User login
From the Hartford HealthCare Cancer Institute, Hartford, CT (Dr. Salner and Dr. Yu), and the Albert Einstein College of Medicine, New York, NY (Mr. Sekerak).
Abstract
- Objective: To explore the extent to which patients with newly diagnosed colorectal cancer (CRC) received standard of care screening for Lynch syndrome (LS), with testing of specimens for loss of expression of mismatch repair (MMR) genes and referral of patients with positive results to a genetic counselor.
- Methods: We conducted a retrospective study using cancer registry data from the Hartford HealthCare Cancer Institute, which is part of a 5-hospital urban health care system. Measures that were included in this study were patient age and gender, date of surgery, pathologic grade, pathologic stage, presence of MMR immunohistochemical test, and presence of genetic counseling and testing for MMR-positive patients
- Results: 432 patients diagnosed with CRC during calendar years 2014 and 2015 were identified. The average age of the patients was 68.2 years and overall 81.3% of patients were screened (range, 30.8%–94.5%). Of the patients with MMR-positive results, 15 (57.7%) received a genetic consult and 10 of these had a germline test. Seven patients (70%) tested positive for LS. Patients who were diagnosed with LS were younger, and the majority were male.
- Conclusion: This study showed that improved implementation strategies for LS screening at HHC hospitals were needed, as MMR testing was not fully implemented across all of our sites. Strategies that led to improved compliance included consensus building, comprehensive communications, embedding the new standard in a series of steps, and subsequent audits with feedback.
Keywords: Lynch syndrome; colorectal cancer; quality; screening; standard of care.
Colorectal cancer (CRC) is the third most common cancer in men and women, accounting for as many as 135,000 new cases and 50,000 cancer deaths per year in the United States.1 These cancers appear to be heterogeneous with multiple molecular subtypes, including chromosomal instability and microsatellite instability (MSI) pathways.2,3 MSI tumors may result from sporadic mutations or constitutional mutations. Lynch syndrome (LS), formerly known as hereditary non-polyposis colorectal cancer, is caused by a germline mutation in 1 of several DNA mismatch repair (MMR) genes or loss of expression of MSH2 due to deletions in the EPCAM gene.4 The MMR genes that have been identified in LS are MLH1, MSH2, MSH6, and PMS2.5-8 The protein products of these genes are essential to maintaining the integrity of the DNA sequence. Importantly for clinical practice, patients who carry gene mutations indicative of LS have a higher risk of certain cancers, namely CRC, pancreatic cancer, and endometrial cancer, among others.8,9
While most occurrences of CRC are sporadic, accounting for roughly 90% of all cases, approximately 5% to 10% of CRCs are caused by inherited genes.10 LS is the most common cause of inherited CRC, accounting for 1% to 3% of all CRC cases.8,10,11 Individuals with LS are likely to have onset of disease at an earlier age and also have a much higher risk for developing CRC, with a lifetime risk of CRC of approximately 70% for men and 45% for women.12,13 Thus, it is important to identify patients who have LS so that they can receive proper surveillance and care (ie, frequency of follow-up and treatment options). It is additionally important for family members of patients with LS to receive proper genetic counseling and genetic testing to better understand their possible predisposition and risk for CRC. CRC screening for LS helps clinicians appropriately personalize patient care, as the adjuvant therapy selection may be influenced by MMR results.3
The National Comprehensive Cancer Network guidelines recommend screening all patients with newly diagnosed CRC for Lynch syndrome. Hartford HealthCare (HHC), a large health care system located in Hartford, CT, has adopted these guidelines at the 5 hospitals within its cancer institute. According to the standard of care, a positive MMR pathology report should result in a referral to a genetic counselor for consultation, and the genetic counselor would recommend genetic testing for germline MMR genes. This quality improvement project sought to evaluate the performance of each of the 5 hospitals in implementing the standard of care for screening for LS in patients with CRC and to determine if the appropriate genetic referrals were made for patients with positive screening results. This study focused on LS screening in patients diagnosed only with CRC.
Data Collection and Analysis
We conducted a retrospective study examining all cases of patients diagnosed with invasive colon or rectal cancer at each of the 5 HHC Cancer Institute hospitals during calendar years 2014 and 2015. The study was developed as a quality improvement project for the HHC cancer centers. The database of patients diagnosed with colon and rectal cancer at HHC was obtained from our cancer registry.
Patients were stratified by hospital and surgeon. The study analyzed multiple factors, including age and gender, date of surgery, pathologic grade, pathologic stage, presence of MMR immunohistochemical (IHC) test, and presence of genetic counseling and testing for MMR-positive patients. Data was extracted from patient charts, pathology reports, and genetic reports. Only patients with primary adenocarcinomas were included in the study. In total, the study comprised 423 cases among the 5 hospitals. Results were tabulated and simple descriptive statistics were utilized to analyze the data.
Results
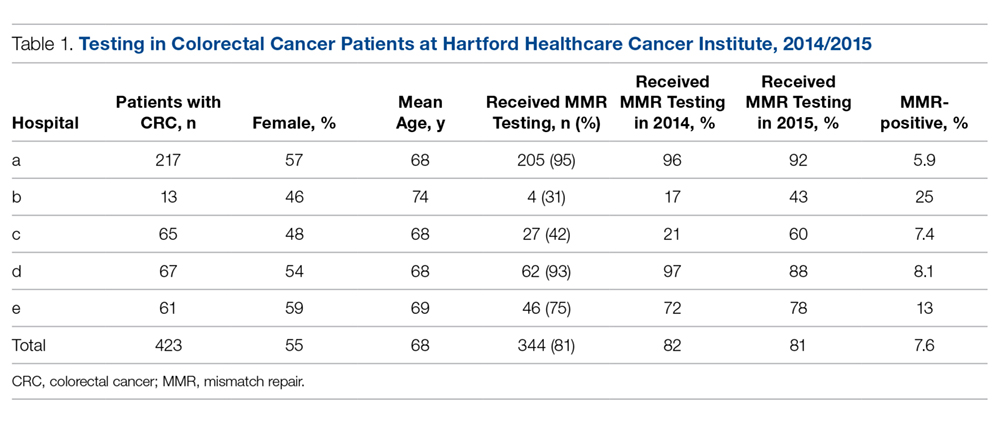
Of the 423 CRC patients treated at HHC during the study period, 45% were male and 55% were female, with an average age of 68.2 years (Table 1). The HHC Cancer Institute performed MMR IHC testing on 81.3% of all patients diagnosed in 2014 and 2015 (range, 30.8% to 94.5%). While the percentage of patients tested overall did not change from 2014 to 2015, it appreciably increased for the lower performing hospitals (Table 1). This improvement resulted from enhanced communication and establishment of pathology protocols for handling the tissue of patients with a cancer diagnosis.
