Falls Veteran Affairs Health Care System (SFVAHCS). Data were extracted from the VA electronic health record (EHR) from January 1, 2005 through December 31, 2015. The study included both currently living and deceased veterans who received ≥ 2 documented outpatient visits at the SFVAHCS during the study time frame. Patients also had to be aged ≥ 60 years at the start of the study period. Patients were excluded if they received only a ≤ 30-day PPI prescription. Patients with dementia related to head trauma, acute intoxication, or other known diseases were excluded.
To analyze the primary endpoint of association between PPI use and dementia, the study compared the rate of dementia in a cohort of veterans who had received an outpatient prescription for a PPI within the study time frame vs the rate of dementia in a random, equal number of veterans who had never been prescribed PPIs within the study time frame. In this study, veterans were classified as having dementia if they had a diagnosis of dementia based on ICD-9 or ICD-10 codes (Table 1), or if they had been prescribed medications used to treat dementia (donepezil, ergoloid mesylates, galantamine, memantine, and rivastigmine).
Secondary endpoints included analysis of the effects of PPI agent, PPI dose, and PPI duration on the risk of dementia. For the PPI dose analysis, cumulative doses were converted into defined daily doses (DDDs) using the World Health Organization calculation to equalize the different potencies of PPI agents (Table 2).14 In addition, the effect of PPI use on vitamin B12 levels was analyzed as an exploratory endpoint to investigate the hypothesis that PPI may be associated with vitamin B12 deficiency, which in turn may be associated with dementia.6,7
Baseline characteristics were collected to determine the variability between the treatment and control group. Data collected included age, gender, past medical history of diseases that may increase risk of dementia, and anticholinergic drug use. Anticholinergic drugs were included if they were classified as having “definite anticholinergic effects” based on the Aging Brain Care Anticholinergic Burden Scale (Appendix).15
Statistical Analysis
The primary endpoint was analyzed using a χ2 for association test. For the secondary endpoints, a χ2 for association test was used for endpoints with nominal data, and the Mood median test was used for endpoints with continuous data. The exploratory endpoint analyzing vitamin B12 levels was analyzed with the Mood median test. A P value of < .05 was defined as being statistically significant. Power analysis was not performed since all veterans who met the criteria were included in the study.
Results
Records of 23,656 veterans were included in the study with 11,828 veterans in both the PPI cohort and the non-PPI cohort (Table 3).
Primary Endpoint
Within the PPI group, 1,119 (9.5%) veterans had dementia compared with only 740 (6.3%) veterans in the non-PPI group. There was a statistically significant association between PPI use and dementia (P < .001). These results yielded an odds ratio of 1.55 for dementia risk in PPI users vs nonusers and a relative risk increase of 51.4% for dementia risk with PPI use compared with no PPI use.
Secondary Endpoints
Users of rabeprazole had the highest rate of dementia (12.8%), followed by lansoprazole (10.9%), omeprazole (9.7%), esomeprazole (7.7%), and pantoprazole (7.0%). The rate of dementia for non-PPI users was 6.3% (P < .001). The median cumulative doses of PPIs were not significant: 597 DDDs (95% CI, 540-630) in the dementia group vs 570 DDDs (95% CI, 540-624) in the nondementia group (P = .79). The median cumulative duration of PPI use in the dementia group was 4.6 years (95% CI, 4.25-4.92) vs 5.3 years (95% CI, 5.08-5.42) in the nondementia group (P < .001).
Exploratory Endpoint
The median B12 level in the PPI group was 521 pg/mL (95% CI, 509-533) compared with 480 pg/mL (95% CI, 465-496) in the non-PPI group (P < .001). However, both groups fell within the normal range for vitamin B12 (200-900 pg/mL).16
Discussion
The aim of this study was to determine whether an association existed between PPI use and dementia. This study showed a statistically significant association between PPI use and dementia within the veteran population. This study also showed a significant association between specific PPI agents and dementia. When analyzing the individual PPI agents, the rabeprazole group yielded the strongest relationship. However, this study was not powered to evaluate and compare risks of dementia between individual PPI agents. More data are needed to determine statistical and clinical significance of associations between individual PPI agents and risk of dementia.
The veterans with dementia had a higher median cumulative PPI dose than did the veterans without dementia; however, the results were not statistically significant. Therefore, the data cannot correlate higher doses of PPI use to increased risk of dementia.
