User login
- Among ER-positive breast cancer patients treated with tamoxifen for 5 years, the annual recurrence rate is cut in half and the annual death rate is reduced by 28%.
- Tamoxifen has been shown to reduce the incidence of breast cancer by 44%, 51%, and 55% in women aged 35 to 49, 50 to 59, and 60 and older, respectively.
- Raloxifene mimics the effects of estrogen on the skeleton and lipids, but acts as a complete estrogen antagonist in the breast and uterus.
- Recent data indicate that 4 years of raloxifene therapy reduces the risk of all ER-positive breast cancers by 72% compared with placebo.
- The ideal candidate for raloxifene is a postmenopausal woman with osteopenia or osteoporosis, no increased risk of thromboembolism, and few or no vasomotor symptoms.
Although it was initially considered a female sex hormone, estrogen is now recognized as a systemic substance that affects every organ system and appears to be important for both men’s and women’s health.1-4 At puberty, elevated circulating estrogen levels transform girls into young women and shape their feminine fig-ures. In boys, estrogen is important in the closure of the epiphyseal plates to arrest postpubertal growth and support bone health throughout life. It also may have beneficial actions on the male cardiovascular system.2
As a systemic hormone, estrogen is important in women for the health of the skeleton, the heart, the integuments, and the brain.1,3 Women who suffer the loss of estrogen through surgery, illness, or early menopause often are advised to consider estrogen therapy to protect these organs from premature failure.
Unfortunately, estrogen replacement therapy (ERT) is a double-edged sword, with significant benefits in some organs and significant risks in others. ERT protects against vasomotor symptoms, urogenital atrophy, osteoporosis, cardiovascular disease, and perhaps Alzheimer’s disease.1,3 Unfortunately, it also increases the risk of venous thromboembolic events, recurrent myocardial infarction and cardiac death5 (in diseased hearts), and cancers of the uterus and breast.4
Of the many diseases that impact the lives of American women, none is more feared than breast cancer, the most common cancer in females and the second leading cause of female cancer mortality.6 The fear of this disease causes many women to decline or discontinue ERT, a decision that can profoundly affect their long-term health.7 Hence, the controversy regarding ERT centers on weighing the risk-benefit balance, i.e., trying to select patients who are more likely to benefit from the hormone and less likely to suffer from its adverse effects.
Physicians—particularly gynecologists who devote their care exclusively to women’s health—have longed for the ideal estrogen that will impart all of the benefits without the risks. This ideal estrogen would relieve women of vasomotor and urogenital symptoms and prevent the dire consequences of osteoporosis, accelerated atherosclerosis, neurogenic deficit, and collagen loss from the skin, without increasing the risk of cancer and thromboembolic phenomena. Some experts believe this ideal may be found in the new class of compounds referred to as selective estrogen receptor modulators (SERMs). Although several SERMs with desirable estrogenic properties are available, none is ideal. Nevertheless, this new class of estrogenic substances—with multiple beneficial effects and fewer risks—represents an important pharma-cotherapeutic advance in the care of postmenopausal women.
Tamoxifen
Tamoxifen citrate (Nolvadex; AstraZeneca, Wilmington, Del) has wide clinical applications in the treatment and prevention of breast cancer. In 1980, tamoxifen was approved by the FDA for postmenopausal women with node-positive breast cancer and for premenopausal women with estrogen-receptor (ER) positive advanced breast cancer. In 1990, the FDA extended the approval of tamoxifen to include pre- and postmenopausal women with node-negative, ER-positive breast cancer.
In patients with node-positive breast cancer, the 10-year-survival rate improves from 50% in control subjects to 61% in patients treated with tamoxifen for 5 years. Similar improvements in survival have been reported with tamoxifen therapy in node-negative breast cancer patients. After 5 years of tamoxifen therapy and a median follow-up of 10 years, the reduction of breast cancer recurrence and death with tamoxifen treatment compared with placebo are 47% and 26%, respectively.8
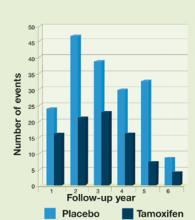
For women with ER-positive tumors (about 70% of all breast cancers), tamoxifen therapy of at least 1 year’s duration results in statistically significant recurrence and survival benefits. These results increase with the duration of treatment up to 5 years. Among ER-positive women treated with tamoxifen for 5 years—the optimal length of therapy—the annual recurrence rate is cut in half and the annual death rate is reduced by 28% (Figure 1).9 Treatment beyond 5 years does not accrue additional benefits and is associated with increased adverse events.10 Tamoxifen has little effect on animal tumors and ER-negative tumors in cell cultures and confers little or no benefit to women with ER-negative tumors.9
In ER-positive tumors, tamoxifen is believed to exert its anti-tumor effects by inhibiting the estrogen-dependent secretion of growth factors and angiogenic factors by the tumor cells, and by inducing programmed death of the tumor cells.9 An understanding of this mechanism, along with the observation that tamoxifen-treated breast cancer patients not only experience a reduction in breast cancer recurrence but also a 47% reduction in contralateral breast cancer, led to the launch of the tamoxifen chemoprevention trials in North America and Europe.
In 1992, the National Surgical Adjuvant Breast and Bowel Project (NSABP) Tamoxifen Breast Cancer Prevention Trial was launched in the United States and Canada. The results of this trial were released early because of the compelling evidence of tamoxifen’s therapeutic efficacy. The drug was found to reduce the incidence of breast cancer by 44%, 51%, and 55% in women aged 35 to 49, 50 to 59, and 60 or older, respectively. Tamoxifen also was found to reduce ductal carcinoma in situ by 50%. Although tamoxifen decreased the overall occurrence of ER-positive tumors by 69%, it did not have a significant impact on the incidence of ER-negative tumors.11
Based on these results, the FDA approved the use of tamoxifen for the primary prevention of breast cancer in high-risk women. It is important to limit the use of tamoxifen to high-risk women because of the potential for serious side effects, which include endometrial cancer, pulmonary embolism, deep vein thrombosis (DVT), and cataract formation.9-11
The issue of whether tamoxifen inhibits the initial development of a tumor or suppresses an occult tumor remains unresolved. It is possible that both mechanisms are involved. This is not merely an academic concern. Questions remain as to whether a suppressed tumor may develop resistance to tamoxifen or even be stimulated by the drug, becoming more virulent during or after discontinuation of therapy. However, given the extensive data and longterm clinical experience and follow-up with tamoxifen, this scenario seems unlikely. In fact, the reduction in the incidence of breast cancer appears to continue for years after the therapy is discontinued.12,13
FIGURE 1Effect of tamoxifen on ER-positive breast cancer
Adapted from: Osborne CK. Tamoxifen in the treatment of breast cancer. N Engl J Med. 1998;339:1609-1618.