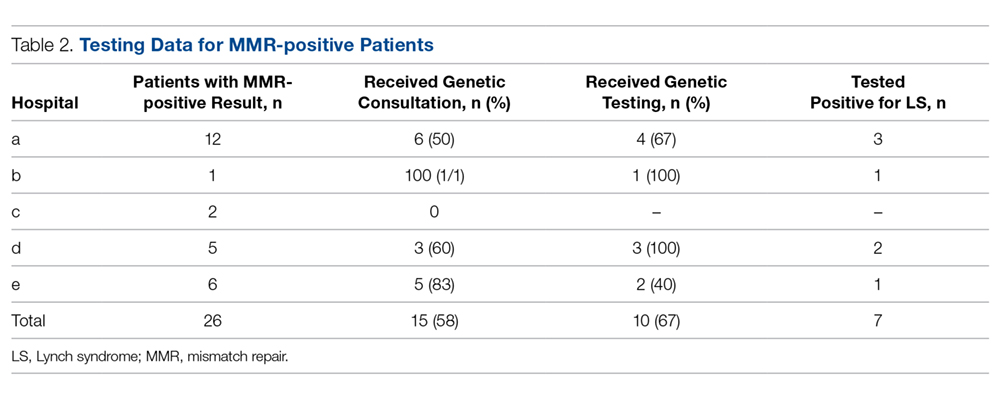
Twenty-six (7.6%) of the 344 specimens tested were IHC abnormal, revealing a loss of 1 or more MMR gene products (Table 2). Of the patients with MMR-positive results, 15 (57.7%) received a genetic consult and 10 of these had a germline test of their MMR genes. Of note, 1 patient had been diagnosed with LS at an outside facility and therefore did not receive a genetic consult; 1 patient was unable to be reached for scheduling of a consult; 2 patients declined genetic testing; and 1 patient did not have their genetic test ordered.
Of the patients who underwent germline testing, 7 (70%) tested positive for LS (Table 2). Five LS patients tested positive for an MLH1 gene mutation, 1 tested positive for an MSH2 mutation, and 1 had a pathogenic variant of unknown significance (VUS) in their MLH1 gene.
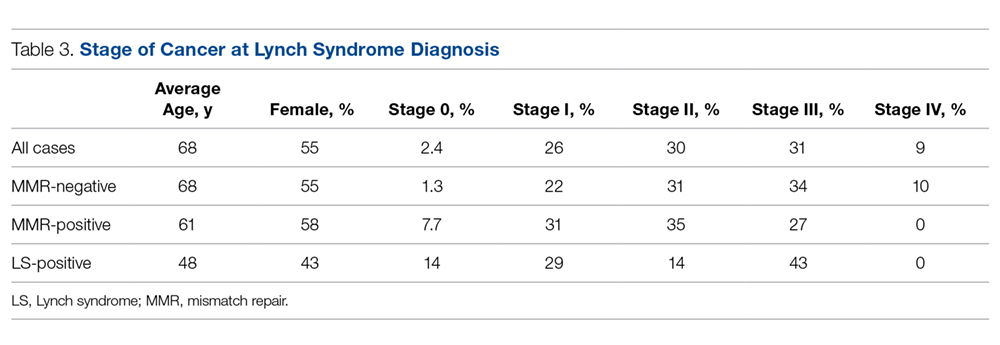
The stage of cancer at diagnosis for MMR-negative, MMR-positive, and LS-positive groups was similar; nearly all patients were stage I, II, or III (Table 3). Compared to patients who were MMR-negative or MMR-positive, LS patients were younger (68.3, 60.9, and 47.6 years, respectively), and the majority were male (44.8%, 42.3%, and 57.1%, respectively).
Discussion
The shifting paradigm of health care delivery in America has led to increasing consolidation of hospitals into larger health care organizations. Consolidation creates a challenge when trying to implement a unified standard of care within distinct hospitals that comprise a health care system. In 2014, HHC integrated 2 additional hospitals into its system, for a total of 5 hospitals. As part of our quality improvement process, we wanted to explore the effect this had on universal MMR tumor screening for CRC patients among the 5 separate pathology departments, recognizing that implementation might take some time as protocols change. Although our Cancer Institute and Pathology Council had approved the universal MMR testing standard for all CCR patients, it was not clear that the standard had been embedded into pathology department standard practice.
The project reported here revealed substantial variance in MMR IHC testing among the 5 hospitals, suggesting the difficulty of implementing a unified standard of care among hospitals with separate groups of pathologists. This variance could result from several issues: lack of embedding the new standard in a series of steps to assure universal compliance; lack of agreement by pathologists on submitting every case; lack of follow-up by pathology staff to forward slides/tissue to the central lab for processing; and concern about privacy issues associated with conducting an unconsented genetic test.
There has been some debate as to whether CRC tumor screening requires consent from the patient.14 Without a clear standard of care for CRC cases, MMR IHC testing might not be ordered if a pathologist deems it necessary for the surgeon to obtain patient consent to the test. When the discrepancy in MMR IHC testing among the hospitals was investigated, we learned that one pathologist performed MMR testing only if a signed patient consent was provided. This revealed a deviation from our CRC protocol and a deficiency of communication within the HHC network. In addition, only 3 of our 5 hospitals routinely had genetic counselors present during the study period, requiring travel for patients at the other 2 hospitals and thus creating a potential barrier to the genetic consultation.
