User login
Metrics from 2017 at the Fayetteville Veterans Affairs Heath Care Center (FVAHCC) Anticoagulation Clinic indicate that 43% of patients with atrial fibrillation (AF) who are prescribed warfarin have difficulty maintaining a therapeutic international normalized ratio (INR). These patients require frequent clinic appointments to adjust their regimens to ensure anticoagulation efficacy. FVAHCC policy requires a patient to return to the clinic for repeat INR evaluation within 5 to 14 days of the visit where INR was outside of the established therapeutic range.1 These frequent INR monitoring appointments increase patient and health care provider burden.
Direct oral anticoagulants (DOACs) are an alternative to warfarin for patients with AF who require anticoagulation. DOACs, which do not require regular efficacy monitoring, can be beneficial to patients who struggle to maintain a therapeutic INR when taking warfarin. FVAHCC policy regarding warfarin therapy monitoring allows for a maximum of 6 weeks between appointments. This period is often extended to 3 to 6 months for patients on DOACs.1
At FVAHCC, patients prescribed warfarin are managed in a centralized Anticoagulation Clinic led by a clinical pharmacy specialist (CPS). When a patient reports for an appointment, a clinical pharmacy technician performs point-of-care INR testing and asks standardized questions regarding therapy, including an assessment of adherence. The CPS then evaluates the patient’s INR test results, adjusts the dosage of warfarin as indicated, and determines appropriate follow-up.
A patient who is prescribed a DOAC is monitored by a CPS who works within a patient aligned care team (PACT). The PACT, a multidisciplinary team providing health care to veterans, includes physicians, nurses, pharmacists, dieticians, and mental health providers. Each CPS covers 3 or 4 PACTs. These pharmacists monitor all aspects of DOAC therapy at regular intervals, including renal and hepatic function, complete blood counts, medication adherence, and adverse effects.
Clinic and patient INR data are tracked using a time in therapeutic range (TTR) report generated by the US Department of Veterans Affairs (VA). The TTR report provides clinical information to enhance patient anticoagulation care.2 The TTR report identifies patients with an active order for warfarin and a diagnosis of AF or venous thromboembolism (VTE) whose INR is within therapeutic range (between 2 and 3) < 60% of the time over the previous 160 days.2 The patient must have had at least 3 INR levels drawn within that time frame for a TTR report calculation.2 The report excludes patients who were first prescribed warfarin within the previous 42 days and those with mechanical heart valves. The TTR report is used by the VA to see concrete facility-level results for quality improvement efforts.2
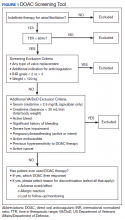
A quality improvement screening tool was developed to identify patients with AF being treated with warfarin who may appropriately transition to DOAC therapy. Anticoagulation Clinic patients were eligible for further evaluation if they had a TTR report level of < 60% and were prescribed indefinite warfarin therapy for AF.
The national VA goal is to have patient TTR report levels read > 60%. Therefore, the primary objective of this project was to improve Anticoagulation Clinic TTR metrics by targeting patients with TTR levels below the national goal.2
Patients who were successfully converted from warfarin to a DOAC were no longer included in Anticoagulation Clinic metrics and instead were followed by a PACT CPS. Thus, it was hypothesized that the average number of monthly Anticoagulation Clinic encounters would decrease on successful implementation of the screening tool. A secondary endpoint of the study evaluated the change in the total number of encounters of those who converted from warfarin to a DOAC.
Fewer clinic encounters could increase time available for the CPS to incorporate other initiatives into workflow and could increase clinic availability for newly referred veterans.
Methods
As this undertaking was considered to be a quality improvement project, institutional review board approval was not required. During an 8-week screening period (August to September 2018), the DOAC screening tool was implemented into the Anticoagulation Clinic workflow. This screening tool (Figure 1) was established based on VA Pharmacy Benefit Management (PBM) Service’s Criteria for Use for Stroke Prevention in Nonvalvular Atrial Fibrillation, a national set of standards used to determine appropriate candidates for DOAC therapy.3
Exclusion criteria included patients with INR goals < 2 or > 3, patients with a diagnosis of VTE, and patients with weight > 120 kg. Patients with a diagnosis of VTE were excluded due to the variability in therapy duration. Weight cutoffs were based on recommendations by the International Society on Thrombosis and Haemostasis. Due to a lack of available data, it was suggested that clinical judgment be used in patients whose weight was > 120 kg.4
During the screening period, weekly TTR reports identified patients in the clinic who had TTR < 60%. When a patient with a TTR report results of < 60% also had a scheduled appointment within a week, a CPS then further reviewed patient eligibility using the DOAC screening tool. On arrival for an appointment, the eligible patient was counseled on DOAC medications and the differences between warfarin and DOACs, including monitoring requirements. Patients had the option to switch to DOAC therapy or remain on warfarin.
