User login
In modern times in the United States, the vesicovaginal fistula (VVF) arises chiefly as a sequela of gynecologic surgery, usually hysterectomy. The injury most likely occurs at the time of dissection of the bladder flap off of the lower uterine segment and upper vagina.1 With increasing use of endoscopy and electrosurgery at the time of hysterectomy,2 the occurrence of VVF is likely to increase. Because of this, fistulas stemming from benign gynecologic surgical activity tend to occur above the trigone near the vaginal cuff.
Current technique of fistula repair involves either a vaginal approach, with the Latsko procedure,3 or an abdominal approach, involving a laparotomy; laparoscopy also is used with increasing frequency.4
Vaginal versus endoscopic approach. The vaginal approach can be straightforward, such as in cases of vault prolapse or a distally located fistula, or more difficult if the fistula is apical in location, especially if the apex is well suspended and the vagina is of normal length. I have found the abdominal approach to be optimal if the fistula is near the cuff and the vagina is of normal length and well suspended.
Classical teaching tells us that the first repair of the VVF is likely to be the most successful, with successively lower cure rates as the number of repair attempts increases. For this reason, I advocate the abdominal approach in most cases of apically placed VVFs.
Surgical approach
Why endoscopic, why robotic? Often, repair of the VVF is complicated by:
-
the challenge of locating the defect in the bladder
-
the technical difficulty in oversewing the bladder, which often must be done on the underside of the bladder, between the vaginal and bladder walls.
To tackle these challenges, an endoscopic approach promises improved visualization, and the robotic approach allows for surgical closure with improved visibility characteristic of endoscopy, while preserving the manual dexterity characteristic of open surgery.
Timing. It is believed that, in order to improve chances of successful surgical repair, the fistula should be approached either immediately (ie, within 1 to 2 weeks of the insult) or delayed by 8 to 12 or more weeks after the causative surgery.5
Preparation. Vesicovaginal fistulas can rarely involve the ureters, and this ureteric involvement needs to be ruled out. Accordingly, the workup of the VVF should begin with a thorough cystoscopic evaluation of the bladder, with retrograde pyelography to evaluate the integrity of the ureters bilaterally.
During this procedure, the location of the fistulous tract should be meticulously mapped. Care should be taken to document the location and extent of the fistula, as well as to identify the presence of multiple or separate tracts. If these tracts are present, they also need also to be catalogued. In my practice, the cystoscopy/retrograde pyelogram is performed as a separate surgical encounter.
Surgical technique
After the fistula is mapped and ureteric integrity is confirmed, the definitive surgical repair is performed. The steps to the surgical approach are straightforward.
1. Insert stents into the fistula and bilaterally into the ureters
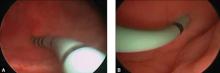
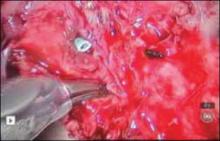
Stenting the fistula permits rapid identification of the fistula tract without the need to enter the bladder separately. The ureteric stents you use should be one color, and the fistula stent should be a second color. I use 5 French yellow stents to cannulate the ureters and a 6 French blue stent for the fistula itself. Insert the fistula stent from the bladder side of the fistula. It should exit through the vagina (FIGURES 1A and 1B). In addition, place a 3-way Foley catheter for drainage and irrigate the bladder when indicated.
Figure 1. Stent insertion
Cystoscopically place a 6 French blue stent into the bladder side of the fistula. (A) The stent as seen from inside the bladder. (B) The stent as seen from the vaginal side.
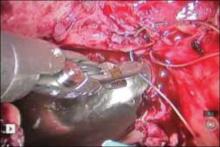
2. Place the ports for optimal access
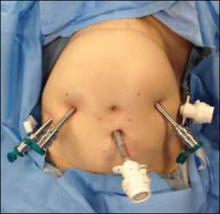
A 0° camera is adequate for visualization. Port placement is similar to that used for robotic sacral colpopexy; I use a supraumbilically placed camera port and three 8-mm robot ports (two on the left and one on the right of the umbilicus). Each port should be separated by about 10 cm. An assistant port is placed to the far right, for passing and retrieving sutures (FIGURE 2). An atraumatic grasper, monopolar scissors, and bipolar Maryland-type forceps are placed within the ports to begin the surgical procedure.