Based on the results of this study and other studies in the literature, we estimated that approximately 7 to 10 MMR-positive cases and 5 to 7 patients with LS may have been missed within the HHC network during the 2 study years as a result of suboptimal MMR testing, genetic counseling, and genetic testing.14-18 These potentially missed cases and diagnoses underscore the importance of implementing a unified standard of care across all large health care organizations. Individualized care, genetic testing, and counseling for patients and families affected by LS lead to more effective monitoring of these patients for disease.
However, our project showed that effective implementation of a standard of care for universal tumor screening for patients with CRC can modify institutional cancer care.15 Notably, hospitals that tested a lower percentage of patients overall improved their MMR testing drastically from 2014 to 2015. This significant increase in MMR testing shows the impact of measuring and disseminating compliance performance information following the institution of a new quality standard within a health care system. Further audits have revealed universal acceptance and use of this testing.
General patient perception of universal tumor screening is positive, and patients understand and endorse the benefits of screening for LS.16 In our study, patients with LS were on average 21 years younger at diagnosis compared to patients who were MMR-negative. Because LS patients are younger at diagnosis of CRC compared to patients who do not have MMR gene mutations and because colonoscopy typically is not initiated until age 50 years, molecular screening and genetic testing of MMR-positive patients is important. Identifying the presence of LS is important for both the patient and their family. Specifically, patients with LS are recommended to receive a screening colonoscopy every 1 to 2 years beginning at age 20 to 25 years.13 Personalizing care and increasing surveillance for patients with LS can help to reduce the morbidity and mortality of CRC and potentially other cancers.
Conclusion
As a result of this study, we recognized that inclusion of pathologists in the discussion is essential but not enough to ensure that all cases will be screened. Rather, a much more detailed series of steps is necessary to ensure compliance, including:
- Gain consensus among clinical leadership in CRC (including surgery, medical oncology, and pathology) that universal screening is necessary.
- Bring the appropriate strategy to pathology department operational managers to ensure that policy is transmitted to all appropriate staff.
- Ensure that involved individuals at newer hospitals in the system have access to the details of cultural discussions that have occurred to develop consensus and the policies and procedures that followed.
- Develop policies and procedures to assure that all appropriate patients are tested, including those who present outside normal hours for emergency surgery (ie, bowel obstruction).
- Develop an audit process to ensure that all patients have been screened and determine where any exceptions might be present.
- Present audit data back to the pathology team and Cancer Institute leadership team, and consider any strategy or operational modifications if needed.
The results of this study also highlight the important role quality studies play in informing health care organizations and improving clinical care. Quality studies assist in changing the culture and practice of institutions and guide the development and implementation of a unified standard of care.
Corresponding author: Andrew L. Salner, MD, Hartford HealthCare Cancer Institute, 80 Seymour Street, Hartford, CT 06102; andrew.salner@hhchealth.org.
Financial disclosures: None.
Funding: This study was funded internally as a quality improvement study.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7-30.
2. Guinney J, Dientsmann R, Wang X, et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 2015;21:1350-1356.
3. Ryan E, Sheehan K, Creavin B, et al. The current value of determining the mismatch repair status of colorectal cancer: A rationale for routine testing. Crit Rev Oncol Hematol. 2017;116:38-57.
4. Koessler T, Oestergaard MZ, Song H, et al. Common variants in mismatch repair genes and risk of colorectal cancer. Gut. 2008;57:1097-101.
5. Quehenberger F, Vasen HF, van Houwelingen HC. Risk of colorectal and endometrial cancer for carriers of mutations of the hMLH1 and hMSH2 gene: correction for ascertainment. J Med Genet. 2005;42:491-496.
6. Senter L, Clendenning M, Sotamaa K, et al. The clinical phenotype of Lynch syndrome due to germ-line PMS2 mutations. Gastroenterology. 2008;135:419-428.
7. Talseth-Palmer BA, McPhillips M, Groombridge C, et al. MSH6 and PMS2 mutation positive Australian Lynch syndrome families: novel mutations, cancer risk and age of diagnosis of colorectal cancer. Hered Cancer Clin Pract. 2010;8(1):5.
8. Bondona V, Bonaiti B, Olschwang S, et al. Cancer risks associated with germline mutations in MLH1, MSH2, MSH6 genes in Lynch syndrome. JAMA. 2011;305:2304-2310.
9. Barrow E, Alduaij W, Robinson L, et al. Colorectal cancer in HNPCC: cumulative lifetime incidence, survival and tumour distribution. A report of 121 families with proven mutations. Clin Genet 2008;74:233-242.
10. Johns LE, Houlston RS. A systematic review and meta-analysis of familial colorectal cancer risk. Am J Gastroenterol. 2001;96:2992–3003.
11. Hampel H, Frankel WL, Martin E, et al. Feasibility of screening for Lynch syndrome among patients with colorectal cancer. J Clin Oncol. 2008;26:5783-5788.
12. Kohlmann W, Gruber SB. Lynch syndrome. 2004 Feb 5 [Updated 2014 May 22]. In: Pagon RA, Adam MP, Ardinger HH, et al, eds. GeneReviews [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2016.
13. Stoffel E, Mukherjee B, Raymond VM, et al. Calculation of risk of colorectal and endometrial cancer among patients with Lynch syndrome. Gastroenterology. 2009;137:1621-1627.
14. Beamer LC, Grant ML, Espenshied CR, et al. Reflex immunohistochemical and microsatellite instability testing of colorectal tumors for Lynch syndrome among US cancer programs and follow-up of abnormal results. J Clin Oncol. 2012;30:1058-1063.
15. Cohen SA, Laurino M, Bowen DJ, et al. Initiation of universal tumor screening for Lynch syndrome in colorectal cancer patients as a model for the implementation of genetic information into clinical oncology practice. Cancer. 2016;122:393-401.