The cumulative duration of PPI use was statistically significant but opposite of the expected outcome. The dementia group had a lower median lifetime duration of PPI use compared with that of the nondementia group. It is difficult to determine the reason for this outcome, but it seems that for this study population, a longer duration of PPI use was not associated with an increased risk of dementia.
Finally, the exploratory endpoint analyzed vitamin B12 levels, since it has been shown that PPI use can lead to vitamin B12 deficiency and that B12 deficiency can lead to dementia.6-8 This study found that the dementia group had significantly higher vitamin B12 levels than the nondementia group. These data suggest that PPI use may not be associated with vitamin B12 deficiency. However, it is important to note that this study was unable to collect data on the use of vitamin B12 supplementation due to the unreliability of over-the-counter (OTC) and non-VA medication use records. Therefore, it is possible that the PPI group had higher rates of B12 deficiency but were effectively treated with B12 supplementation. More research is needed to determine the exact relationship between PPI use, vitamin B12 deficiency, and dementia risk.
Strengths/Limitations
Strengths of this study that support its findings include the large population size. Additionally, the use of the VA EHR allowed for a complete drug dispensing history to be collected, which improves reliability of the data.
This study also had some limitations. First, the causal relationship of PPI use and dementia cannot be proven using a retrospective cohort design. This study’s design can show association, but it cannot prove causation. Also, due to the retrospective design, exposure to PPI use could not be randomized; thus, correlation between PPI use and dementia may be explained by confounding variables that are not captured within this study. This is especially true since the baseline characteristics were not equally distributed between the 2 groups. In fact, the PPI group had higher rates of many clinical comorbidities. This imbalance may have skewed the results of the primary endpoint. Lastly, OTC PPI use and non-VA PPI prescriptions were not available. Therefore, some of the patients included in the non-PPI group may have been PPI users if they received PPIs from OTC or non-VA sources, which could skew the results.
Conclusion
This study showed a significant association between PPI use and dementia within the veteran study population. The study also showed a significant association between PPI use and dementia within the secondary endpoint of individual PPI agent. Higher cumulative dose and duration of PPI use did not seem to increase risk of dementia. Finally, PPI use was not associated with significantly low vitamin B12 levels. More studies are needed to determine causation of dementia and its risk factors.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Sioux Falls VA Health Care System.
1. Savarino V, Dulbecco P, de Bortoli N, Ottonello A, Savarino E. The appropriate use of proton pump inhibitors (PPIs): need for a reappraisal. Eur J Intern Med. 2017;37:19-24.
2. Heidelbaugh J, Goldberg K, Inadomi J. Magnitude and economic effect of overuse of antisecretory therapy in the ambulatory care setting. Am J Manag Care. 2010;16(9):e228-e234.
3. Heidelbaugh JJ, Kim AH, Chang R. Walker PC. Overutilization of proton-pump inhibitors: what the clinician needs to know. Therap Adv Gastroenterol. 2012;5(4):219-232.
4. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, (DSM-5). American Psychiatric Association: Washington, DC; 2013.
5. World Health Organization. Dementia. http://www.who.int/mediacentre/factsheets/fs362/en/. Published December 12, 2017. Accessed March 10, 2019.
6. Vogiatzoglou A, Smith AD, Nurk E, et al. Cognitive function in an elderly population: interaction between vitamin B12 status, depression, and apolipoprotein E ε4: the Hordaland Homocysteine Study. Psychosom Med. 2013;75(1):20-29.
7. Lam JR, Schneider JL, Zhao W, Corley DA. Proton pump inhibitor and histamine 2 receptor antagonist use and vitamin B12 deficiency. JAMA. 2013;310(22):2435-2442.
8. Badiola N, Alcalde V, Pujol A, et al. The proton-pump inhibitor lansoprazole enhances amyloid beta production. PLoS One. 2013;8(3):e58837.
9. Haenisch B, von Holt K, Wiese B, et al. Risk of dementia in elderly patients with the use of proton pump inhibitors. Eur Arch Psychiatry Clin Neurosci. 2015;265(5):419-428.
10. Gomm W, von Holt K, Thomé F, et al. Association between proton pump inhibitors with risk of dementia. A pharmacoepidemiological claims data analysis. JAMA Neurol. 2016;73(4):410-416.
11. Tai SY, Chien CY, Wu DC, et al. Risk of dementia from proton pump inhibitor use in Asian population: a nationwide cohort study in Taiwan. PLoS One. 2017;12(2):e0171006.