The change in the average number of monthly Anticoagulation Clinic encounters for 3 months prior to the screening period (May to July 2018) and 2 months following screening (October to November 2018) was evaluated to measure the impact of the DOAC screening tool. The total number of encounters in the clinic was assessed using the monthly VA reports and were averaged for each period. Then data from the 2 periods were compared.
The monthly encounter reports, a data tool that monitors the number of unique visits per veteran each calendar month, also were used to generate a secondary endpoint showing the number of encounters in the Anticoagulation Clinic associated with patients who switched to a DOAC, including visits prior to changing therapy, and before and after the screening period.
Student’s t test was used to compare the change in encounter frequency before and after screening tool implementation for both primary and secondary endpoints. α was defined as .05 a priori. Continuous data were presented as means and standard deviations. Data were calculated with Microsoft Excel 2016.
Results
For the 3 months before the 8-week screening period, an average of 476 Anticoagulation Clinic encounters per month were documented. Two months of data following the screening period averaged 546 encounters per month. There were an average of 70 additional encounters per month after screening tool implementation (P = .15), reflecting the study’s primary objective.
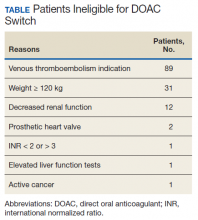
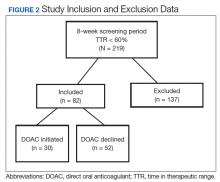
A total of 219 patients in the Anticoagulation Clinic were identified as having a TTR report results of < 60% during the 8-week screening period (Figure 2). Eighty-two of those patients (37.4%) were considered eligible to switch from warfarin to DOAC therapy. Thirty of those eligible patients (13.7%) switched to a DOAC. A total of 107 clinic encounters (22.5%) was associated with these 30 patients prior to screening and 32 associated encounters (5.9%) following screening (P = .01). Of the remaining 137 patients (62.6%) who were ineligible for DOAC therapy, the most common reason for disqualification was a diagnosis of VTE (Table).
Discussion
The general results of this quality improvement project showed that implementation of a screening tool designed to identify patients eligible for DOAC therapy did not decrease the average number of Anticoagulation Clinic encounters. Thirty of 82 eligible patients (36.6%) decided to switch to DOAC therapy during the study period. For those 30 patients, there was a statistically significant decrease in the number of individual clinic encounters. This suggests that the screening tool may positively impact Anticoagulation Clinic metrics when evaluating individual patients, potentially increasing clinic appointment availability.
Confounding Factors
Multiple confounding factors may have affected this project’s results. First, Class I recall for point-of-care test strips used by the clinic was mandated by the US Food and Drug Administration on November 1, 2018.5 Before the recall, investigators found that many nontherapeutic INRs using point-of-care testing later showed results that were within the therapeutic INR range using same-day venous blood collection. This may have led to increases in falsely recorded nontherapeutic INRs and lowered TTR report results. Initially, the project was designed to collect monthly clinic encounter data for 3 months following the 8-week screening period; however, data collection was stopped after 2 months because of the test strip recall.
In addition, in early December 2018, all patients were moved from the Anticoagulation Clinic to the Anticoagulation Telephone Clinic that uses venous blood draws and telephone appointments. Data from venous blood draw results had previously been excluded from this project because results were not available on the same day. Patients in this program are contacted by telephone rather than being offered a face-to-face appointment, thus reducing in-clinic encounters.
Another confounding factor was a FVAHCC policy change in August 2018 requiring that any patient initiated on a DOAC make a onetime visit to the Anticoagulation Clinic prior to establishing care with a PACT CPS. Investigators were unable to exclude these patients from monthly encounter data. Some patients transitioning from warfarin to DOAC therapy were required to continue receiving anticoagulation monitoring from the clinic because of limited PACT CPS clinic availability, thus further increasing postscreening encounters.
Health care providers outside of the Anticoagulation Clinic and uninvolved with the quality improvement project also were switching patients from warfarin to DOAC therapies. Although this may have affected encounter data positively, investigators cannot guarantee these patients would have met criteria outlined by the screening tool.
In September 2018 Hurricane Florence disrupted health care delivery during the 8-week screening period. This event disrupted numerous clinic appointments. Although screening of patients was completed during the 8-week screening period, some patients did not switch to DOAC therapies until November 2018.
Secondary Endpoint Results
Promising results can be seen by specifically looking at the secondary endpoint: the number of encounters associated with patients who chose DOAC therapy. There were 107 encounters associated with the 30 patients who switched to a DOAC prior to screening and only 32 associated encounters after screening, a reduction of 70.1%. This suggests that multiple appointment slots were freed when the screening tool led to successful conversion from warfarin to a DOAC. Further assessment is warranted.