Figure 2. Ideal port placement
Place the camera port supraunbilically, with two 8-mm robot ports
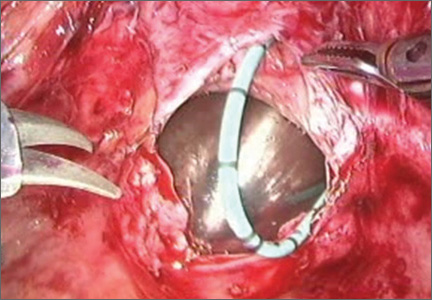
3. Place a vaginal stent to aid dissection
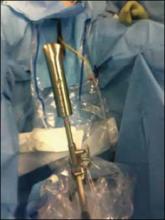
The stent should be a sterile cylinder and have a diameter of 2 cm to 5 cm (to match the vaginal caliber). The tip should be rounded and flattened, with an extended handle available for manipulating the stent. The handle can be held by an assistant or attached to an external uterine positioning system (FIGURE 3); I use the Uterine Positioning SystemTM (Cooper Surgical, Trumbull, Connecticut).
4. Incise the vaginal cuff
Transversely incise the vaginal cuff with the monopolar scissors (VIDEO 1).
This allows entry into the vagina at the apex. The blue stent in the fistula should be visible at the anterior vaginal wall, as demonstrated in FIGURE 4.
5. Dissect the vaginal wall
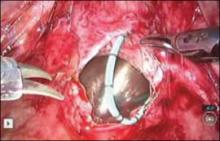
Dissect the anterior vaginal wall down to the fistula, and dissect the bladder off of the vaginal wall for about 1.5 cm to 2 cm around the fistula tract (FIGURE 5 and VIDEO 2).
6. Cut the stent
Cut the stent passing thru the fistula, to move it out of the way.
7. Close the bladder
Stitch the bladder in a running fashion using three layers of 3-0 rapid absorbable synthetic suture (FIGURE 6 and Video 3 . I prefer polyglactin 910 (Vicryl; Ethicon, Somerville, New Jersey) because it is easier to handle.
FIGURE 6: Close the bladderStitch the bladder in a running fashion using three layers of 3-0 rapid absorbable synthetic suture. Keep the closure line free of tension.
8. Close the vaginal side of the fistula
Stitch the vaginal side of the fistula in a running fashion with 2-0 absorbable synthetic suture.
9. Verify closure
Check for watertight closure by retrofilling the bladder with 100 mL of sterile milk (obtained from the labor/delivery suite). Observe the suture line for any evidence of milk leakage. (Sterile milk does not stain the tissues, and this preserves tissue visibility. For this reason, milk is preferable to indigo carmine or methylene blue.)
10. Remove the stents from the bladder
Cystoscopically remove all stents.
11. Close the laparoscopic ports
12. Follow up to ensure surgical success
Leave the indwelling Foley catheter in place for 2 to 3 weeks. After such time, remove the catheter and perform voiding cystourethrogram to document bladder wall integrity.
Discussion
I have described a systematic approach to robotic VVF repair. The robotic portion of the procedure should require about 60 to 90 minutes in the absence of significant adhesions. The technique is amenable to a laparoscopic approach, when performed by an appropriately skilled operator.
Final takeaways. Important takeaways to this repair include:
- Stent the fistula to make it easy to find intraoperatively.
- Enter the vagina from above to rapidly locate the fistula tract.
- Use sterile milk to fill the bladder to look for leaks. This works without staining the tissues.
- Minimize tension on the bladder suture line.
In modern times in the United States, the vesicovaginal fistula (VVF) arises chiefly as a sequela of gynecologic surgery, usually hysterectomy. The injury most likely occurs at the time of dissection of the bladder flap off of the lower uterine segment and upper vagina.1 With increasing use of endoscopy and electrosurgery at the time of hysterectomy,2 the occurrence of VVF is likely to increase. Because of this, fistulas stemming from benign gynecologic surgical activity tend to occur above the trigone near the vaginal cuff.
