User login
even when done in accordance with endoscope reprocessing standards, according to results of a prospective, multisite investigation.
All clinically used bronchoscopes evaluated in the study had residual contamination after reprocessing, and more than half showed microbial growth, the researchers reported in the journal CHEST.
These findings suggest that systematic changes are needed to improve cleaning and disinfection and to avoid the retention of bioburden, said researcher Cori L. Ofstead, MSPH, and her coinvestigators (Chest 2018 Nov;154[5]:1024-34).
“Evidence-based, bronchoscope-specific reprocessing and maintenance guidelines are needed, along with quality management programs to ensure that these complex processes are carried out effectively,” Ms. Ofstead and her colleagues said in their report.
Institutions also should consider shifting from high-level disinfection (HLD) to sterilization to reduce patient exposure to contaminated bronchoscopes, they added.
The study was conducted in three large, tertiary hospitals that contributed a total of 24 clinically used devices. That total comprised nine therapeutic, nine pediatric, and six endobronchial ultrasound (EBUS) bronchoscopes that were all reprocessed in accordance with each institution’s standard practices.
Proteins were detected in 100% of the bronchoscopes after HLD, according to researchers.
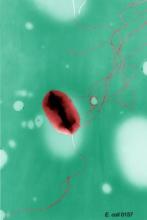
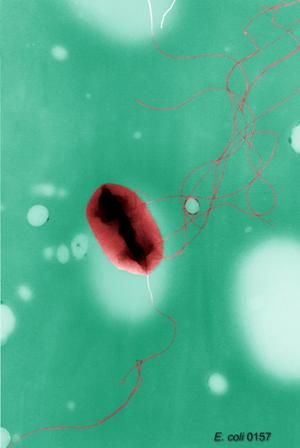
Looking at 20 paired postcleaning and post-HLD samples, the researchers found microbial growth in 11 of 20 (55%) manually-cleaned bronchoscopes and 14 of 24 (58%) bronchoscopes after HLD. The post-HLD samples included mold and recognized pathogens such as Escherichia coli, as well as normal flora and environmental bacteria, they said.
All 24 of the bronchoscopes had visible irregularities, including brown, red, or oily residue, retained fluid, debris in channels, scratches, or damage at insertion tubes and distal ends, they added.
Substandard reprocessing practices were found at two of the three participating institutions, according to the investigators. At one site, technicians reused syringes to flush channels with alcohol stored in an uncovered bowl during the day, according to the report, and bronchoscopes at that site were dried with reused towels and stored in a cabinet without active ventilation.
“Nursing staff were observed handling patient-ready bronchoscopes with bare hands,” the investigators reported.
Although clinical outcomes were not measured, the contamination, microbial growth, and defects observed in this study are “worrisome,” according to authors, because of the high infection risk in many patients undergoing bronchoscopy, and because of the infectious outbreaks and patient deaths linked to contaminated bronchoscopes in previous investigations.
Research funding for the study was provided by 3M Company. Study materials were provided by 3M Company and Healthmark Industries. Ms. Ofstead and several coauthors reported employment with Ofstead & Associates, which has received research funding and speaking fees related to infection prevention from 3M Company, Healthmark Industries, Advanced Sterilization Products (Johnson & Johnson), and others.
The senior author of the study was J. Scott Ferguson, MD, of the division of pulmonary and critical care medicine at the University of Wisconsin School of Medicine and Public Health, Madison. Dr. Ferguson provided disclosures related to NewWave Medical, Pharmaceutical Product Development, Oncocyte, Concordia, and PneumRx.
SOURCE: Ofstead C et al. Chest. 2018 Nov;154(5):1024-34.
Results of this study, which document biological contamination of inadequately reprocessed bronchoscopes, are provocative and “alarming,” warranting attention from physicians, paramedical staff, administrators, and manufacturers, Atul C. Mehta, MBBS, and Thomas Gildea, MD, wrote in an editorial (Chest 2018 Nov;154[5]:1001-3).