16. Hunter JE, Zepp JM, Gilmore MJ, et al. Universal tumor screening for Lynch syndrome: Assessment of the perspectives of patients with colorectal cancer regarding benefits and barriers. Cancer. 2015;121:3281-3289.
17. Jasperson KW, Tuohy TM, Neklason DW, Burt RW. Hereditary and familial colon cancer. Gastroenterology. 2010;138:2044-2058.
18. Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Working Group (2009). Recommendations from the EGAPP Working Group: genetic testing strategies in newly diagnosed individuals with colorectal cancer aimed at reducing morbidity and mortality from Lynch syndrome relatives. Genet. Med. 2009;11:35–41
From the Hartford HealthCare Cancer Institute, Hartford, CT (Dr. Salner and Dr. Yu), and the Albert Einstein College of Medicine, New York, NY (Mr. Sekerak).
Abstract
- Objective: To explore the extent to which patients with newly diagnosed colorectal cancer (CRC) received standard of care screening for Lynch syndrome (LS), with testing of specimens for loss of expression of mismatch repair (MMR) genes and referral of patients with positive results to a genetic counselor.
- Methods: We conducted a retrospective study using cancer registry data from the Hartford HealthCare Cancer Institute, which is part of a 5-hospital urban health care system. Measures that were included in this study were patient age and gender, date of surgery, pathologic grade, pathologic stage, presence of MMR immunohistochemical test, and presence of genetic counseling and testing for MMR-positive patients
- Results: 432 patients diagnosed with CRC during calendar years 2014 and 2015 were identified. The average age of the patients was 68.2 years and overall 81.3% of patients were screened (range, 30.8%–94.5%). Of the patients with MMR-positive results, 15 (57.7%) received a genetic consult and 10 of these had a germline test. Seven patients (70%) tested positive for LS. Patients who were diagnosed with LS were younger, and the majority were male.
- Conclusion: This study showed that improved implementation strategies for LS screening at HHC hospitals were needed, as MMR testing was not fully implemented across all of our sites. Strategies that led to improved compliance included consensus building, comprehensive communications, embedding the new standard in a series of steps, and subsequent audits with feedback.
Keywords: Lynch syndrome; colorectal cancer; quality; screening; standard of care.
Colorectal cancer (CRC) is the third most common cancer in men and women, accounting for as many as 135,000 new cases and 50,000 cancer deaths per year in the United States.1 These cancers appear to be heterogeneous with multiple molecular subtypes, including chromosomal instability and microsatellite instability (MSI) pathways.2,3 MSI tumors may result from sporadic mutations or constitutional mutations. Lynch syndrome (LS), formerly known as hereditary non-polyposis colorectal cancer, is caused by a germline mutation in 1 of several DNA mismatch repair (MMR) genes or loss of expression of MSH2 due to deletions in the EPCAM gene.4 The MMR genes that have been identified in LS are MLH1, MSH2, MSH6, and PMS2.5-8 The protein products of these genes are essential to maintaining the integrity of the DNA sequence. Importantly for clinical practice, patients who carry gene mutations indicative of LS have a higher risk of certain cancers, namely CRC, pancreatic cancer, and endometrial cancer, among others.8,9
While most occurrences of CRC are sporadic, accounting for roughly 90% of all cases, approximately 5% to 10% of CRCs are caused by inherited genes.10 LS is the most common cause of inherited CRC, accounting for 1% to 3% of all CRC cases.8,10,11 Individuals with LS are likely to have onset of disease at an earlier age and also have a much higher risk for developing CRC, with a lifetime risk of CRC of approximately 70% for men and 45% for women.12,13 Thus, it is important to identify patients who have LS so that they can receive proper surveillance and care (ie, frequency of follow-up and treatment options). It is additionally important for family members of patients with LS to receive proper genetic counseling and genetic testing to better understand their possible predisposition and risk for CRC. CRC screening for LS helps clinicians appropriately personalize patient care, as the adjuvant therapy selection may be influenced by MMR results.3
The National Comprehensive Cancer Network guidelines recommend screening all patients with newly diagnosed CRC for Lynch syndrome. Hartford HealthCare (HHC), a large health care system located in Hartford, CT, has adopted these guidelines at the 5 hospitals within its cancer institute. According to the standard of care, a positive MMR pathology report should result in a referral to a genetic counselor for consultation, and the genetic counselor would recommend genetic testing for germline MMR genes. This quality improvement project sought to evaluate the performance of each of the 5 hospitals in implementing the standard of care for screening for LS in patients with CRC and to determine if the appropriate genetic referrals were made for patients with positive screening results. This study focused on LS screening in patients diagnosed only with CRC.
Data Collection and Analysis
We conducted a retrospective study examining all cases of patients diagnosed with invasive colon or rectal cancer at each of the 5 HHC Cancer Institute hospitals during calendar years 2014 and 2015. The study was developed as a quality improvement project for the HHC cancer centers. The database of patients diagnosed with colon and rectal cancer at HHC was obtained from our cancer registry.
Patients were stratified by hospital and surgeon. The study analyzed multiple factors, including age and gender, date of surgery, pathologic grade, pathologic stage, presence of MMR immunohistochemical (IHC) test, and presence of genetic counseling and testing for MMR-positive patients. Data was extracted from patient charts, pathology reports, and genetic reports. Only patients with primary adenocarcinomas were included in the study. In total, the study comprised 423 cases among the 5 hospitals. Results were tabulated and simple descriptive statistics were utilized to analyze the data.
Results
Of the 423 CRC patients treated at HHC during the study period, 45% were male and 55% were female, with an average age of 68.2 years (Table 1). The HHC Cancer Institute performed MMR IHC testing on 81.3% of all patients diagnosed in 2014 and 2015 (range, 30.8% to 94.5%). While the percentage of patients tested overall did not change from 2014 to 2015, it appreciably increased for the lower performing hospitals (Table 1). This improvement resulted from enhanced communication and establishment of pathology protocols for handling the tissue of patients with a cancer diagnosis.