Future Project Development
Future areas for quality improvement project development include expanding project criteria to include patients taking warfarin for VTE. Eighty-nine of 137 patients (65%) who were deemed ineligible to switch to DOAC therapy were excluded due to a diagnosis of VTE. There are existing VA/Department of Defense Criteria for Use for DOAC use in VTE recommendations. Straightforward modification of the screening tool could include this patient group and may be especially useful for patients on indefinite warfarin therapy for recurrent VTE who have poor TTR report results.6
Given the number of confounding factors caused by unforeseen changes to the Anticoagulation Clinic workflow, use of the DOAC screening tool was placed on hold at the conclusion of data collection. This limited the ability to analyze encounter data in the months following project conclusion. Future plans include reimplementation of the screening tool with minor adjustments to include patients on warfarin for VTE and patients with a TTR report results above 60%.
Conclusion
This quality improvement project sought to determine the impact of a screening tool on effecting Anticoagulation Clinic encounter metrics. Results of this project show that the screening tool was unsuccessful in reducing the number of overall clinic encounters. Some promise was shown when evaluating clinic encounters for patients who switched anticoagulation therapies. Numerous confounding factors may have contributed to these results.
1. US Department of Veterans Affairs, Fayetteville Veterans Affairs Health Care Center. MCM 11-188 Anticoagulation Management Program. Revised July 11, 2017. [Source not verified.]
2. US Department of Veterans Affairs, Pharmacy Benefits Management Clinical Pharmacy Practice Office. Anticoagulation percent time in therapeutic range reports. https://spsites.cdw.va.gov/sites/PBM_CPPO/Pages/AnticoagulationTTR.aspx. Revised May 24, 2017. Accessed April 20, 2020. [Source not verified.]
3. US Department of Veterans Affairs, VA Pharmacy Benefits Management Services, Medical Advisory Panel, and VISN Pharmacist Executives. Direct oral anticoagulants (DOACs). Dabigatran (Pradaxa), rivaroxaban (Xarelto), apixaban (Eliquis) and edoxaban (SAVAYSA) criteria for use for stroke prevention in nonvalvular atrial fibrillation (AF). https://www.pbm.va.gov/apps/VANationalFormulary/. Updated December 2017. Accessed April 30, 2020.
4. Martin K, Beyer-Westendorf J, Davidson BL, Huisman MV, Sandset PM, Moll S. Use of the direct oral anticoagulants in obese patients: guidance from the SSC of the ISTH. J Thromb Haemost. 2016;14(6):1308-1313.
5. US Food and Drug Administration. Roche Diagnostics recalls CoaguChek XS PT Test Strips due to naccurate INR test results. https://www.fda.gov/MedicalDevices/Safety/ListofRecalls/ucm624822.htm. Published November 1, 2018. Accessed April 16, 2019.
6. US Department of Veterans Affairs, VA Pharmacy Benefits Management Services, Medical Advisory Panel, and VISN Pharmacist Executives. Direct oral anticoagulants (DOACs) (formerly called TSOACs) dabigatran (Pradaxa), rivaroxaban (Xarelto), apixaban (Eliquis), and edoxaban(Savaysa) criteria for use for *treatment of venous thromboembolism (VTE)* https://www.pbm.va.gov/apps/VANationalFormulary/. Updated December 2017. Accessed April 30, 2020.
Metrics from 2017 at the Fayetteville Veterans Affairs Heath Care Center (FVAHCC) Anticoagulation Clinic indicate that 43% of patients with atrial fibrillation (AF) who are prescribed warfarin have difficulty maintaining a therapeutic international normalized ratio (INR). These patients require frequent clinic appointments to adjust their regimens to ensure anticoagulation efficacy. FVAHCC policy requires a patient to return to the clinic for repeat INR evaluation within 5 to 14 days of the visit where INR was outside of the established therapeutic range.1 These frequent INR monitoring appointments increase patient and health care provider burden.
Direct oral anticoagulants (DOACs) are an alternative to warfarin for patients with AF who require anticoagulation. DOACs, which do not require regular efficacy monitoring, can be beneficial to patients who struggle to maintain a therapeutic INR when taking warfarin. FVAHCC policy regarding warfarin therapy monitoring allows for a maximum of 6 weeks between appointments. This period is often extended to 3 to 6 months for patients on DOACs.1
At FVAHCC, patients prescribed warfarin are managed in a centralized Anticoagulation Clinic led by a clinical pharmacy specialist (CPS). When a patient reports for an appointment, a clinical pharmacy technician performs point-of-care INR testing and asks standardized questions regarding therapy, including an assessment of adherence. The CPS then evaluates the patient’s INR test results, adjusts the dosage of warfarin as indicated, and determines appropriate follow-up.