A breach in the disinfection protocol is the “most common culprit” behind the spread of infection during bronchoscopy, they noted.
“In our opinion, the interventional pulmonology community has buried its head in the sand regarding this issue,” they wrote.
While cases of true infection related to bronchoscopy “can seldom be differentiated” in the literature, it is nevertheless “mandatory” that biologic contamination from one patient to another be avoided, they wrote.
One dismaying observation in the present study is that human proteins were detected in the working channels of all of bronchoscopes after high-level disinfection (HLD), Dr. Mehta and Dr. Gildea stated.
“It is critical that if HLD remains the standard of care and is sufficient, it must be done properly,” they wrote. “This study raises concerns that HLD itself may not be sufficient, and we have no options for disposable specialty bronchoscopes.”
Unfortunately, requiring sterilized bronchoscopes is time-consuming and impractical for a busy bronchoscopy practice, according to the authors, while disposable bronchoscopes need to be established as clinically effective and cost effective.
In the meantime, the authors recommended that clinicians proactively consider initiatives to ensure patient safety, including formally assessing HLD processes, examining all bronchoscopes for visible damage, and ensuring that HLD guidelines are met or exceeded.
Dr. Mehta and Dr. Gildea are with the department of pulmonary medicine at Cleveland Clinic. Neither author reported conflicts of interest related to the editorial, which was published in Chest.
Results of this study, which document biological contamination of inadequately reprocessed bronchoscopes, are provocative and “alarming,” warranting attention from physicians, paramedical staff, administrators, and manufacturers, Atul C. Mehta, MBBS, and Thomas Gildea, MD, wrote in an editorial (Chest 2018 Nov;154[5]:1001-3).
A breach in the disinfection protocol is the “most common culprit” behind the spread of infection during bronchoscopy, they noted.
“In our opinion, the interventional pulmonology community has buried its head in the sand regarding this issue,” they wrote.
While cases of true infection related to bronchoscopy “can seldom be differentiated” in the literature, it is nevertheless “mandatory” that biologic contamination from one patient to another be avoided, they wrote.
One dismaying observation in the present study is that human proteins were detected in the working channels of all of bronchoscopes after high-level disinfection (HLD), Dr. Mehta and Dr. Gildea stated.
“It is critical that if HLD remains the standard of care and is sufficient, it must be done properly,” they wrote. “This study raises concerns that HLD itself may not be sufficient, and we have no options for disposable specialty bronchoscopes.”
Unfortunately, requiring sterilized bronchoscopes is time-consuming and impractical for a busy bronchoscopy practice, according to the authors, while disposable bronchoscopes need to be established as clinically effective and cost effective.
In the meantime, the authors recommended that clinicians proactively consider initiatives to ensure patient safety, including formally assessing HLD processes, examining all bronchoscopes for visible damage, and ensuring that HLD guidelines are met or exceeded.
Dr. Mehta and Dr. Gildea are with the department of pulmonary medicine at Cleveland Clinic. Neither author reported conflicts of interest related to the editorial, which was published in Chest.
Results of this study, which document biological contamination of inadequately reprocessed bronchoscopes, are provocative and “alarming,” warranting attention from physicians, paramedical staff, administrators, and manufacturers, Atul C. Mehta, MBBS, and Thomas Gildea, MD, wrote in an editorial (Chest 2018 Nov;154[5]:1001-3).
A breach in the disinfection protocol is the “most common culprit” behind the spread of infection during bronchoscopy, they noted.
“In our opinion, the interventional pulmonology community has buried its head in the sand regarding this issue,” they wrote.
While cases of true infection related to bronchoscopy “can seldom be differentiated” in the literature, it is nevertheless “mandatory” that biologic contamination from one patient to another be avoided, they wrote.