Twenty-six (7.6%) of the 344 specimens tested were IHC abnormal, revealing a loss of 1 or more MMR gene products (Table 2). Of the patients with MMR-positive results, 15 (57.7%) received a genetic consult and 10 of these had a germline test of their MMR genes. Of note, 1 patient had been diagnosed with LS at an outside facility and therefore did not receive a genetic consult; 1 patient was unable to be reached for scheduling of a consult; 2 patients declined genetic testing; and 1 patient did not have their genetic test ordered.
Of the patients who underwent germline testing, 7 (70%) tested positive for LS (Table 2). Five LS patients tested positive for an MLH1 gene mutation, 1 tested positive for an MSH2 mutation, and 1 had a pathogenic variant of unknown significance (VUS) in their MLH1 gene.
The stage of cancer at diagnosis for MMR-negative, MMR-positive, and LS-positive groups was similar; nearly all patients were stage I, II, or III (Table 3). Compared to patients who were MMR-negative or MMR-positive, LS patients were younger (68.3, 60.9, and 47.6 years, respectively), and the majority were male (44.8%, 42.3%, and 57.1%, respectively).
Discussion
The shifting paradigm of health care delivery in America has led to increasing consolidation of hospitals into larger health care organizations. Consolidation creates a challenge when trying to implement a unified standard of care within distinct hospitals that comprise a health care system. In 2014, HHC integrated 2 additional hospitals into its system, for a total of 5 hospitals. As part of our quality improvement process, we wanted to explore the effect this had on universal MMR tumor screening for CRC patients among the 5 separate pathology departments, recognizing that implementation might take some time as protocols change. Although our Cancer Institute and Pathology Council had approved the universal MMR testing standard for all CCR patients, it was not clear that the standard had been embedded into pathology department standard practice.
The project reported here revealed substantial variance in MMR IHC testing among the 5 hospitals, suggesting the difficulty of implementing a unified standard of care among hospitals with separate groups of pathologists. This variance could result from several issues: lack of embedding the new standard in a series of steps to assure universal compliance; lack of agreement by pathologists on submitting every case; lack of follow-up by pathology staff to forward slides/tissue to the central lab for processing; and concern about privacy issues associated with conducting an unconsented genetic test.
There has been some debate as to whether CRC tumor screening requires consent from the patient.14 Without a clear standard of care for CRC cases, MMR IHC testing might not be ordered if a pathologist deems it necessary for the surgeon to obtain patient consent to the test. When the discrepancy in MMR IHC testing among the hospitals was investigated, we learned that one pathologist performed MMR testing only if a signed patient consent was provided. This revealed a deviation from our CRC protocol and a deficiency of communication within the HHC network. In addition, only 3 of our 5 hospitals routinely had genetic counselors present during the study period, requiring travel for patients at the other 2 hospitals and thus creating a potential barrier to the genetic consultation.
Based on the results of this study and other studies in the literature, we estimated that approximately 7 to 10 MMR-positive cases and 5 to 7 patients with LS may have been missed within the HHC network during the 2 study years as a result of suboptimal MMR testing, genetic counseling, and genetic testing.14-18 These potentially missed cases and diagnoses underscore the importance of implementing a unified standard of care across all large health care organizations. Individualized care, genetic testing, and counseling for patients and families affected by LS lead to more effective monitoring of these patients for disease.
However, our project showed that effective implementation of a standard of care for universal tumor screening for patients with CRC can modify institutional cancer care.15 Notably, hospitals that tested a lower percentage of patients overall improved their MMR testing drastically from 2014 to 2015. This significant increase in MMR testing shows the impact of measuring and disseminating compliance performance information following the institution of a new quality standard within a health care system. Further audits have revealed universal acceptance and use of this testing.
General patient perception of universal tumor screening is positive, and patients understand and endorse the benefits of screening for LS.16 In our study, patients with LS were on average 21 years younger at diagnosis compared to patients who were MMR-negative. Because LS patients are younger at diagnosis of CRC compared to patients who do not have MMR gene mutations and because colonoscopy typically is not initiated until age 50 years, molecular screening and genetic testing of MMR-positive patients is important. Identifying the presence of LS is important for both the patient and their family. Specifically, patients with LS are recommended to receive a screening colonoscopy every 1 to 2 years beginning at age 20 to 25 years.13 Personalizing care and increasing surveillance for patients with LS can help to reduce the morbidity and mortality of CRC and potentially other cancers.
Conclusion
As a result of this study, we recognized that inclusion of pathologists in the discussion is essential but not enough to ensure that all cases will be screened. Rather, a much more detailed series of steps is necessary to ensure compliance, including:
- Gain consensus among clinical leadership in CRC (including surgery, medical oncology, and pathology) that universal screening is necessary.
- Bring the appropriate strategy to pathology department operational managers to ensure that policy is transmitted to all appropriate staff.
- Ensure that involved individuals at newer hospitals in the system have access to the details of cultural discussions that have occurred to develop consensus and the policies and procedures that followed.
- Develop policies and procedures to assure that all appropriate patients are tested, including those who present outside normal hours for emergency surgery (ie, bowel obstruction).
- Develop an audit process to ensure that all patients have been screened and determine where any exceptions might be present.
- Present audit data back to the pathology team and Cancer Institute leadership team, and consider any strategy or operational modifications if needed.
The results of this study also highlight the important role quality studies play in informing health care organizations and improving clinical care. Quality studies assist in changing the culture and practice of institutions and guide the development and implementation of a unified standard of care.
Corresponding author: Andrew L. Salner, MD, Hartford HealthCare Cancer Institute, 80 Seymour Street, Hartford, CT 06102; andrew.salner@hhchealth.org.
Financial disclosures: None.
Funding: This study was funded internally as a quality improvement study.
From the Hartford HealthCare Cancer Institute, Hartford, CT (Dr. Salner and Dr. Yu), and the Albert Einstein College of Medicine, New York, NY (Mr. Sekerak).