A patient who is prescribed a DOAC is monitored by a CPS who works within a patient aligned care team (PACT). The PACT, a multidisciplinary team providing health care to veterans, includes physicians, nurses, pharmacists, dieticians, and mental health providers. Each CPS covers 3 or 4 PACTs. These pharmacists monitor all aspects of DOAC therapy at regular intervals, including renal and hepatic function, complete blood counts, medication adherence, and adverse effects.
Clinic and patient INR data are tracked using a time in therapeutic range (TTR) report generated by the US Department of Veterans Affairs (VA). The TTR report provides clinical information to enhance patient anticoagulation care.2 The TTR report identifies patients with an active order for warfarin and a diagnosis of AF or venous thromboembolism (VTE) whose INR is within therapeutic range (between 2 and 3) < 60% of the time over the previous 160 days.2 The patient must have had at least 3 INR levels drawn within that time frame for a TTR report calculation.2 The report excludes patients who were first prescribed warfarin within the previous 42 days and those with mechanical heart valves. The TTR report is used by the VA to see concrete facility-level results for quality improvement efforts.2
A quality improvement screening tool was developed to identify patients with AF being treated with warfarin who may appropriately transition to DOAC therapy. Anticoagulation Clinic patients were eligible for further evaluation if they had a TTR report level of < 60% and were prescribed indefinite warfarin therapy for AF.
The national VA goal is to have patient TTR report levels read > 60%. Therefore, the primary objective of this project was to improve Anticoagulation Clinic TTR metrics by targeting patients with TTR levels below the national goal.2
Patients who were successfully converted from warfarin to a DOAC were no longer included in Anticoagulation Clinic metrics and instead were followed by a PACT CPS. Thus, it was hypothesized that the average number of monthly Anticoagulation Clinic encounters would decrease on successful implementation of the screening tool. A secondary endpoint of the study evaluated the change in the total number of encounters of those who converted from warfarin to a DOAC.
Fewer clinic encounters could increase time available for the CPS to incorporate other initiatives into workflow and could increase clinic availability for newly referred veterans.
Methods
As this undertaking was considered to be a quality improvement project, institutional review board approval was not required. During an 8-week screening period (August to September 2018), the DOAC screening tool was implemented into the Anticoagulation Clinic workflow. This screening tool (Figure 1) was established based on VA Pharmacy Benefit Management (PBM) Service’s Criteria for Use for Stroke Prevention in Nonvalvular Atrial Fibrillation, a national set of standards used to determine appropriate candidates for DOAC therapy.3
Exclusion criteria included patients with INR goals < 2 or > 3, patients with a diagnosis of VTE, and patients with weight > 120 kg. Patients with a diagnosis of VTE were excluded due to the variability in therapy duration. Weight cutoffs were based on recommendations by the International Society on Thrombosis and Haemostasis. Due to a lack of available data, it was suggested that clinical judgment be used in patients whose weight was > 120 kg.4
During the screening period, weekly TTR reports identified patients in the clinic who had TTR < 60%. When a patient with a TTR report results of < 60% also had a scheduled appointment within a week, a CPS then further reviewed patient eligibility using the DOAC screening tool. On arrival for an appointment, the eligible patient was counseled on DOAC medications and the differences between warfarin and DOACs, including monitoring requirements. Patients had the option to switch to DOAC therapy or remain on warfarin.
The change in the average number of monthly Anticoagulation Clinic encounters for 3 months prior to the screening period (May to July 2018) and 2 months following screening (October to November 2018) was evaluated to measure the impact of the DOAC screening tool. The total number of encounters in the clinic was assessed using the monthly VA reports and were averaged for each period. Then data from the 2 periods were compared.
The monthly encounter reports, a data tool that monitors the number of unique visits per veteran each calendar month, also were used to generate a secondary endpoint showing the number of encounters in the Anticoagulation Clinic associated with patients who switched to a DOAC, including visits prior to changing therapy, and before and after the screening period.
Student’s t test was used to compare the change in encounter frequency before and after screening tool implementation for both primary and secondary endpoints. α was defined as .05 a priori. Continuous data were presented as means and standard deviations. Data were calculated with Microsoft Excel 2016.
Results
For the 3 months before the 8-week screening period, an average of 476 Anticoagulation Clinic encounters per month were documented. Two months of data following the screening period averaged 546 encounters per month. There were an average of 70 additional encounters per month after screening tool implementation (P = .15), reflecting the study’s primary objective.