One dismaying observation in the present study is that human proteins were detected in the working channels of all of bronchoscopes after high-level disinfection (HLD), Dr. Mehta and Dr. Gildea stated.
“It is critical that if HLD remains the standard of care and is sufficient, it must be done properly,” they wrote. “This study raises concerns that HLD itself may not be sufficient, and we have no options for disposable specialty bronchoscopes.”
Unfortunately, requiring sterilized bronchoscopes is time-consuming and impractical for a busy bronchoscopy practice, according to the authors, while disposable bronchoscopes need to be established as clinically effective and cost effective.
In the meantime, the authors recommended that clinicians proactively consider initiatives to ensure patient safety, including formally assessing HLD processes, examining all bronchoscopes for visible damage, and ensuring that HLD guidelines are met or exceeded.
Dr. Mehta and Dr. Gildea are with the department of pulmonary medicine at Cleveland Clinic. Neither author reported conflicts of interest related to the editorial, which was published in Chest.
even when done in accordance with endoscope reprocessing standards, according to results of a prospective, multisite investigation.
All clinically used bronchoscopes evaluated in the study had residual contamination after reprocessing, and more than half showed microbial growth, the researchers reported in the journal CHEST.
These findings suggest that systematic changes are needed to improve cleaning and disinfection and to avoid the retention of bioburden, said researcher Cori L. Ofstead, MSPH, and her coinvestigators (Chest 2018 Nov;154[5]:1024-34).
“Evidence-based, bronchoscope-specific reprocessing and maintenance guidelines are needed, along with quality management programs to ensure that these complex processes are carried out effectively,” Ms. Ofstead and her colleagues said in their report.
Institutions also should consider shifting from high-level disinfection (HLD) to sterilization to reduce patient exposure to contaminated bronchoscopes, they added.
The study was conducted in three large, tertiary hospitals that contributed a total of 24 clinically used devices. That total comprised nine therapeutic, nine pediatric, and six endobronchial ultrasound (EBUS) bronchoscopes that were all reprocessed in accordance with each institution’s standard practices.
Proteins were detected in 100% of the bronchoscopes after HLD, according to researchers.
Looking at 20 paired postcleaning and post-HLD samples, the researchers found microbial growth in 11 of 20 (55%) manually-cleaned bronchoscopes and 14 of 24 (58%) bronchoscopes after HLD. The post-HLD samples included mold and recognized pathogens such as Escherichia coli, as well as normal flora and environmental bacteria, they said.
All 24 of the bronchoscopes had visible irregularities, including brown, red, or oily residue, retained fluid, debris in channels, scratches, or damage at insertion tubes and distal ends, they added.
Substandard reprocessing practices were found at two of the three participating institutions, according to the investigators. At one site, technicians reused syringes to flush channels with alcohol stored in an uncovered bowl during the day, according to the report, and bronchoscopes at that site were dried with reused towels and stored in a cabinet without active ventilation.
“Nursing staff were observed handling patient-ready bronchoscopes with bare hands,” the investigators reported.
Although clinical outcomes were not measured, the contamination, microbial growth, and defects observed in this study are “worrisome,” according to authors, because of the high infection risk in many patients undergoing bronchoscopy, and because of the infectious outbreaks and patient deaths linked to contaminated bronchoscopes in previous investigations.
Research funding for the study was provided by 3M Company. Study materials were provided by 3M Company and Healthmark Industries. Ms. Ofstead and several coauthors reported employment with Ofstead & Associates, which has received research funding and speaking fees related to infection prevention from 3M Company, Healthmark Industries, Advanced Sterilization Products (Johnson & Johnson), and others.
The senior author of the study was J. Scott Ferguson, MD, of the division of pulmonary and critical care medicine at the University of Wisconsin School of Medicine and Public Health, Madison. Dr. Ferguson provided disclosures related to NewWave Medical, Pharmaceutical Product Development, Oncocyte, Concordia, and PneumRx.