Abstract
- Objective: To explore the extent to which patients with newly diagnosed colorectal cancer (CRC) received standard of care screening for Lynch syndrome (LS), with testing of specimens for loss of expression of mismatch repair (MMR) genes and referral of patients with positive results to a genetic counselor.
- Methods: We conducted a retrospective study using cancer registry data from the Hartford HealthCare Cancer Institute, which is part of a 5-hospital urban health care system. Measures that were included in this study were patient age and gender, date of surgery, pathologic grade, pathologic stage, presence of MMR immunohistochemical test, and presence of genetic counseling and testing for MMR-positive patients
- Results: 432 patients diagnosed with CRC during calendar years 2014 and 2015 were identified. The average age of the patients was 68.2 years and overall 81.3% of patients were screened (range, 30.8%–94.5%). Of the patients with MMR-positive results, 15 (57.7%) received a genetic consult and 10 of these had a germline test. Seven patients (70%) tested positive for LS. Patients who were diagnosed with LS were younger, and the majority were male.
- Conclusion: This study showed that improved implementation strategies for LS screening at HHC hospitals were needed, as MMR testing was not fully implemented across all of our sites. Strategies that led to improved compliance included consensus building, comprehensive communications, embedding the new standard in a series of steps, and subsequent audits with feedback.
Keywords: Lynch syndrome; colorectal cancer; quality; screening; standard of care.
Colorectal cancer (CRC) is the third most common cancer in men and women, accounting for as many as 135,000 new cases and 50,000 cancer deaths per year in the United States.1 These cancers appear to be heterogeneous with multiple molecular subtypes, including chromosomal instability and microsatellite instability (MSI) pathways.2,3 MSI tumors may result from sporadic mutations or constitutional mutations. Lynch syndrome (LS), formerly known as hereditary non-polyposis colorectal cancer, is caused by a germline mutation in 1 of several DNA mismatch repair (MMR) genes or loss of expression of MSH2 due to deletions in the EPCAM gene.4 The MMR genes that have been identified in LS are MLH1, MSH2, MSH6, and PMS2.5-8 The protein products of these genes are essential to maintaining the integrity of the DNA sequence. Importantly for clinical practice, patients who carry gene mutations indicative of LS have a higher risk of certain cancers, namely CRC, pancreatic cancer, and endometrial cancer, among others.8,9
While most occurrences of CRC are sporadic, accounting for roughly 90% of all cases, approximately 5% to 10% of CRCs are caused by inherited genes.10 LS is the most common cause of inherited CRC, accounting for 1% to 3% of all CRC cases.8,10,11 Individuals with LS are likely to have onset of disease at an earlier age and also have a much higher risk for developing CRC, with a lifetime risk of CRC of approximately 70% for men and 45% for women.12,13 Thus, it is important to identify patients who have LS so that they can receive proper surveillance and care (ie, frequency of follow-up and treatment options). It is additionally important for family members of patients with LS to receive proper genetic counseling and genetic testing to better understand their possible predisposition and risk for CRC. CRC screening for LS helps clinicians appropriately personalize patient care, as the adjuvant therapy selection may be influenced by MMR results.3
The National Comprehensive Cancer Network guidelines recommend screening all patients with newly diagnosed CRC for Lynch syndrome. Hartford HealthCare (HHC), a large health care system located in Hartford, CT, has adopted these guidelines at the 5 hospitals within its cancer institute. According to the standard of care, a positive MMR pathology report should result in a referral to a genetic counselor for consultation, and the genetic counselor would recommend genetic testing for germline MMR genes. This quality improvement project sought to evaluate the performance of each of the 5 hospitals in implementing the standard of care for screening for LS in patients with CRC and to determine if the appropriate genetic referrals were made for patients with positive screening results. This study focused on LS screening in patients diagnosed only with CRC.
Data Collection and Analysis
We conducted a retrospective study examining all cases of patients diagnosed with invasive colon or rectal cancer at each of the 5 HHC Cancer Institute hospitals during calendar years 2014 and 2015. The study was developed as a quality improvement project for the HHC cancer centers. The database of patients diagnosed with colon and rectal cancer at HHC was obtained from our cancer registry.
Patients were stratified by hospital and surgeon. The study analyzed multiple factors, including age and gender, date of surgery, pathologic grade, pathologic stage, presence of MMR immunohistochemical (IHC) test, and presence of genetic counseling and testing for MMR-positive patients. Data was extracted from patient charts, pathology reports, and genetic reports. Only patients with primary adenocarcinomas were included in the study. In total, the study comprised 423 cases among the 5 hospitals. Results were tabulated and simple descriptive statistics were utilized to analyze the data.
Results
Of the 423 CRC patients treated at HHC during the study period, 45% were male and 55% were female, with an average age of 68.2 years (Table 1). The HHC Cancer Institute performed MMR IHC testing on 81.3% of all patients diagnosed in 2014 and 2015 (range, 30.8% to 94.5%). While the percentage of patients tested overall did not change from 2014 to 2015, it appreciably increased for the lower performing hospitals (Table 1). This improvement resulted from enhanced communication and establishment of pathology protocols for handling the tissue of patients with a cancer diagnosis.
Twenty-six (7.6%) of the 344 specimens tested were IHC abnormal, revealing a loss of 1 or more MMR gene products (Table 2). Of the patients with MMR-positive results, 15 (57.7%) received a genetic consult and 10 of these had a germline test of their MMR genes. Of note, 1 patient had been diagnosed with LS at an outside facility and therefore did not receive a genetic consult; 1 patient was unable to be reached for scheduling of a consult; 2 patients declined genetic testing; and 1 patient did not have their genetic test ordered.
Of the patients who underwent germline testing, 7 (70%) tested positive for LS (Table 2). Five LS patients tested positive for an MLH1 gene mutation, 1 tested positive for an MSH2 mutation, and 1 had a pathogenic variant of unknown significance (VUS) in their MLH1 gene.