A total of 219 patients in the Anticoagulation Clinic were identified as having a TTR report results of < 60% during the 8-week screening period (Figure 2). Eighty-two of those patients (37.4%) were considered eligible to switch from warfarin to DOAC therapy. Thirty of those eligible patients (13.7%) switched to a DOAC. A total of 107 clinic encounters (22.5%) was associated with these 30 patients prior to screening and 32 associated encounters (5.9%) following screening (P = .01). Of the remaining 137 patients (62.6%) who were ineligible for DOAC therapy, the most common reason for disqualification was a diagnosis of VTE (Table).
Discussion
The general results of this quality improvement project showed that implementation of a screening tool designed to identify patients eligible for DOAC therapy did not decrease the average number of Anticoagulation Clinic encounters. Thirty of 82 eligible patients (36.6%) decided to switch to DOAC therapy during the study period. For those 30 patients, there was a statistically significant decrease in the number of individual clinic encounters. This suggests that the screening tool may positively impact Anticoagulation Clinic metrics when evaluating individual patients, potentially increasing clinic appointment availability.
Confounding Factors
Multiple confounding factors may have affected this project’s results. First, Class I recall for point-of-care test strips used by the clinic was mandated by the US Food and Drug Administration on November 1, 2018.5 Before the recall, investigators found that many nontherapeutic INRs using point-of-care testing later showed results that were within the therapeutic INR range using same-day venous blood collection. This may have led to increases in falsely recorded nontherapeutic INRs and lowered TTR report results. Initially, the project was designed to collect monthly clinic encounter data for 3 months following the 8-week screening period; however, data collection was stopped after 2 months because of the test strip recall.
In addition, in early December 2018, all patients were moved from the Anticoagulation Clinic to the Anticoagulation Telephone Clinic that uses venous blood draws and telephone appointments. Data from venous blood draw results had previously been excluded from this project because results were not available on the same day. Patients in this program are contacted by telephone rather than being offered a face-to-face appointment, thus reducing in-clinic encounters.
Another confounding factor was a FVAHCC policy change in August 2018 requiring that any patient initiated on a DOAC make a onetime visit to the Anticoagulation Clinic prior to establishing care with a PACT CPS. Investigators were unable to exclude these patients from monthly encounter data. Some patients transitioning from warfarin to DOAC therapy were required to continue receiving anticoagulation monitoring from the clinic because of limited PACT CPS clinic availability, thus further increasing postscreening encounters.
Health care providers outside of the Anticoagulation Clinic and uninvolved with the quality improvement project also were switching patients from warfarin to DOAC therapies. Although this may have affected encounter data positively, investigators cannot guarantee these patients would have met criteria outlined by the screening tool.
In September 2018 Hurricane Florence disrupted health care delivery during the 8-week screening period. This event disrupted numerous clinic appointments. Although screening of patients was completed during the 8-week screening period, some patients did not switch to DOAC therapies until November 2018.
Secondary Endpoint Results
Promising results can be seen by specifically looking at the secondary endpoint: the number of encounters associated with patients who chose DOAC therapy. There were 107 encounters associated with the 30 patients who switched to a DOAC prior to screening and only 32 associated encounters after screening, a reduction of 70.1%. This suggests that multiple appointment slots were freed when the screening tool led to successful conversion from warfarin to a DOAC. Further assessment is warranted.
Future Project Development
Future areas for quality improvement project development include expanding project criteria to include patients taking warfarin for VTE. Eighty-nine of 137 patients (65%) who were deemed ineligible to switch to DOAC therapy were excluded due to a diagnosis of VTE. There are existing VA/Department of Defense Criteria for Use for DOAC use in VTE recommendations. Straightforward modification of the screening tool could include this patient group and may be especially useful for patients on indefinite warfarin therapy for recurrent VTE who have poor TTR report results.6
Given the number of confounding factors caused by unforeseen changes to the Anticoagulation Clinic workflow, use of the DOAC screening tool was placed on hold at the conclusion of data collection. This limited the ability to analyze encounter data in the months following project conclusion. Future plans include reimplementation of the screening tool with minor adjustments to include patients on warfarin for VTE and patients with a TTR report results above 60%.
Conclusion
This quality improvement project sought to determine the impact of a screening tool on effecting Anticoagulation Clinic encounter metrics. Results of this project show that the screening tool was unsuccessful in reducing the number of overall clinic encounters. Some promise was shown when evaluating clinic encounters for patients who switched anticoagulation therapies. Numerous confounding factors may have contributed to these results.
Metrics from 2017 at the Fayetteville Veterans Affairs Heath Care Center (FVAHCC) Anticoagulation Clinic indicate that 43% of patients with atrial fibrillation (AF) who are prescribed warfarin have difficulty maintaining a therapeutic international normalized ratio (INR). These patients require frequent clinic appointments to adjust their regimens to ensure anticoagulation efficacy. FVAHCC policy requires a patient to return to the clinic for repeat INR evaluation within 5 to 14 days of the visit where INR was outside of the established therapeutic range.1 These frequent INR monitoring appointments increase patient and health care provider burden.