Raloxifene
Raloxifene hydrochloride (Evista; Eli Lilly and Company, Indianapolis, Ind) was originally investigated for the treatment of breast cancer and was found to be similar to tamoxifen in its anti-tumor activity. Raloxifene was subsequently studied for its skeletal effects and was approved for osteoporosis prevention in postmenopausal women in December 1997, and for fracture prevention in 1999.
The agent mimics the effects of estrogen on the skeleton and lipids. However, it acts as a complete estrogen antagonist in the breast and the uterus, making it a more desirable SERM in the management of menopausal women with an intact uterus.
The Multiple Outcomes of Raloxifene Evaluation (MORE) trial was a randomized, placebo-controlled, multicenter study involving 7,705 postmenopausal women with osteoporosis at baseline. Although bone metabolism and fractures were the primary endpoints, many other outcomes were evaluated, including uterine and endometrial effects, bleeding, breast cancer incidence, lipid levels, clotting factors, patient tolerance, and adverse events.14-18 The results of these studies support the classification of raloxifene as a SERM with several desirable estrogen-agonistic and -antagonistic effects. Even so, it is far from perfect.
Effects on bone. In the MORE trial, both placebo- and raloxifene-treated postmenopausal women received 500 mg of supplemental calcium and 400 to 600 IU of cholecalciferol (vitamin D) daily. In these postmenopausal women with osteoporosis, a daily dose of 60 mg raloxifene increased bone mineral density (BMD) in the spine by 2.6% and in the femoral neck by 2.1% compared with placebo. The assessment of biochemical bone markers indicated that raloxifene increases BMD by decreasing bone turnover. Indeed, markers of bone resorption and formation—urinary type I collagen Ctelopeptide and serum osteocalcin—were significantly decreased with raloxifene within 3 months of therapy, similar to estrogen.14,19
Raloxifene also reduced vertebral fractures in postmenopausal women with osteoporosis. Vertebral fractures occurred in 10.1% of women randomized to placebo versus 6.6% of women receiving 60 mg raloxifene daily. Although raloxifene decreased the risk of new vertebral fractures regardless of whether a spinal fracture was present at baseline, the absolute percentage decrease in the fracture rate was higher in women who did not have a fracture at baseline.14 This finding underscores the importance of diagnosing and treating osteoporosis before the development of a fracture.
One interesting observation, which is true for raloxifene and other antiresorptive agents, particularly calcitonin, is that the magnitude of the reduction in vertebral fractures is greater than would have been predicted by the modest improvement in BMD observed during therapy. This suggests that antiresorptive agents may influence bone quality, perhaps through its architectural structure, in ways that cannot be ascertained by the assessment of BMD alone. Bone quality refers to skeletal factors that strongly impact the structural properties of bone, independent of the quantity of bone, assessed by BMD. Specific factors that contribute to the structural competence of bone include its microarchitecture, degree of bone mineralization, state of the organic matrix, and rate of bone turnover. The effects of antiresorptive agents on these factors may explain why such modest improvements in BMD—i.e., 2% to 6%—result in reductions in fracture risks in the range of 30% to 50%.
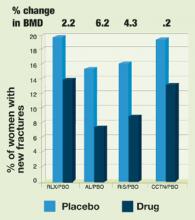
Figure 2 shows the mean changes in BMD and the corresponding reduction in vertebral fractures for the therapeutic agents currently approved for the prevention and treatment of osteoporosis. Despite significant differences in BMD increases, the reductions in vertebral fracture rates are quite comparable. In the MORE trial, there was no difference in the incidence of nontraumatic fractures at sites other than the spine, possibly because of the small number of nonvertebral fractures that occurred during the study period, which yielded insufficient power to detect a difference. A subset of patients in the MORE study is being followed for a longer time to further evaluate the incidence of nonvertebral fractures.
Effects on cardiovascular health. Raloxifene’s effects on total and low-density lipoprotein (LDL) cholesterol are similar to those of estrogen, with decreases of 11% and 6%, respectively. Unlike estrogen, which increases the circulating levels of both high-density lipoprotein (HDL) cholesterol and triglycerides, raloxifene causes an insignificant increase in HDL and a slight decrease in triglyceride levels. Serum levels of lipoprotein(a) are reduced much more with ERT (16.3%) than raloxifene therapy (4.1%), whereas fibrinogen levels are reduced more with raloxifene (12.2%) than ERT (2.8%).15
The effects of raloxifene on 2 additional independent risk factors for cardiovascular disease were recently reported. Elevated levels of C-reactive protein, a circulating marker of inflammation, are associated with a significantly greater risk of myocardial infarction. Also, elevated levels of homocysteine predict a greater risk of coronary artery disease. While ERT significantly increases circulating levels of C-reactive protein (by as much as 84%), raloxifene lacks a significant effect. Like estrogen, raloxifene significantly lowers homocysteine levels by 6% to 8%.16
The changes in serum levels of the several markers of cardiovascular health are summarized for both ERT and raloxifene in Table 1. Overall, raloxifene therapy is associated with favorable changes in the serum levels of several of these markers, suggesting that the SERM may substantially reduce the risk of heart disease in postmenopausal women. However, conclusive proof would require a clinical trial with cardiovascular events as the definitive endpoints. Such an investigation is currently being carried out in the Raloxifene Use for the Heart (RUTH) trial. The results should indicate whether these favorable biochemical effects are indeed associated with a reduction in the incidence of cardiovascular disease.
Effects on the endometrium. Unlike tamoxifen and toremifene (Fareston; Schering-Plough, Kenilworth, NJ), which stimulate endometrial proliferation and increase the risk of endometrial cancer, raloxifene has no stimulatory effects on either the endometrium or the myometrium.17,18 In postmenopausal women receiving raloxifene, the incidence of vaginal bleeding is the same as in women on placebo. In the MORE trial, raloxifene did not increase the risk of endometrial cancer, endometrial hyperplasia, or uterine bleeding over a follow-up period of 40 months.20 Several other studies have evaluated the endometrial response to raloxifene—compared with placebo or ERT—for periods ranging from 12 to 24 months. These studies have consistently reported that raloxifene has no stimulatory effects on the uterus of postmenopausal women.17,18,21 Thus, it seems clear that postmenopausal patients on raloxifene need not worry about an increased risk of polyps, endometrial proliferation, hyperplasia, or cancer. Their endometrial thickness and uterine volume will not increase, nor will they experience a greater incidence of vaginal bleeding than untreated women.5,17,18,20
In postmenopausal women, the administration of raloxifene does not require the addition of a progestin or routine endometrial monitoring by biopsy or ultrasonography. Any bleeding during raloxifene therapy should therefore be promptly evaluated, since it is unlikely to be related to the SERM.