The stage of cancer at diagnosis for MMR-negative, MMR-positive, and LS-positive groups was similar; nearly all patients were stage I, II, or III (Table 3). Compared to patients who were MMR-negative or MMR-positive, LS patients were younger (68.3, 60.9, and 47.6 years, respectively), and the majority were male (44.8%, 42.3%, and 57.1%, respectively).
Discussion
The shifting paradigm of health care delivery in America has led to increasing consolidation of hospitals into larger health care organizations. Consolidation creates a challenge when trying to implement a unified standard of care within distinct hospitals that comprise a health care system. In 2014, HHC integrated 2 additional hospitals into its system, for a total of 5 hospitals. As part of our quality improvement process, we wanted to explore the effect this had on universal MMR tumor screening for CRC patients among the 5 separate pathology departments, recognizing that implementation might take some time as protocols change. Although our Cancer Institute and Pathology Council had approved the universal MMR testing standard for all CCR patients, it was not clear that the standard had been embedded into pathology department standard practice.
The project reported here revealed substantial variance in MMR IHC testing among the 5 hospitals, suggesting the difficulty of implementing a unified standard of care among hospitals with separate groups of pathologists. This variance could result from several issues: lack of embedding the new standard in a series of steps to assure universal compliance; lack of agreement by pathologists on submitting every case; lack of follow-up by pathology staff to forward slides/tissue to the central lab for processing; and concern about privacy issues associated with conducting an unconsented genetic test.
There has been some debate as to whether CRC tumor screening requires consent from the patient.14 Without a clear standard of care for CRC cases, MMR IHC testing might not be ordered if a pathologist deems it necessary for the surgeon to obtain patient consent to the test. When the discrepancy in MMR IHC testing among the hospitals was investigated, we learned that one pathologist performed MMR testing only if a signed patient consent was provided. This revealed a deviation from our CRC protocol and a deficiency of communication within the HHC network. In addition, only 3 of our 5 hospitals routinely had genetic counselors present during the study period, requiring travel for patients at the other 2 hospitals and thus creating a potential barrier to the genetic consultation.
Based on the results of this study and other studies in the literature, we estimated that approximately 7 to 10 MMR-positive cases and 5 to 7 patients with LS may have been missed within the HHC network during the 2 study years as a result of suboptimal MMR testing, genetic counseling, and genetic testing.14-18 These potentially missed cases and diagnoses underscore the importance of implementing a unified standard of care across all large health care organizations. Individualized care, genetic testing, and counseling for patients and families affected by LS lead to more effective monitoring of these patients for disease.
However, our project showed that effective implementation of a standard of care for universal tumor screening for patients with CRC can modify institutional cancer care.15 Notably, hospitals that tested a lower percentage of patients overall improved their MMR testing drastically from 2014 to 2015. This significant increase in MMR testing shows the impact of measuring and disseminating compliance performance information following the institution of a new quality standard within a health care system. Further audits have revealed universal acceptance and use of this testing.
General patient perception of universal tumor screening is positive, and patients understand and endorse the benefits of screening for LS.16 In our study, patients with LS were on average 21 years younger at diagnosis compared to patients who were MMR-negative. Because LS patients are younger at diagnosis of CRC compared to patients who do not have MMR gene mutations and because colonoscopy typically is not initiated until age 50 years, molecular screening and genetic testing of MMR-positive patients is important. Identifying the presence of LS is important for both the patient and their family. Specifically, patients with LS are recommended to receive a screening colonoscopy every 1 to 2 years beginning at age 20 to 25 years.13 Personalizing care and increasing surveillance for patients with LS can help to reduce the morbidity and mortality of CRC and potentially other cancers.
Conclusion
As a result of this study, we recognized that inclusion of pathologists in the discussion is essential but not enough to ensure that all cases will be screened. Rather, a much more detailed series of steps is necessary to ensure compliance, including:
- Gain consensus among clinical leadership in CRC (including surgery, medical oncology, and pathology) that universal screening is necessary.
- Bring the appropriate strategy to pathology department operational managers to ensure that policy is transmitted to all appropriate staff.
- Ensure that involved individuals at newer hospitals in the system have access to the details of cultural discussions that have occurred to develop consensus and the policies and procedures that followed.
- Develop policies and procedures to assure that all appropriate patients are tested, including those who present outside normal hours for emergency surgery (ie, bowel obstruction).
- Develop an audit process to ensure that all patients have been screened and determine where any exceptions might be present.
- Present audit data back to the pathology team and Cancer Institute leadership team, and consider any strategy or operational modifications if needed.
The results of this study also highlight the important role quality studies play in informing health care organizations and improving clinical care. Quality studies assist in changing the culture and practice of institutions and guide the development and implementation of a unified standard of care.
Corresponding author: Andrew L. Salner, MD, Hartford HealthCare Cancer Institute, 80 Seymour Street, Hartford, CT 06102; andrew.salner@hhchealth.org.
Financial disclosures: None.
Funding: This study was funded internally as a quality improvement study.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7-30.
2. Guinney J, Dientsmann R, Wang X, et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 2015;21:1350-1356.
3. Ryan E, Sheehan K, Creavin B, et al. The current value of determining the mismatch repair status of colorectal cancer: A rationale for routine testing. Crit Rev Oncol Hematol. 2017;116:38-57.
4. Koessler T, Oestergaard MZ, Song H, et al. Common variants in mismatch repair genes and risk of colorectal cancer. Gut. 2008;57:1097-101.
5. Quehenberger F, Vasen HF, van Houwelingen HC. Risk of colorectal and endometrial cancer for carriers of mutations of the hMLH1 and hMSH2 gene: correction for ascertainment. J Med Genet. 2005;42:491-496.
6. Senter L, Clendenning M, Sotamaa K, et al. The clinical phenotype of Lynch syndrome due to germ-line PMS2 mutations. Gastroenterology. 2008;135:419-428.
7. Talseth-Palmer BA, McPhillips M, Groombridge C, et al. MSH6 and PMS2 mutation positive Australian Lynch syndrome families: novel mutations, cancer risk and age of diagnosis of colorectal cancer. Hered Cancer Clin Pract. 2010;8(1):5.