12. Goldstein FC, Steenland K, Zhao L, Wharton W, Levey AI, Hajjar I. Proton pump inhibitors and risk of mild cognitive impairment and dementia. J Am Geriatr Soc. 2017;65(9):1969-1674.
13. Taipale H, Tolppanen AM, Tiihonen M. Tanskanen A, Tiihonen J, Hartikainen S. No association between proton pump inhibitor use and risk of Alzheimer’s disease. Am J Gastroenterol. 2017;112(12):1801-1808.
14. World Health Organization Collaborating Centre for Drug Statistics Methodology. Definition and general considerations. https://www.whocc.no/ddd/definition_and_general_considera/. Updated February 7, 2018. Accessed March 13, 2019.
15. Indiana University Center for Aging Research, Aging Brain Program. Anticholinergic cognitive burden scale. http://www.idhca.org/wp-content/uploads/2018/02/DESAI_ACB_scale_-_Legal_size_paper.pdf. Updated 2012. Accessed March 10, 2019.
16. US National Library of Medicine, MedlinePlus. Vitamin B12 level. https://medlineplus.gov/ency/article/003705.htm. Updated March 7, 2019. Accessed March 13, 2019.
1. Savarino V, Dulbecco P, de Bortoli N, Ottonello A, Savarino E. The appropriate use of proton pump inhibitors (PPIs): need for a reappraisal. Eur J Intern Med. 2017;37:19-24.
2. Heidelbaugh J, Goldberg K, Inadomi J. Magnitude and economic effect of overuse of antisecretory therapy in the ambulatory care setting. Am J Manag Care. 2010;16(9):e228-e234.
3. Heidelbaugh JJ, Kim AH, Chang R. Walker PC. Overutilization of proton-pump inhibitors: what the clinician needs to know. Therap Adv Gastroenterol. 2012;5(4):219-232.
4. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, (DSM-5). American Psychiatric Association: Washington, DC; 2013.
5. World Health Organization. Dementia. http://www.who.int/mediacentre/factsheets/fs362/en/. Published December 12, 2017. Accessed March 10, 2019.
6. Vogiatzoglou A, Smith AD, Nurk E, et al. Cognitive function in an elderly population: interaction between vitamin B12 status, depression, and apolipoprotein E ε4: the Hordaland Homocysteine Study. Psychosom Med. 2013;75(1):20-29.
7. Lam JR, Schneider JL, Zhao W, Corley DA. Proton pump inhibitor and histamine 2 receptor antagonist use and vitamin B12 deficiency. JAMA. 2013;310(22):2435-2442.
8. Badiola N, Alcalde V, Pujol A, et al. The proton-pump inhibitor lansoprazole enhances amyloid beta production. PLoS One. 2013;8(3):e58837.
9. Haenisch B, von Holt K, Wiese B, et al. Risk of dementia in elderly patients with the use of proton pump inhibitors. Eur Arch Psychiatry Clin Neurosci. 2015;265(5):419-428.
10. Gomm W, von Holt K, Thomé F, et al. Association between proton pump inhibitors with risk of dementia. A pharmacoepidemiological claims data analysis. JAMA Neurol. 2016;73(4):410-416.
11. Tai SY, Chien CY, Wu DC, et al. Risk of dementia from proton pump inhibitor use in Asian population: a nationwide cohort study in Taiwan. PLoS One. 2017;12(2):e0171006.
12. Goldstein FC, Steenland K, Zhao L, Wharton W, Levey AI, Hajjar I. Proton pump inhibitors and risk of mild cognitive impairment and dementia. J Am Geriatr Soc. 2017;65(9):1969-1674.
13. Taipale H, Tolppanen AM, Tiihonen M. Tanskanen A, Tiihonen J, Hartikainen S. No association between proton pump inhibitor use and risk of Alzheimer’s disease. Am J Gastroenterol. 2017;112(12):1801-1808.
14. World Health Organization Collaborating Centre for Drug Statistics Methodology. Definition and general considerations. https://www.whocc.no/ddd/definition_and_general_considera/. Updated February 7, 2018. Accessed March 13, 2019.
15. Indiana University Center for Aging Research, Aging Brain Program. Anticholinergic cognitive burden scale. http://www.idhca.org/wp-content/uploads/2018/02/DESAI_ACB_scale_-_Legal_size_paper.pdf. Updated 2012. Accessed March 10, 2019.
16. US National Library of Medicine, MedlinePlus. Vitamin B12 level. https://medlineplus.gov/ency/article/003705.htm. Updated March 7, 2019. Accessed March 13, 2019.