Effects on the breast. In contrast to ERT, raloxifene does not increase the frequency of breast pain or tenderness in postmenopausal women. In addition, recent data suggest that raloxifene may reduce the risk of breast cancer.
Like tamoxifen, raloxifene inhibits the growth of mammary tumors in rodents and the growth of the human MCF-7 breast cancer cell line in vitro.9,22 The latest follow-up data from the MORE trial indicate that 4 years of raloxifene therapy reduces the risk of all breast cancers by 72% compared with placebo. At the 4-year mark of the MORE trial, a total of 79 breast cancer cases occurred—4.7 cases per 1,000 patient-years in the placebo group compared with 1.3 cases per 1,000 patient-years in the raloxifene group. The protective effect was essentially restricted to ER-positive cancers. As in previous observations, women with the highest serum estrogen levels had the highest BMD and the highest incidence of breast cancer. These same women experienced the greatest reduction in breast cancer risk with raloxifene therapy.
Because raloxifene has not yet been studied directly as a prophylactic, it cannot be recommended for the prevention of breast cancer. Several other issues regarding raloxifene and breast cancer prevention also need to be addressed. For example, the efficacy rates of raloxifene in women at increased risk for breast cancer and in younger postmenopausal women are unknown. We also lack data regarding the optimal duration of raloxifene therapy for maximal, sustained protection against breast cancer.
These issues are expected to be resolved at the completion of the Study of Tamoxifen and Raloxifene (STAR) trial. This randomized, double-blind comparison of tamoxifen and raloxifene is the largest breast-cancer prevention study ever conducted, involving more than 300 institutions throughout the U.S., Canada, and Puerto Rico and approximately 22,000 postmenopausal women. Participants include women 35 to 59 years of age who face an increased risk of breast cancer and women 60 and older with no additional risk factors. These women are given either 20 mg tamoxifen daily or 60 mg raloxifene daily for 5 years. The study, which was launched in 1999 and is expected to continue for 5 to 10 years, should provide definitive data regarding the role of these SERMs in the prevention of breast cancer and in preventing disease in general in postmenopausal women.
Adverse effects. Although raloxifene exerts several desirable estrogen-agonist and -antagonist effects, it also exerts several undesirable effects (Table 2), which may preclude its administration in a large segment of postmenopausal women. Perhaps its most worrisome side effect is the increased risk of venous thromboembolic events, which has been reported to be approximately 3 times the risk incurred by women on placebo.20,22 (This level of risk is similar to that reported for estrogen use.21) Raloxifene does not improve vasomotor symptoms or vaginal dryness. Leg cramps have been reported in 6% of women on raloxifene versus 2% of women on placebo.23
Raloxifene’s effects on the central nervous system (CNS) are largely unknown and seem to differ from those of estrogen. In an in vitro system using cultured rat neurons derived from areas of the brain important for memory, raloxifene was neuroprotective at low concentrations but neurotoxic at high concentrations. Other animal studies have reported that raloxifene, like estrogen, may have a beneficial effect on cholinergic transmission within the brain and induce neurite outgrowth in ER-positive rat neuronal cell lines.12,24 Because it does not decrease the incidence of hot flushes, some experts believe that raloxifene may exert anti-estrogenic effects on the CNS. However there are few clinical studies of the effects of raloxifene on the CNS in women. The few that exist indicate neither beneficial nor adverse effectson cognition, mood, or memory.25 No clinical data on raloxifene and the risk of Alzheimer’s disease are currently available.
FIGURE 2Effect of bone therapy on vertebral fracture rates
RLX = raloxifene, AL = alendronate, RIS = risedronate, CCTN = calcitonin, PBO = placeboTABLE 1
Effects of raloxifene and ERT on markers of cardiovascular disease risk in healthy postmenopausal women
| Marker | Raloxifene | ERT |
|---|---|---|
| LDL cholesterol | -12 | -14 |
| HDL cholesterol | 0 | +10 |
| HDL2 | +15 | +33 |
| Triglycerides | -4 | +20 |
| Lipoprotein(a) | -7 | -19 |
| Fibrinogen | -10 | -1 |
| Homocysteine | -8 | -6.6 |
| C-reactive protein | -4 | +84.1 |
| Data are reported as percent change compared with placebo except for homocysteine and C-reactive protein, which are reported as percent change from baseline. | ||
| Source: Walsh B, et al. Effects of raloxifene on serum lipids and coagulation factors in healthy postmenopausal women. JAMA. 1998;279:1445-1451. Walsh B, et al. The effects of hormone replacement therapy and raloxifene on C-reactive protein and homocysteine in healthy postmenopausal women: a randomized controlled trial. J Clin Endocrinol Metab. 2000;85:214-218. | ||
TABLE 2
Adverse events reported by postmenopausal women in controlled trials of raloxifene
| Adverse event | Raloxifene | Placebo | P value |
|---|---|---|---|
| Vasomotor symptoms | 9.7% | 6.4% | .01 |
| Leg cramps | 7% | 3.7% | <.001 |
| Influenza syndrome | 13.4% | 11.4% | <.001 |
| Peripheral edema | 5.2% | 4.4% | <.01 |
| Endometrial cavity fluid | 8.1% | 5.7% | .02 |
| Venous thromboembolism | 1% | 0.3% | <.001 |
| Source: Ettinger B, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. JAMA. 1999;282:637-644. Nilsen J, et al. Raloxifene induces neurite outgrowth in estrogen receptor positive PC12 cells. Menopause. 1998;5:211-216. | |||
Patient selection
The candidates for tamoxifen are clearly defined. These include ER-positive breast-cancer patients as well as pre- and postmenopausal women at significant risk for the disease. There currently is no other indication for tamoxifen therapy in the U.S. In other countries, the drug is used—as clomiphene citrate is in the U.S.—to induce ovulation. Because of the risk of venous thromboem-bolism, it is important to limit the use of tamoxifen for the prevention of breast cancer to women at significant risk for the disease.