Direct oral anticoagulants (DOACs) are an alternative to warfarin for patients with AF who require anticoagulation. DOACs, which do not require regular efficacy monitoring, can be beneficial to patients who struggle to maintain a therapeutic INR when taking warfarin. FVAHCC policy regarding warfarin therapy monitoring allows for a maximum of 6 weeks between appointments. This period is often extended to 3 to 6 months for patients on DOACs.1
At FVAHCC, patients prescribed warfarin are managed in a centralized Anticoagulation Clinic led by a clinical pharmacy specialist (CPS). When a patient reports for an appointment, a clinical pharmacy technician performs point-of-care INR testing and asks standardized questions regarding therapy, including an assessment of adherence. The CPS then evaluates the patient’s INR test results, adjusts the dosage of warfarin as indicated, and determines appropriate follow-up.
A patient who is prescribed a DOAC is monitored by a CPS who works within a patient aligned care team (PACT). The PACT, a multidisciplinary team providing health care to veterans, includes physicians, nurses, pharmacists, dieticians, and mental health providers. Each CPS covers 3 or 4 PACTs. These pharmacists monitor all aspects of DOAC therapy at regular intervals, including renal and hepatic function, complete blood counts, medication adherence, and adverse effects.
Clinic and patient INR data are tracked using a time in therapeutic range (TTR) report generated by the US Department of Veterans Affairs (VA). The TTR report provides clinical information to enhance patient anticoagulation care.2 The TTR report identifies patients with an active order for warfarin and a diagnosis of AF or venous thromboembolism (VTE) whose INR is within therapeutic range (between 2 and 3) < 60% of the time over the previous 160 days.2 The patient must have had at least 3 INR levels drawn within that time frame for a TTR report calculation.2 The report excludes patients who were first prescribed warfarin within the previous 42 days and those with mechanical heart valves. The TTR report is used by the VA to see concrete facility-level results for quality improvement efforts.2
A quality improvement screening tool was developed to identify patients with AF being treated with warfarin who may appropriately transition to DOAC therapy. Anticoagulation Clinic patients were eligible for further evaluation if they had a TTR report level of < 60% and were prescribed indefinite warfarin therapy for AF.
The national VA goal is to have patient TTR report levels read > 60%. Therefore, the primary objective of this project was to improve Anticoagulation Clinic TTR metrics by targeting patients with TTR levels below the national goal.2
Patients who were successfully converted from warfarin to a DOAC were no longer included in Anticoagulation Clinic metrics and instead were followed by a PACT CPS. Thus, it was hypothesized that the average number of monthly Anticoagulation Clinic encounters would decrease on successful implementation of the screening tool. A secondary endpoint of the study evaluated the change in the total number of encounters of those who converted from warfarin to a DOAC.
Fewer clinic encounters could increase time available for the CPS to incorporate other initiatives into workflow and could increase clinic availability for newly referred veterans.
Methods
As this undertaking was considered to be a quality improvement project, institutional review board approval was not required. During an 8-week screening period (August to September 2018), the DOAC screening tool was implemented into the Anticoagulation Clinic workflow. This screening tool (Figure 1) was established based on VA Pharmacy Benefit Management (PBM) Service’s Criteria for Use for Stroke Prevention in Nonvalvular Atrial Fibrillation, a national set of standards used to determine appropriate candidates for DOAC therapy.3
Exclusion criteria included patients with INR goals < 2 or > 3, patients with a diagnosis of VTE, and patients with weight > 120 kg. Patients with a diagnosis of VTE were excluded due to the variability in therapy duration. Weight cutoffs were based on recommendations by the International Society on Thrombosis and Haemostasis. Due to a lack of available data, it was suggested that clinical judgment be used in patients whose weight was > 120 kg.4
During the screening period, weekly TTR reports identified patients in the clinic who had TTR < 60%. When a patient with a TTR report results of < 60% also had a scheduled appointment within a week, a CPS then further reviewed patient eligibility using the DOAC screening tool. On arrival for an appointment, the eligible patient was counseled on DOAC medications and the differences between warfarin and DOACs, including monitoring requirements. Patients had the option to switch to DOAC therapy or remain on warfarin.
The change in the average number of monthly Anticoagulation Clinic encounters for 3 months prior to the screening period (May to July 2018) and 2 months following screening (October to November 2018) was evaluated to measure the impact of the DOAC screening tool. The total number of encounters in the clinic was assessed using the monthly VA reports and were averaged for each period. Then data from the 2 periods were compared.