Current technique of fistula repair involves either a vaginal approach, with the Latsko procedure,3 or an abdominal approach, involving a laparotomy; laparoscopy also is used with increasing frequency.4
Vaginal versus endoscopic approach. The vaginal approach can be straightforward, such as in cases of vault prolapse or a distally located fistula, or more difficult if the fistula is apical in location, especially if the apex is well suspended and the vagina is of normal length. I have found the abdominal approach to be optimal if the fistula is near the cuff and the vagina is of normal length and well suspended.
Classical teaching tells us that the first repair of the VVF is likely to be the most successful, with successively lower cure rates as the number of repair attempts increases. For this reason, I advocate the abdominal approach in most cases of apically placed VVFs.
Surgical approach
Why endoscopic, why robotic? Often, repair of the VVF is complicated by:
-
the challenge of locating the defect in the bladder
-
the technical difficulty in oversewing the bladder, which often must be done on the underside of the bladder, between the vaginal and bladder walls.
To tackle these challenges, an endoscopic approach promises improved visualization, and the robotic approach allows for surgical closure with improved visibility characteristic of endoscopy, while preserving the manual dexterity characteristic of open surgery.
Timing. It is believed that, in order to improve chances of successful surgical repair, the fistula should be approached either immediately (ie, within 1 to 2 weeks of the insult) or delayed by 8 to 12 or more weeks after the causative surgery.5
Preparation. Vesicovaginal fistulas can rarely involve the ureters, and this ureteric involvement needs to be ruled out. Accordingly, the workup of the VVF should begin with a thorough cystoscopic evaluation of the bladder, with retrograde pyelography to evaluate the integrity of the ureters bilaterally.
During this procedure, the location of the fistulous tract should be meticulously mapped. Care should be taken to document the location and extent of the fistula, as well as to identify the presence of multiple or separate tracts. If these tracts are present, they also need also to be catalogued. In my practice, the cystoscopy/retrograde pyelogram is performed as a separate surgical encounter.
Surgical technique
After the fistula is mapped and ureteric integrity is confirmed, the definitive surgical repair is performed. The steps to the surgical approach are straightforward.
1. Insert stents into the fistula and bilaterally into the ureters
Stenting the fistula permits rapid identification of the fistula tract without the need to enter the bladder separately. The ureteric stents you use should be one color, and the fistula stent should be a second color. I use 5 French yellow stents to cannulate the ureters and a 6 French blue stent for the fistula itself. Insert the fistula stent from the bladder side of the fistula. It should exit through the vagina (FIGURES 1A and 1B). In addition, place a 3-way Foley catheter for drainage and irrigate the bladder when indicated.
Figure 1. Stent insertion
Cystoscopically place a 6 French blue stent into the bladder side of the fistula. (A) The stent as seen from inside the bladder. (B) The stent as seen from the vaginal side.
2. Place the ports for optimal access
A 0° camera is adequate for visualization. Port placement is similar to that used for robotic sacral colpopexy; I use a supraumbilically placed camera port and three 8-mm robot ports (two on the left and one on the right of the umbilicus). Each port should be separated by about 10 cm. An assistant port is placed to the far right, for passing and retrieving sutures (FIGURE 2). An atraumatic grasper, monopolar scissors, and bipolar Maryland-type forceps are placed within the ports to begin the surgical procedure.
Figure 2. Ideal port placement
Place the camera port supraunbilically, with two 8-mm robot ports
3. Place a vaginal stent to aid dissection
The stent should be a sterile cylinder and have a diameter of 2 cm to 5 cm (to match the vaginal caliber). The tip should be rounded and flattened, with an extended handle available for manipulating the stent. The handle can be held by an assistant or attached to an external uterine positioning system (FIGURE 3); I use the Uterine Positioning SystemTM (Cooper Surgical, Trumbull, Connecticut).
4. Incise the vaginal cuff
Transversely incise the vaginal cuff with the monopolar scissors (VIDEO 1).
This allows entry into the vagina at the apex. The blue stent in the fistula should be visible at the anterior vaginal wall, as demonstrated in FIGURE 4.
5. Dissect the vaginal wall
Dissect the anterior vaginal wall down to the fistula, and dissect the bladder off of the vaginal wall for about 1.5 cm to 2 cm around the fistula tract (FIGURE 5 and VIDEO 2).