The best candidates for raloxifene are not as easily defined because several other therapeutic options are available for the same indications. For example, for the prevention and treatment of osteoporosis, options include the bisphosphonates (alendronate and risedronate), calcitonin, ERT, and raloxifene. However, only ERT and raloxifene have other beneficial effects that may be important to postmenopausal women (Table 3). Consequently, to prevent or treat osteoporosis in a postmenopausal woman with vasomotor symptoms and/or urogenital atrophic changes, the best option appears to be ERT, which not only protects against bone loss but relieves menopausal symptoms as well. A similar woman without menopausal symptoms who is concerned about or at risk for breast cancer might best be treated with raloxifene, which not only confers bone protection but may diminish breast cancer risk. However, if the same woman has a history of venous thromboembolism, the bisphosphonates or calcitonin might be better options, since both ERT and raloxifene would expose her to an unnecessarily high risk of a thromboembolic event.
The ideal candidate for raloxifene appears to be a postmenopausal woman with osteopenia or osteoporosis who has no increased risk of thromboembolism and few or no vasomotor symptoms. Raloxifene would be especially suitable for such a candidate if she has experienced any bleeding on ERT or fears an increased risk of breast cancer. This would include women who have been on ERT for 5 years or more, since the use of ERT for more than 5 years significantly increases the risk of breast cancer.4
In my practice, the women who are happiest with raloxifene tend to be older (4 or more years past menopause), concerned about osteoporosis and cardiovascular symptoms, and very glad to be free of vaginal bleeding while on the drug. Since the 2 most common reasons for discontinuing ERT—vaginal bleeding and the fear of breast cancer—are non-issues when raloxifene is given, compliance is likely to be high. The few cases of vaginal dryness that occur usually can be treated with lubricants, small doses of vaginal estrogen tablets, or the vaginal estrogen ring.
TABLE 3
Treatment options for osteoporosis: benefits and side effects
| Agent | Benefits | Side effects |
|---|---|---|
| ERT | Effective, safe, multidimensional.* Eases vasomotor and urogenital symptoms, improves mood, protects cardiovascular health | Bleeding, blood clots, breast cancer |
| Raloxifene | Effective, safe, multidimensional. Improves lipid profile. No bleeding or breast cancer risk | Blood clots. No relief of hot flushes or vaginal dryness |
| Alendronate, risedronate | Effective, safe, multidimensional | Difficult to take. Esophageal and gastric irritation, bleeding |
| Calcitonin | Effective, safe, unidimensional. Analgesic effects in patients with compression fractures | Insignificant |
| *The term “multidimensional” suggests that the agent affects more than 1 organ system. | ||
Conclusion
Thanks to a dramatic increase in life expectancy, American women can expect to live 30 or more years beyond the average age of menopause. With aging, the risk of health problems increases progressively. Several of these problems are specifically related to or augmented by estrogen deficiency. Despite several health benefits of ERT, its use remains very low (less than 30% in postmenopausal women) because of side effects and concerns about safety, bleeding, and breast cancer.7
SERMs—particularly raloxifene—provide many of estrogen’s positive effects on bone metabolism and lipids and other markers of cardiovascular disease without increasing the risk of bleeding or breast cancer. In fact, recent data suggest that raloxifene not only fails to increase the risk of breast cancer but actually may be protective against the disease.
SERMs do not necessarily replace older therapies such as ERT, but they do enhance our ability to modify and improve therapies, individualize regimens, and boost compliance. This is especially important with long-term preventive therapy, which requires a high level of acceptance and commitment by both patient and physician. Because of their multiple beneficial effects and favorable long-term risk-benefit profile, SERMs represent an important therapeutic advance in the field of women’s health.
Dr. Luciano reports that he serves on the speakers’ bureaus for Eli Lilly and Co., Wyeth Labs, and Pharmacia, and that he receives research grants from Pharmacia.
1. Grady D, Rubin SM, Petitti DB, et al. Hormone therapy to prevent disease and prolong life in menopausal women. Ann Intern Med. 1992;117(12):1016-1037.
2. Sudhir K, Komesaroff PA. Cardiovascular actions of estrogen in men. J Clin Endocrinol Metab. 1999;84:3411-3415.
3. Barrett-Connors E, Grady D. Hormone replacement therapy, heart disease and other considerations. Annu Rev Public Health. 1998;19:55-72.
4. Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormone replacement therapy: collaborative reanalysis of data from 51 epidemiologic studies of 52,705 women with breast cancer and 108,411 women without breast cancer. Lancet. 1997;350:1047-1059.
5. Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA. 1998;280:605-613.
6. Cancer Facts and Figures—1997. Atlanta: American Cancer Society; 1997.
7. Berman RS, et al. Risk factors associated with women’s compliance with estrogen replacement therapy. J Women’s Health. 1997;6:219-226.
8. Early Breast Cancer Trialists’ Collaborative Group. Tamoxifen for early breast cancer: an overview of the randomized trials. Lancet. 1998;351:1451-1467.
9. Osborne CK. Tamoxifen in the treatment of breast cancer. N Engl J Med. 1998;339:1609-1618.
10. Fisher B, et al. Five versus more than five years of tamoxifen therapy for breast cancer patients with negative lymph nodes and estrogen-positive tumors. J Natl Cancer Inst. 1996;88:1529-1542.
11. Fisher B, et al. Tamoxifen for the prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 1998;90:1371-1388.
12. Baker VL, Leitman D, Jaffe RB. Selective estrogen receptor modulators in reproductive medicine and biology. Obstet Gynecol Survey. 2000;55(7 Suppl 2):S21-S47.
13. Early Breast Cancer Collaborative Trial Group. Tamoxifen for early breast cancer: an overview of the randomized trials. Lancet. 1998;351:1451-1467.
14. Ettinger B, Black D, Mitlak B, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. JAMA. 1999;282:637-644.
15. Walsh B, Kuller L, Wild R, et al. Effects of raloxifene on serum lipids and coagulation factors in healthy postmenopausal women. JAMA. 1998;279:1445-1451.
16. Walsh B, Paul S, Wild R, et al. The effects of hormone replacement therapy and raloxifene on C-reactive protein and homocysteine in healthy postmenopausal women: a randomized controlled trial. J Clin Endocrinol Metab. 2000;85:214-218.
17. Goldstein SR, Scheele WH, Rajagopalan SK, et al. A 12-month comparative study of raloxifene, estrogen and placebo on the postmenopausal endometrium. Obstet Gynecol. 2000;95:95-103.
18. Cohen FJ, Watts S, Shah A, et al. Uterine effects of 3-year raloxifene therapy in postmenopausal women younger than age 60. Obstet Gynecol. 2000;95:104-110.