The monthly encounter reports, a data tool that monitors the number of unique visits per veteran each calendar month, also were used to generate a secondary endpoint showing the number of encounters in the Anticoagulation Clinic associated with patients who switched to a DOAC, including visits prior to changing therapy, and before and after the screening period.
Student’s t test was used to compare the change in encounter frequency before and after screening tool implementation for both primary and secondary endpoints. α was defined as .05 a priori. Continuous data were presented as means and standard deviations. Data were calculated with Microsoft Excel 2016.
Results
For the 3 months before the 8-week screening period, an average of 476 Anticoagulation Clinic encounters per month were documented. Two months of data following the screening period averaged 546 encounters per month. There were an average of 70 additional encounters per month after screening tool implementation (P = .15), reflecting the study’s primary objective.
A total of 219 patients in the Anticoagulation Clinic were identified as having a TTR report results of < 60% during the 8-week screening period (Figure 2). Eighty-two of those patients (37.4%) were considered eligible to switch from warfarin to DOAC therapy. Thirty of those eligible patients (13.7%) switched to a DOAC. A total of 107 clinic encounters (22.5%) was associated with these 30 patients prior to screening and 32 associated encounters (5.9%) following screening (P = .01). Of the remaining 137 patients (62.6%) who were ineligible for DOAC therapy, the most common reason for disqualification was a diagnosis of VTE (Table).
Discussion
The general results of this quality improvement project showed that implementation of a screening tool designed to identify patients eligible for DOAC therapy did not decrease the average number of Anticoagulation Clinic encounters. Thirty of 82 eligible patients (36.6%) decided to switch to DOAC therapy during the study period. For those 30 patients, there was a statistically significant decrease in the number of individual clinic encounters. This suggests that the screening tool may positively impact Anticoagulation Clinic metrics when evaluating individual patients, potentially increasing clinic appointment availability.
Confounding Factors
Multiple confounding factors may have affected this project’s results. First, Class I recall for point-of-care test strips used by the clinic was mandated by the US Food and Drug Administration on November 1, 2018.5 Before the recall, investigators found that many nontherapeutic INRs using point-of-care testing later showed results that were within the therapeutic INR range using same-day venous blood collection. This may have led to increases in falsely recorded nontherapeutic INRs and lowered TTR report results. Initially, the project was designed to collect monthly clinic encounter data for 3 months following the 8-week screening period; however, data collection was stopped after 2 months because of the test strip recall.
In addition, in early December 2018, all patients were moved from the Anticoagulation Clinic to the Anticoagulation Telephone Clinic that uses venous blood draws and telephone appointments. Data from venous blood draw results had previously been excluded from this project because results were not available on the same day. Patients in this program are contacted by telephone rather than being offered a face-to-face appointment, thus reducing in-clinic encounters.
Another confounding factor was a FVAHCC policy change in August 2018 requiring that any patient initiated on a DOAC make a onetime visit to the Anticoagulation Clinic prior to establishing care with a PACT CPS. Investigators were unable to exclude these patients from monthly encounter data. Some patients transitioning from warfarin to DOAC therapy were required to continue receiving anticoagulation monitoring from the clinic because of limited PACT CPS clinic availability, thus further increasing postscreening encounters.
Health care providers outside of the Anticoagulation Clinic and uninvolved with the quality improvement project also were switching patients from warfarin to DOAC therapies. Although this may have affected encounter data positively, investigators cannot guarantee these patients would have met criteria outlined by the screening tool.
In September 2018 Hurricane Florence disrupted health care delivery during the 8-week screening period. This event disrupted numerous clinic appointments. Although screening of patients was completed during the 8-week screening period, some patients did not switch to DOAC therapies until November 2018.
Secondary Endpoint Results
Promising results can be seen by specifically looking at the secondary endpoint: the number of encounters associated with patients who chose DOAC therapy. There were 107 encounters associated with the 30 patients who switched to a DOAC prior to screening and only 32 associated encounters after screening, a reduction of 70.1%. This suggests that multiple appointment slots were freed when the screening tool led to successful conversion from warfarin to a DOAC. Further assessment is warranted.
Future Project Development
Future areas for quality improvement project development include expanding project criteria to include patients taking warfarin for VTE. Eighty-nine of 137 patients (65%) who were deemed ineligible to switch to DOAC therapy were excluded due to a diagnosis of VTE. There are existing VA/Department of Defense Criteria for Use for DOAC use in VTE recommendations. Straightforward modification of the screening tool could include this patient group and may be especially useful for patients on indefinite warfarin therapy for recurrent VTE who have poor TTR report results.6
Given the number of confounding factors caused by unforeseen changes to the Anticoagulation Clinic workflow, use of the DOAC screening tool was placed on hold at the conclusion of data collection. This limited the ability to analyze encounter data in the months following project conclusion. Future plans include reimplementation of the screening tool with minor adjustments to include patients on warfarin for VTE and patients with a TTR report results above 60%.