6. Cut the stent
Cut the stent passing thru the fistula, to move it out of the way.
7. Close the bladder
Stitch the bladder in a running fashion using three layers of 3-0 rapid absorbable synthetic suture (FIGURE 6 and Video 3 . I prefer polyglactin 910 (Vicryl; Ethicon, Somerville, New Jersey) because it is easier to handle.
FIGURE 6: Close the bladderStitch the bladder in a running fashion using three layers of 3-0 rapid absorbable synthetic suture. Keep the closure line free of tension.
8. Close the vaginal side of the fistula
Stitch the vaginal side of the fistula in a running fashion with 2-0 absorbable synthetic suture.
9. Verify closure
Check for watertight closure by retrofilling the bladder with 100 mL of sterile milk (obtained from the labor/delivery suite). Observe the suture line for any evidence of milk leakage. (Sterile milk does not stain the tissues, and this preserves tissue visibility. For this reason, milk is preferable to indigo carmine or methylene blue.)
10. Remove the stents from the bladder
Cystoscopically remove all stents.
11. Close the laparoscopic ports
12. Follow up to ensure surgical success
Leave the indwelling Foley catheter in place for 2 to 3 weeks. After such time, remove the catheter and perform voiding cystourethrogram to document bladder wall integrity.
Discussion
I have described a systematic approach to robotic VVF repair. The robotic portion of the procedure should require about 60 to 90 minutes in the absence of significant adhesions. The technique is amenable to a laparoscopic approach, when performed by an appropriately skilled operator.
Final takeaways. Important takeaways to this repair include:
- Stent the fistula to make it easy to find intraoperatively.
- Enter the vagina from above to rapidly locate the fistula tract.
- Use sterile milk to fill the bladder to look for leaks. This works without staining the tissues.
- Minimize tension on the bladder suture line.
In modern times in the United States, the vesicovaginal fistula (VVF) arises chiefly as a sequela of gynecologic surgery, usually hysterectomy. The injury most likely occurs at the time of dissection of the bladder flap off of the lower uterine segment and upper vagina.1 With increasing use of endoscopy and electrosurgery at the time of hysterectomy,2 the occurrence of VVF is likely to increase. Because of this, fistulas stemming from benign gynecologic surgical activity tend to occur above the trigone near the vaginal cuff.
Current technique of fistula repair involves either a vaginal approach, with the Latsko procedure,3 or an abdominal approach, involving a laparotomy; laparoscopy also is used with increasing frequency.4
Vaginal versus endoscopic approach. The vaginal approach can be straightforward, such as in cases of vault prolapse or a distally located fistula, or more difficult if the fistula is apical in location, especially if the apex is well suspended and the vagina is of normal length. I have found the abdominal approach to be optimal if the fistula is near the cuff and the vagina is of normal length and well suspended.
Classical teaching tells us that the first repair of the VVF is likely to be the most successful, with successively lower cure rates as the number of repair attempts increases. For this reason, I advocate the abdominal approach in most cases of apically placed VVFs.
Surgical approach
Why endoscopic, why robotic? Often, repair of the VVF is complicated by:
-
the challenge of locating the defect in the bladder
-
the technical difficulty in oversewing the bladder, which often must be done on the underside of the bladder, between the vaginal and bladder walls.
To tackle these challenges, an endoscopic approach promises improved visualization, and the robotic approach allows for surgical closure with improved visibility characteristic of endoscopy, while preserving the manual dexterity characteristic of open surgery.
Timing. It is believed that, in order to improve chances of successful surgical repair, the fistula should be approached either immediately (ie, within 1 to 2 weeks of the insult) or delayed by 8 to 12 or more weeks after the causative surgery.5
Preparation. Vesicovaginal fistulas can rarely involve the ureters, and this ureteric involvement needs to be ruled out. Accordingly, the workup of the VVF should begin with a thorough cystoscopic evaluation of the bladder, with retrograde pyelography to evaluate the integrity of the ureters bilaterally.
During this procedure, the location of the fistulous tract should be meticulously mapped. Care should be taken to document the location and extent of the fistula, as well as to identify the presence of multiple or separate tracts. If these tracts are present, they also need also to be catalogued. In my practice, the cystoscopy/retrograde pyelogram is performed as a separate surgical encounter.