19. Delmas P, Bjarnason N, Mitlak B, et al. Effects of raloxifene on bone mineral density, serum cholesterol concentrations, and uterine endometrium in postmenopausal women. N Engl J Med. 1997;337:1641-1647.
20. Cummings S, Eckert S, Krueger K, et al. The effect of raloxifene on risk of breast cancer in postmenopausal women. JAMA. 1999;281:2189-2197.
21. Daly E, Vessey MP, Hawkins MM, et al. Risk of venous thromboembolism in users of hormone replacement therapy. Lancet. 1996;348:977-980.
22. Bryant H, Glasebrook A, Yang N, et al. A pharmacologic review of raloxifene. J Bone Miner Metab. 1995;13:75-78.
23. Davies G, et al. Adverse events reported by postmenopausal women in controlled trials with raloxifene. Obstet Gynecol. 1999;93:558-565.
24. Nilsen J, Mor G, Naftolin F. Raloxifene induces neurite outgrowth in estrogen receptor positive PC12 cells. Menopause. 1998;5:211-216.
25. Evista [package insert]. Indianapolis, Ind: Eli Lilly and Company; 1997.
- Among ER-positive breast cancer patients treated with tamoxifen for 5 years, the annual recurrence rate is cut in half and the annual death rate is reduced by 28%.
- Tamoxifen has been shown to reduce the incidence of breast cancer by 44%, 51%, and 55% in women aged 35 to 49, 50 to 59, and 60 and older, respectively.
- Raloxifene mimics the effects of estrogen on the skeleton and lipids, but acts as a complete estrogen antagonist in the breast and uterus.
- Recent data indicate that 4 years of raloxifene therapy reduces the risk of all ER-positive breast cancers by 72% compared with placebo.
- The ideal candidate for raloxifene is a postmenopausal woman with osteopenia or osteoporosis, no increased risk of thromboembolism, and few or no vasomotor symptoms.
Although it was initially considered a female sex hormone, estrogen is now recognized as a systemic substance that affects every organ system and appears to be important for both men’s and women’s health.1-4 At puberty, elevated circulating estrogen levels transform girls into young women and shape their feminine fig-ures. In boys, estrogen is important in the closure of the epiphyseal plates to arrest postpubertal growth and support bone health throughout life. It also may have beneficial actions on the male cardiovascular system.2
As a systemic hormone, estrogen is important in women for the health of the skeleton, the heart, the integuments, and the brain.1,3 Women who suffer the loss of estrogen through surgery, illness, or early menopause often are advised to consider estrogen therapy to protect these organs from premature failure.
Unfortunately, estrogen replacement therapy (ERT) is a double-edged sword, with significant benefits in some organs and significant risks in others. ERT protects against vasomotor symptoms, urogenital atrophy, osteoporosis, cardiovascular disease, and perhaps Alzheimer’s disease.1,3 Unfortunately, it also increases the risk of venous thromboembolic events, recurrent myocardial infarction and cardiac death5 (in diseased hearts), and cancers of the uterus and breast.4
Of the many diseases that impact the lives of American women, none is more feared than breast cancer, the most common cancer in females and the second leading cause of female cancer mortality.6 The fear of this disease causes many women to decline or discontinue ERT, a decision that can profoundly affect their long-term health.7 Hence, the controversy regarding ERT centers on weighing the risk-benefit balance, i.e., trying to select patients who are more likely to benefit from the hormone and less likely to suffer from its adverse effects.
Physicians—particularly gynecologists who devote their care exclusively to women’s health—have longed for the ideal estrogen that will impart all of the benefits without the risks. This ideal estrogen would relieve women of vasomotor and urogenital symptoms and prevent the dire consequences of osteoporosis, accelerated atherosclerosis, neurogenic deficit, and collagen loss from the skin, without increasing the risk of cancer and thromboembolic phenomena. Some experts believe this ideal may be found in the new class of compounds referred to as selective estrogen receptor modulators (SERMs). Although several SERMs with desirable estrogenic properties are available, none is ideal. Nevertheless, this new class of estrogenic substances—with multiple beneficial effects and fewer risks—represents an important pharma-cotherapeutic advance in the care of postmenopausal women.
Tamoxifen
Tamoxifen citrate (Nolvadex; AstraZeneca, Wilmington, Del) has wide clinical applications in the treatment and prevention of breast cancer. In 1980, tamoxifen was approved by the FDA for postmenopausal women with node-positive breast cancer and for premenopausal women with estrogen-receptor (ER) positive advanced breast cancer. In 1990, the FDA extended the approval of tamoxifen to include pre- and postmenopausal women with node-negative, ER-positive breast cancer.
In patients with node-positive breast cancer, the 10-year-survival rate improves from 50% in control subjects to 61% in patients treated with tamoxifen for 5 years. Similar improvements in survival have been reported with tamoxifen therapy in node-negative breast cancer patients. After 5 years of tamoxifen therapy and a median follow-up of 10 years, the reduction of breast cancer recurrence and death with tamoxifen treatment compared with placebo are 47% and 26%, respectively.8
For women with ER-positive tumors (about 70% of all breast cancers), tamoxifen therapy of at least 1 year’s duration results in statistically significant recurrence and survival benefits. These results increase with the duration of treatment up to 5 years. Among ER-positive women treated with tamoxifen for 5 years—the optimal length of therapy—the annual recurrence rate is cut in half and the annual death rate is reduced by 28% (Figure 1).9 Treatment beyond 5 years does not accrue additional benefits and is associated with increased adverse events.10 Tamoxifen has little effect on animal tumors and ER-negative tumors in cell cultures and confers little or no benefit to women with ER-negative tumors.9
In ER-positive tumors, tamoxifen is believed to exert its anti-tumor effects by inhibiting the estrogen-dependent secretion of growth factors and angiogenic factors by the tumor cells, and by inducing programmed death of the tumor cells.9 An understanding of this mechanism, along with the observation that tamoxifen-treated breast cancer patients not only experience a reduction in breast cancer recurrence but also a 47% reduction in contralateral breast cancer, led to the launch of the tamoxifen chemoprevention trials in North America and Europe.