8. Bondona V, Bonaiti B, Olschwang S, et al. Cancer risks associated with germline mutations in MLH1, MSH2, MSH6 genes in Lynch syndrome. JAMA. 2011;305:2304-2310.
9. Barrow E, Alduaij W, Robinson L, et al. Colorectal cancer in HNPCC: cumulative lifetime incidence, survival and tumour distribution. A report of 121 families with proven mutations. Clin Genet 2008;74:233-242.
10. Johns LE, Houlston RS. A systematic review and meta-analysis of familial colorectal cancer risk. Am J Gastroenterol. 2001;96:2992–3003.
11. Hampel H, Frankel WL, Martin E, et al. Feasibility of screening for Lynch syndrome among patients with colorectal cancer. J Clin Oncol. 2008;26:5783-5788.
12. Kohlmann W, Gruber SB. Lynch syndrome. 2004 Feb 5 [Updated 2014 May 22]. In: Pagon RA, Adam MP, Ardinger HH, et al, eds. GeneReviews [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2016.
13. Stoffel E, Mukherjee B, Raymond VM, et al. Calculation of risk of colorectal and endometrial cancer among patients with Lynch syndrome. Gastroenterology. 2009;137:1621-1627.
14. Beamer LC, Grant ML, Espenshied CR, et al. Reflex immunohistochemical and microsatellite instability testing of colorectal tumors for Lynch syndrome among US cancer programs and follow-up of abnormal results. J Clin Oncol. 2012;30:1058-1063.
15. Cohen SA, Laurino M, Bowen DJ, et al. Initiation of universal tumor screening for Lynch syndrome in colorectal cancer patients as a model for the implementation of genetic information into clinical oncology practice. Cancer. 2016;122:393-401.
16. Hunter JE, Zepp JM, Gilmore MJ, et al. Universal tumor screening for Lynch syndrome: Assessment of the perspectives of patients with colorectal cancer regarding benefits and barriers. Cancer. 2015;121:3281-3289.
17. Jasperson KW, Tuohy TM, Neklason DW, Burt RW. Hereditary and familial colon cancer. Gastroenterology. 2010;138:2044-2058.
18. Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Working Group (2009). Recommendations from the EGAPP Working Group: genetic testing strategies in newly diagnosed individuals with colorectal cancer aimed at reducing morbidity and mortality from Lynch syndrome relatives. Genet. Med. 2009;11:35–41
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7-30.
2. Guinney J, Dientsmann R, Wang X, et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 2015;21:1350-1356.
3. Ryan E, Sheehan K, Creavin B, et al. The current value of determining the mismatch repair status of colorectal cancer: A rationale for routine testing. Crit Rev Oncol Hematol. 2017;116:38-57.
4. Koessler T, Oestergaard MZ, Song H, et al. Common variants in mismatch repair genes and risk of colorectal cancer. Gut. 2008;57:1097-101.
5. Quehenberger F, Vasen HF, van Houwelingen HC. Risk of colorectal and endometrial cancer for carriers of mutations of the hMLH1 and hMSH2 gene: correction for ascertainment. J Med Genet. 2005;42:491-496.
6. Senter L, Clendenning M, Sotamaa K, et al. The clinical phenotype of Lynch syndrome due to germ-line PMS2 mutations. Gastroenterology. 2008;135:419-428.
7. Talseth-Palmer BA, McPhillips M, Groombridge C, et al. MSH6 and PMS2 mutation positive Australian Lynch syndrome families: novel mutations, cancer risk and age of diagnosis of colorectal cancer. Hered Cancer Clin Pract. 2010;8(1):5.
8. Bondona V, Bonaiti B, Olschwang S, et al. Cancer risks associated with germline mutations in MLH1, MSH2, MSH6 genes in Lynch syndrome. JAMA. 2011;305:2304-2310.
9. Barrow E, Alduaij W, Robinson L, et al. Colorectal cancer in HNPCC: cumulative lifetime incidence, survival and tumour distribution. A report of 121 families with proven mutations. Clin Genet 2008;74:233-242.
10. Johns LE, Houlston RS. A systematic review and meta-analysis of familial colorectal cancer risk. Am J Gastroenterol. 2001;96:2992–3003.
11. Hampel H, Frankel WL, Martin E, et al. Feasibility of screening for Lynch syndrome among patients with colorectal cancer. J Clin Oncol. 2008;26:5783-5788.
12. Kohlmann W, Gruber SB. Lynch syndrome. 2004 Feb 5 [Updated 2014 May 22]. In: Pagon RA, Adam MP, Ardinger HH, et al, eds. GeneReviews [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2016.
13. Stoffel E, Mukherjee B, Raymond VM, et al. Calculation of risk of colorectal and endometrial cancer among patients with Lynch syndrome. Gastroenterology. 2009;137:1621-1627.
14. Beamer LC, Grant ML, Espenshied CR, et al. Reflex immunohistochemical and microsatellite instability testing of colorectal tumors for Lynch syndrome among US cancer programs and follow-up of abnormal results. J Clin Oncol. 2012;30:1058-1063.
15. Cohen SA, Laurino M, Bowen DJ, et al. Initiation of universal tumor screening for Lynch syndrome in colorectal cancer patients as a model for the implementation of genetic information into clinical oncology practice. Cancer. 2016;122:393-401.
16. Hunter JE, Zepp JM, Gilmore MJ, et al. Universal tumor screening for Lynch syndrome: Assessment of the perspectives of patients with colorectal cancer regarding benefits and barriers. Cancer. 2015;121:3281-3289.
17. Jasperson KW, Tuohy TM, Neklason DW, Burt RW. Hereditary and familial colon cancer. Gastroenterology. 2010;138:2044-2058.
18. Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Working Group (2009). Recommendations from the EGAPP Working Group: genetic testing strategies in newly diagnosed individuals with colorectal cancer aimed at reducing morbidity and mortality from Lynch syndrome relatives. Genet. Med. 2009;11:35–41