Conclusion
This quality improvement project sought to determine the impact of a screening tool on effecting Anticoagulation Clinic encounter metrics. Results of this project show that the screening tool was unsuccessful in reducing the number of overall clinic encounters. Some promise was shown when evaluating clinic encounters for patients who switched anticoagulation therapies. Numerous confounding factors may have contributed to these results.
1. US Department of Veterans Affairs, Fayetteville Veterans Affairs Health Care Center. MCM 11-188 Anticoagulation Management Program. Revised July 11, 2017. [Source not verified.]
2. US Department of Veterans Affairs, Pharmacy Benefits Management Clinical Pharmacy Practice Office. Anticoagulation percent time in therapeutic range reports. https://spsites.cdw.va.gov/sites/PBM_CPPO/Pages/AnticoagulationTTR.aspx. Revised May 24, 2017. Accessed April 20, 2020. [Source not verified.]
3. US Department of Veterans Affairs, VA Pharmacy Benefits Management Services, Medical Advisory Panel, and VISN Pharmacist Executives. Direct oral anticoagulants (DOACs). Dabigatran (Pradaxa), rivaroxaban (Xarelto), apixaban (Eliquis) and edoxaban (SAVAYSA) criteria for use for stroke prevention in nonvalvular atrial fibrillation (AF). https://www.pbm.va.gov/apps/VANationalFormulary/. Updated December 2017. Accessed April 30, 2020.
4. Martin K, Beyer-Westendorf J, Davidson BL, Huisman MV, Sandset PM, Moll S. Use of the direct oral anticoagulants in obese patients: guidance from the SSC of the ISTH. J Thromb Haemost. 2016;14(6):1308-1313.
5. US Food and Drug Administration. Roche Diagnostics recalls CoaguChek XS PT Test Strips due to naccurate INR test results. https://www.fda.gov/MedicalDevices/Safety/ListofRecalls/ucm624822.htm. Published November 1, 2018. Accessed April 16, 2019.
6. US Department of Veterans Affairs, VA Pharmacy Benefits Management Services, Medical Advisory Panel, and VISN Pharmacist Executives. Direct oral anticoagulants (DOACs) (formerly called TSOACs) dabigatran (Pradaxa), rivaroxaban (Xarelto), apixaban (Eliquis), and edoxaban(Savaysa) criteria for use for *treatment of venous thromboembolism (VTE)* https://www.pbm.va.gov/apps/VANationalFormulary/. Updated December 2017. Accessed April 30, 2020.
1. US Department of Veterans Affairs, Fayetteville Veterans Affairs Health Care Center. MCM 11-188 Anticoagulation Management Program. Revised July 11, 2017. [Source not verified.]
2. US Department of Veterans Affairs, Pharmacy Benefits Management Clinical Pharmacy Practice Office. Anticoagulation percent time in therapeutic range reports. https://spsites.cdw.va.gov/sites/PBM_CPPO/Pages/AnticoagulationTTR.aspx. Revised May 24, 2017. Accessed April 20, 2020. [Source not verified.]
3. US Department of Veterans Affairs, VA Pharmacy Benefits Management Services, Medical Advisory Panel, and VISN Pharmacist Executives. Direct oral anticoagulants (DOACs). Dabigatran (Pradaxa), rivaroxaban (Xarelto), apixaban (Eliquis) and edoxaban (SAVAYSA) criteria for use for stroke prevention in nonvalvular atrial fibrillation (AF). https://www.pbm.va.gov/apps/VANationalFormulary/. Updated December 2017. Accessed April 30, 2020.
4. Martin K, Beyer-Westendorf J, Davidson BL, Huisman MV, Sandset PM, Moll S. Use of the direct oral anticoagulants in obese patients: guidance from the SSC of the ISTH. J Thromb Haemost. 2016;14(6):1308-1313.
5. US Food and Drug Administration. Roche Diagnostics recalls CoaguChek XS PT Test Strips due to naccurate INR test results. https://www.fda.gov/MedicalDevices/Safety/ListofRecalls/ucm624822.htm. Published November 1, 2018. Accessed April 16, 2019.
6. US Department of Veterans Affairs, VA Pharmacy Benefits Management Services, Medical Advisory Panel, and VISN Pharmacist Executives. Direct oral anticoagulants (DOACs) (formerly called TSOACs) dabigatran (Pradaxa), rivaroxaban (Xarelto), apixaban (Eliquis), and edoxaban(Savaysa) criteria for use for *treatment of venous thromboembolism (VTE)* https://www.pbm.va.gov/apps/VANationalFormulary/. Updated December 2017. Accessed April 30, 2020.