In 1992, the National Surgical Adjuvant Breast and Bowel Project (NSABP) Tamoxifen Breast Cancer Prevention Trial was launched in the United States and Canada. The results of this trial were released early because of the compelling evidence of tamoxifen’s therapeutic efficacy. The drug was found to reduce the incidence of breast cancer by 44%, 51%, and 55% in women aged 35 to 49, 50 to 59, and 60 or older, respectively. Tamoxifen also was found to reduce ductal carcinoma in situ by 50%. Although tamoxifen decreased the overall occurrence of ER-positive tumors by 69%, it did not have a significant impact on the incidence of ER-negative tumors.11
Based on these results, the FDA approved the use of tamoxifen for the primary prevention of breast cancer in high-risk women. It is important to limit the use of tamoxifen to high-risk women because of the potential for serious side effects, which include endometrial cancer, pulmonary embolism, deep vein thrombosis (DVT), and cataract formation.9-11
The issue of whether tamoxifen inhibits the initial development of a tumor or suppresses an occult tumor remains unresolved. It is possible that both mechanisms are involved. This is not merely an academic concern. Questions remain as to whether a suppressed tumor may develop resistance to tamoxifen or even be stimulated by the drug, becoming more virulent during or after discontinuation of therapy. However, given the extensive data and longterm clinical experience and follow-up with tamoxifen, this scenario seems unlikely. In fact, the reduction in the incidence of breast cancer appears to continue for years after the therapy is discontinued.12,13
FIGURE 1Effect of tamoxifen on ER-positive breast cancer
Adapted from: Osborne CK. Tamoxifen in the treatment of breast cancer. N Engl J Med. 1998;339:1609-1618.
Raloxifene
Raloxifene hydrochloride (Evista; Eli Lilly and Company, Indianapolis, Ind) was originally investigated for the treatment of breast cancer and was found to be similar to tamoxifen in its anti-tumor activity. Raloxifene was subsequently studied for its skeletal effects and was approved for osteoporosis prevention in postmenopausal women in December 1997, and for fracture prevention in 1999.
The agent mimics the effects of estrogen on the skeleton and lipids. However, it acts as a complete estrogen antagonist in the breast and the uterus, making it a more desirable SERM in the management of menopausal women with an intact uterus.
The Multiple Outcomes of Raloxifene Evaluation (MORE) trial was a randomized, placebo-controlled, multicenter study involving 7,705 postmenopausal women with osteoporosis at baseline. Although bone metabolism and fractures were the primary endpoints, many other outcomes were evaluated, including uterine and endometrial effects, bleeding, breast cancer incidence, lipid levels, clotting factors, patient tolerance, and adverse events.14-18 The results of these studies support the classification of raloxifene as a SERM with several desirable estrogen-agonistic and -antagonistic effects. Even so, it is far from perfect.
Effects on bone. In the MORE trial, both placebo- and raloxifene-treated postmenopausal women received 500 mg of supplemental calcium and 400 to 600 IU of cholecalciferol (vitamin D) daily. In these postmenopausal women with osteoporosis, a daily dose of 60 mg raloxifene increased bone mineral density (BMD) in the spine by 2.6% and in the femoral neck by 2.1% compared with placebo. The assessment of biochemical bone markers indicated that raloxifene increases BMD by decreasing bone turnover. Indeed, markers of bone resorption and formation—urinary type I collagen Ctelopeptide and serum osteocalcin—were significantly decreased with raloxifene within 3 months of therapy, similar to estrogen.14,19
Raloxifene also reduced vertebral fractures in postmenopausal women with osteoporosis. Vertebral fractures occurred in 10.1% of women randomized to placebo versus 6.6% of women receiving 60 mg raloxifene daily. Although raloxifene decreased the risk of new vertebral fractures regardless of whether a spinal fracture was present at baseline, the absolute percentage decrease in the fracture rate was higher in women who did not have a fracture at baseline.14 This finding underscores the importance of diagnosing and treating osteoporosis before the development of a fracture.
One interesting observation, which is true for raloxifene and other antiresorptive agents, particularly calcitonin, is that the magnitude of the reduction in vertebral fractures is greater than would have been predicted by the modest improvement in BMD observed during therapy. This suggests that antiresorptive agents may influence bone quality, perhaps through its architectural structure, in ways that cannot be ascertained by the assessment of BMD alone. Bone quality refers to skeletal factors that strongly impact the structural properties of bone, independent of the quantity of bone, assessed by BMD. Specific factors that contribute to the structural competence of bone include its microarchitecture, degree of bone mineralization, state of the organic matrix, and rate of bone turnover. The effects of antiresorptive agents on these factors may explain why such modest improvements in BMD—i.e., 2% to 6%—result in reductions in fracture risks in the range of 30% to 50%.
Figure 2 shows the mean changes in BMD and the corresponding reduction in vertebral fractures for the therapeutic agents currently approved for the prevention and treatment of osteoporosis. Despite significant differences in BMD increases, the reductions in vertebral fracture rates are quite comparable. In the MORE trial, there was no difference in the incidence of nontraumatic fractures at sites other than the spine, possibly because of the small number of nonvertebral fractures that occurred during the study period, which yielded insufficient power to detect a difference. A subset of patients in the MORE study is being followed for a longer time to further evaluate the incidence of nonvertebral fractures.
Effects on cardiovascular health. Raloxifene’s effects on total and low-density lipoprotein (LDL) cholesterol are similar to those of estrogen, with decreases of 11% and 6%, respectively. Unlike estrogen, which increases the circulating levels of both high-density lipoprotein (HDL) cholesterol and triglycerides, raloxifene causes an insignificant increase in HDL and a slight decrease in triglyceride levels. Serum levels of lipoprotein(a) are reduced much more with ERT (16.3%) than raloxifene therapy (4.1%), whereas fibrinogen levels are reduced more with raloxifene (12.2%) than ERT (2.8%).15
The effects of raloxifene on 2 additional independent risk factors for cardiovascular disease were recently reported. Elevated levels of C-reactive protein, a circulating marker of inflammation, are associated with a significantly greater risk of myocardial infarction. Also, elevated levels of homocysteine predict a greater risk of coronary artery disease. While ERT significantly increases circulating levels of C-reactive protein (by as much as 84%), raloxifene lacks a significant effect. Like estrogen, raloxifene significantly lowers homocysteine levels by 6% to 8%.16
The changes in serum levels of the several markers of cardiovascular health are summarized for both ERT and raloxifene in Table 1. Overall, raloxifene therapy is associated with favorable changes in the serum levels of several of these markers, suggesting that the SERM may substantially reduce the risk of heart disease in postmenopausal women. However, conclusive proof would require a clinical trial with cardiovascular events as the definitive endpoints. Such an investigation is currently being carried out in the Raloxifene Use for the Heart (RUTH) trial. The results should indicate whether these favorable biochemical effects are indeed associated with a reduction in the incidence of cardiovascular disease.