SOURCE: Ofstead C et al. Chest. 2018 Nov;154(5):1024-34.
even when done in accordance with endoscope reprocessing standards, according to results of a prospective, multisite investigation.
All clinically used bronchoscopes evaluated in the study had residual contamination after reprocessing, and more than half showed microbial growth, the researchers reported in the journal CHEST.
These findings suggest that systematic changes are needed to improve cleaning and disinfection and to avoid the retention of bioburden, said researcher Cori L. Ofstead, MSPH, and her coinvestigators (Chest 2018 Nov;154[5]:1024-34).
“Evidence-based, bronchoscope-specific reprocessing and maintenance guidelines are needed, along with quality management programs to ensure that these complex processes are carried out effectively,” Ms. Ofstead and her colleagues said in their report.
Institutions also should consider shifting from high-level disinfection (HLD) to sterilization to reduce patient exposure to contaminated bronchoscopes, they added.
The study was conducted in three large, tertiary hospitals that contributed a total of 24 clinically used devices. That total comprised nine therapeutic, nine pediatric, and six endobronchial ultrasound (EBUS) bronchoscopes that were all reprocessed in accordance with each institution’s standard practices.
Proteins were detected in 100% of the bronchoscopes after HLD, according to researchers.
Looking at 20 paired postcleaning and post-HLD samples, the researchers found microbial growth in 11 of 20 (55%) manually-cleaned bronchoscopes and 14 of 24 (58%) bronchoscopes after HLD. The post-HLD samples included mold and recognized pathogens such as Escherichia coli, as well as normal flora and environmental bacteria, they said.
All 24 of the bronchoscopes had visible irregularities, including brown, red, or oily residue, retained fluid, debris in channels, scratches, or damage at insertion tubes and distal ends, they added.
Substandard reprocessing practices were found at two of the three participating institutions, according to the investigators. At one site, technicians reused syringes to flush channels with alcohol stored in an uncovered bowl during the day, according to the report, and bronchoscopes at that site were dried with reused towels and stored in a cabinet without active ventilation.
“Nursing staff were observed handling patient-ready bronchoscopes with bare hands,” the investigators reported.
Although clinical outcomes were not measured, the contamination, microbial growth, and defects observed in this study are “worrisome,” according to authors, because of the high infection risk in many patients undergoing bronchoscopy, and because of the infectious outbreaks and patient deaths linked to contaminated bronchoscopes in previous investigations.
Research funding for the study was provided by 3M Company. Study materials were provided by 3M Company and Healthmark Industries. Ms. Ofstead and several coauthors reported employment with Ofstead & Associates, which has received research funding and speaking fees related to infection prevention from 3M Company, Healthmark Industries, Advanced Sterilization Products (Johnson & Johnson), and others.
The senior author of the study was J. Scott Ferguson, MD, of the division of pulmonary and critical care medicine at the University of Wisconsin School of Medicine and Public Health, Madison. Dr. Ferguson provided disclosures related to NewWave Medical, Pharmaceutical Product Development, Oncocyte, Concordia, and PneumRx.
SOURCE: Ofstead C et al. Chest. 2018 Nov;154(5):1024-34.
FROM CHEST
Key clinical point: Bronchoscope reprocessing was ineffective, even when performed in accordance with endoscope reprocessing standards.
Major finding: After high-level disinfection, residual contamination was found in 100% of bronchoscopes, while microbial growth was seen in 58%.
Study details: Observation and testing of 24 bronchoscopes contributed by three tertiary care hospitals.
Disclosures: 3M Company and Healthmark Industries provided study materials. Ms. Ofstead and several coauthors reported employment with Ofstead & Associates, which has received funding related to infection prevention from these two companies, among others. The senior study author disclosed ties to NewWave Medical, Pharmaceutical Product Development, Oncocyte, Concordia, and PneumRx.
Source: Ofstead C et al. Chest. 2018 Nov;154(5):1024-34.