Surgical technique
After the fistula is mapped and ureteric integrity is confirmed, the definitive surgical repair is performed. The steps to the surgical approach are straightforward.
1. Insert stents into the fistula and bilaterally into the ureters
Stenting the fistula permits rapid identification of the fistula tract without the need to enter the bladder separately. The ureteric stents you use should be one color, and the fistula stent should be a second color. I use 5 French yellow stents to cannulate the ureters and a 6 French blue stent for the fistula itself. Insert the fistula stent from the bladder side of the fistula. It should exit through the vagina (FIGURES 1A and 1B). In addition, place a 3-way Foley catheter for drainage and irrigate the bladder when indicated.
Figure 1. Stent insertion
Cystoscopically place a 6 French blue stent into the bladder side of the fistula. (A) The stent as seen from inside the bladder. (B) The stent as seen from the vaginal side.
2. Place the ports for optimal access
A 0° camera is adequate for visualization. Port placement is similar to that used for robotic sacral colpopexy; I use a supraumbilically placed camera port and three 8-mm robot ports (two on the left and one on the right of the umbilicus). Each port should be separated by about 10 cm. An assistant port is placed to the far right, for passing and retrieving sutures (FIGURE 2). An atraumatic grasper, monopolar scissors, and bipolar Maryland-type forceps are placed within the ports to begin the surgical procedure.
Figure 2. Ideal port placement
Place the camera port supraunbilically, with two 8-mm robot ports
3. Place a vaginal stent to aid dissection
The stent should be a sterile cylinder and have a diameter of 2 cm to 5 cm (to match the vaginal caliber). The tip should be rounded and flattened, with an extended handle available for manipulating the stent. The handle can be held by an assistant or attached to an external uterine positioning system (FIGURE 3); I use the Uterine Positioning SystemTM (Cooper Surgical, Trumbull, Connecticut).
4. Incise the vaginal cuff
Transversely incise the vaginal cuff with the monopolar scissors (VIDEO 1).
This allows entry into the vagina at the apex. The blue stent in the fistula should be visible at the anterior vaginal wall, as demonstrated in FIGURE 4.
5. Dissect the vaginal wall
Dissect the anterior vaginal wall down to the fistula, and dissect the bladder off of the vaginal wall for about 1.5 cm to 2 cm around the fistula tract (FIGURE 5 and VIDEO 2).
6. Cut the stent
Cut the stent passing thru the fistula, to move it out of the way.
7. Close the bladder
Stitch the bladder in a running fashion using three layers of 3-0 rapid absorbable synthetic suture (FIGURE 6 and Video 3 . I prefer polyglactin 910 (Vicryl; Ethicon, Somerville, New Jersey) because it is easier to handle.
FIGURE 6: Close the bladderStitch the bladder in a running fashion using three layers of 3-0 rapid absorbable synthetic suture. Keep the closure line free of tension.
8. Close the vaginal side of the fistula
Stitch the vaginal side of the fistula in a running fashion with 2-0 absorbable synthetic suture.
9. Verify closure
Check for watertight closure by retrofilling the bladder with 100 mL of sterile milk (obtained from the labor/delivery suite). Observe the suture line for any evidence of milk leakage. (Sterile milk does not stain the tissues, and this preserves tissue visibility. For this reason, milk is preferable to indigo carmine or methylene blue.)
10. Remove the stents from the bladder
Cystoscopically remove all stents.
11. Close the laparoscopic ports
12. Follow up to ensure surgical success
Leave the indwelling Foley catheter in place for 2 to 3 weeks. After such time, remove the catheter and perform voiding cystourethrogram to document bladder wall integrity.
Discussion
I have described a systematic approach to robotic VVF repair. The robotic portion of the procedure should require about 60 to 90 minutes in the absence of significant adhesions. The technique is amenable to a laparoscopic approach, when performed by an appropriately skilled operator.
Final takeaways. Important takeaways to this repair include:
- Stent the fistula to make it easy to find intraoperatively.
- Enter the vagina from above to rapidly locate the fistula tract.
- Use sterile milk to fill the bladder to look for leaks. This works without staining the tissues.
- Minimize tension on the bladder suture line.