Effects on the endometrium. Unlike tamoxifen and toremifene (Fareston; Schering-Plough, Kenilworth, NJ), which stimulate endometrial proliferation and increase the risk of endometrial cancer, raloxifene has no stimulatory effects on either the endometrium or the myometrium.17,18 In postmenopausal women receiving raloxifene, the incidence of vaginal bleeding is the same as in women on placebo. In the MORE trial, raloxifene did not increase the risk of endometrial cancer, endometrial hyperplasia, or uterine bleeding over a follow-up period of 40 months.20 Several other studies have evaluated the endometrial response to raloxifene—compared with placebo or ERT—for periods ranging from 12 to 24 months. These studies have consistently reported that raloxifene has no stimulatory effects on the uterus of postmenopausal women.17,18,21 Thus, it seems clear that postmenopausal patients on raloxifene need not worry about an increased risk of polyps, endometrial proliferation, hyperplasia, or cancer. Their endometrial thickness and uterine volume will not increase, nor will they experience a greater incidence of vaginal bleeding than untreated women.5,17,18,20
In postmenopausal women, the administration of raloxifene does not require the addition of a progestin or routine endometrial monitoring by biopsy or ultrasonography. Any bleeding during raloxifene therapy should therefore be promptly evaluated, since it is unlikely to be related to the SERM.
Effects on the breast. In contrast to ERT, raloxifene does not increase the frequency of breast pain or tenderness in postmenopausal women. In addition, recent data suggest that raloxifene may reduce the risk of breast cancer.
Like tamoxifen, raloxifene inhibits the growth of mammary tumors in rodents and the growth of the human MCF-7 breast cancer cell line in vitro.9,22 The latest follow-up data from the MORE trial indicate that 4 years of raloxifene therapy reduces the risk of all breast cancers by 72% compared with placebo. At the 4-year mark of the MORE trial, a total of 79 breast cancer cases occurred—4.7 cases per 1,000 patient-years in the placebo group compared with 1.3 cases per 1,000 patient-years in the raloxifene group. The protective effect was essentially restricted to ER-positive cancers. As in previous observations, women with the highest serum estrogen levels had the highest BMD and the highest incidence of breast cancer. These same women experienced the greatest reduction in breast cancer risk with raloxifene therapy.
Because raloxifene has not yet been studied directly as a prophylactic, it cannot be recommended for the prevention of breast cancer. Several other issues regarding raloxifene and breast cancer prevention also need to be addressed. For example, the efficacy rates of raloxifene in women at increased risk for breast cancer and in younger postmenopausal women are unknown. We also lack data regarding the optimal duration of raloxifene therapy for maximal, sustained protection against breast cancer.
These issues are expected to be resolved at the completion of the Study of Tamoxifen and Raloxifene (STAR) trial. This randomized, double-blind comparison of tamoxifen and raloxifene is the largest breast-cancer prevention study ever conducted, involving more than 300 institutions throughout the U.S., Canada, and Puerto Rico and approximately 22,000 postmenopausal women. Participants include women 35 to 59 years of age who face an increased risk of breast cancer and women 60 and older with no additional risk factors. These women are given either 20 mg tamoxifen daily or 60 mg raloxifene daily for 5 years. The study, which was launched in 1999 and is expected to continue for 5 to 10 years, should provide definitive data regarding the role of these SERMs in the prevention of breast cancer and in preventing disease in general in postmenopausal women.
Adverse effects. Although raloxifene exerts several desirable estrogen-agonist and -antagonist effects, it also exerts several undesirable effects (Table 2), which may preclude its administration in a large segment of postmenopausal women. Perhaps its most worrisome side effect is the increased risk of venous thromboembolic events, which has been reported to be approximately 3 times the risk incurred by women on placebo.20,22 (This level of risk is similar to that reported for estrogen use.21) Raloxifene does not improve vasomotor symptoms or vaginal dryness. Leg cramps have been reported in 6% of women on raloxifene versus 2% of women on placebo.23
Raloxifene’s effects on the central nervous system (CNS) are largely unknown and seem to differ from those of estrogen. In an in vitro system using cultured rat neurons derived from areas of the brain important for memory, raloxifene was neuroprotective at low concentrations but neurotoxic at high concentrations. Other animal studies have reported that raloxifene, like estrogen, may have a beneficial effect on cholinergic transmission within the brain and induce neurite outgrowth in ER-positive rat neuronal cell lines.12,24 Because it does not decrease the incidence of hot flushes, some experts believe that raloxifene may exert anti-estrogenic effects on the CNS. However there are few clinical studies of the effects of raloxifene on the CNS in women. The few that exist indicate neither beneficial nor adverse effectson cognition, mood, or memory.25 No clinical data on raloxifene and the risk of Alzheimer’s disease are currently available.
FIGURE 2Effect of bone therapy on vertebral fracture rates
RLX = raloxifene, AL = alendronate, RIS = risedronate, CCTN = calcitonin, PBO = placeboTABLE 1
Effects of raloxifene and ERT on markers of cardiovascular disease risk in healthy postmenopausal women
| Marker | Raloxifene | ERT |
|---|---|---|
| LDL cholesterol | -12 | -14 |
| HDL cholesterol | 0 | +10 |
| HDL2 | +15 | +33 |
| Triglycerides | -4 | +20 |
| Lipoprotein(a) | -7 | -19 |
| Fibrinogen | -10 | -1 |
| Homocysteine | -8 | -6.6 |
| C-reactive protein | -4 | +84.1 |
| Data are reported as percent change compared with placebo except for homocysteine and C-reactive protein, which are reported as percent change from baseline. | ||
| Source: Walsh B, et al. Effects of raloxifene on serum lipids and coagulation factors in healthy postmenopausal women. JAMA. 1998;279:1445-1451. Walsh B, et al. The effects of hormone replacement therapy and raloxifene on C-reactive protein and homocysteine in healthy postmenopausal women: a randomized controlled trial. J Clin Endocrinol Metab. 2000;85:214-218. | ||
TABLE 2
Adverse events reported by postmenopausal women in controlled trials of raloxifene
| Adverse event | Raloxifene | Placebo | P value |
|---|---|---|---|
| Vasomotor symptoms | 9.7% | 6.4% | .01 |
| Leg cramps | 7% | 3.7% | <.001 |
| Influenza syndrome | 13.4% | 11.4% | <.001 |
| Peripheral edema | 5.2% | 4.4% | <.01 |
| Endometrial cavity fluid | 8.1% | 5.7% | .02 |
| Venous thromboembolism | 1% | 0.3% | <.001 |
| Source: Ettinger B, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. JAMA. 1999;282:637-644. Nilsen J, et al. Raloxifene induces neurite outgrowth in estrogen receptor positive PC12 cells. Menopause. 1998;5:211-216. | |||
Patient selection
The candidates for tamoxifen are clearly defined. These include ER-positive breast-cancer patients as well as pre- and postmenopausal women at significant risk for the disease. There currently is no other indication for tamoxifen therapy in the U.S. In other countries, the drug is used—as clomiphene citrate is in the U.S.—to induce ovulation. Because of the risk of venous thromboem-bolism, it is important to limit the use of tamoxifen for the prevention of breast cancer to women at significant risk for the disease.
The best candidates for raloxifene are not as easily defined because several other therapeutic options are available for the same indications. For example, for the prevention and treatment of osteoporosis, options include the bisphosphonates (alendronate and risedronate), calcitonin, ERT, and raloxifene. However, only ERT and raloxifene have other beneficial effects that may be important to postmenopausal women (Table 3). Consequently, to prevent or treat osteoporosis in a postmenopausal woman with vasomotor symptoms and/or urogenital atrophic changes, the best option appears to be ERT, which not only protects against bone loss but relieves menopausal symptoms as well. A similar woman without menopausal symptoms who is concerned about or at risk for breast cancer might best be treated with raloxifene, which not only confers bone protection but may diminish breast cancer risk. However, if the same woman has a history of venous thromboembolism, the bisphosphonates or calcitonin might be better options, since both ERT and raloxifene would expose her to an unnecessarily high risk of a thromboembolic event.
The ideal candidate for raloxifene appears to be a postmenopausal woman with osteopenia or osteoporosis who has no increased risk of thromboembolism and few or no vasomotor symptoms. Raloxifene would be especially suitable for such a candidate if she has experienced any bleeding on ERT or fears an increased risk of breast cancer. This would include women who have been on ERT for 5 years or more, since the use of ERT for more than 5 years significantly increases the risk of breast cancer.4
In my practice, the women who are happiest with raloxifene tend to be older (4 or more years past menopause), concerned about osteoporosis and cardiovascular symptoms, and very glad to be free of vaginal bleeding while on the drug. Since the 2 most common reasons for discontinuing ERT—vaginal bleeding and the fear of breast cancer—are non-issues when raloxifene is given, compliance is likely to be high. The few cases of vaginal dryness that occur usually can be treated with lubricants, small doses of vaginal estrogen tablets, or the vaginal estrogen ring.
TABLE 3
Treatment options for osteoporosis: benefits and side effects
| Agent | Benefits | Side effects |
|---|---|---|
| ERT | Effective, safe, multidimensional.* Eases vasomotor and urogenital symptoms, improves mood, protects cardiovascular health | Bleeding, blood clots, breast cancer |
| Raloxifene | Effective, safe, multidimensional. Improves lipid profile. No bleeding or breast cancer risk | Blood clots. No relief of hot flushes or vaginal dryness |
| Alendronate, risedronate | Effective, safe, multidimensional | Difficult to take. Esophageal and gastric irritation, bleeding |
| Calcitonin | Effective, safe, unidimensional. Analgesic effects in patients with compression fractures | Insignificant |
| *The term “multidimensional” suggests that the agent affects more than 1 organ system. | ||
Conclusion
Thanks to a dramatic increase in life expectancy, American women can expect to live 30 or more years beyond the average age of menopause. With aging, the risk of health problems increases progressively. Several of these problems are specifically related to or augmented by estrogen deficiency. Despite several health benefits of ERT, its use remains very low (less than 30% in postmenopausal women) because of side effects and concerns about safety, bleeding, and breast cancer.7
SERMs—particularly raloxifene—provide many of estrogen’s positive effects on bone metabolism and lipids and other markers of cardiovascular disease without increasing the risk of bleeding or breast cancer. In fact, recent data suggest that raloxifene not only fails to increase the risk of breast cancer but actually may be protective against the disease.
SERMs do not necessarily replace older therapies such as ERT, but they do enhance our ability to modify and improve therapies, individualize regimens, and boost compliance. This is especially important with long-term preventive therapy, which requires a high level of acceptance and commitment by both patient and physician. Because of their multiple beneficial effects and favorable long-term risk-benefit profile, SERMs represent an important therapeutic advance in the field of women’s health.
Dr. Luciano reports that he serves on the speakers’ bureaus for Eli Lilly and Co., Wyeth Labs, and Pharmacia, and that he receives research grants from Pharmacia.
- Among ER-positive breast cancer patients treated with tamoxifen for 5 years, the annual recurrence rate is cut in half and the annual death rate is reduced by 28%.
- Tamoxifen has been shown to reduce the incidence of breast cancer by 44%, 51%, and 55% in women aged 35 to 49, 50 to 59, and 60 and older, respectively.
- Raloxifene mimics the effects of estrogen on the skeleton and lipids, but acts as a complete estrogen antagonist in the breast and uterus.
- Recent data indicate that 4 years of raloxifene therapy reduces the risk of all ER-positive breast cancers by 72% compared with placebo.
- The ideal candidate for raloxifene is a postmenopausal woman with osteopenia or osteoporosis, no increased risk of thromboembolism, and few or no vasomotor symptoms.
Although it was initially considered a female sex hormone, estrogen is now recognized as a systemic substance that affects every organ system and appears to be important for both men’s and women’s health.1-4 At puberty, elevated circulating estrogen levels transform girls into young women and shape their feminine fig-ures. In boys, estrogen is important in the closure of the epiphyseal plates to arrest postpubertal growth and support bone health throughout life. It also may have beneficial actions on the male cardiovascular system.2
As a systemic hormone, estrogen is important in women for the health of the skeleton, the heart, the integuments, and the brain.1,3 Women who suffer the loss of estrogen through surgery, illness, or early menopause often are advised to consider estrogen therapy to protect these organs from premature failure.
Unfortunately, estrogen replacement therapy (ERT) is a double-edged sword, with significant benefits in some organs and significant risks in others. ERT protects against vasomotor symptoms, urogenital atrophy, osteoporosis, cardiovascular disease, and perhaps Alzheimer’s disease.1,3 Unfortunately, it also increases the risk of venous thromboembolic events, recurrent myocardial infarction and cardiac death5 (in diseased hearts), and cancers of the uterus and breast.4
Of the many diseases that impact the lives of American women, none is more feared than breast cancer, the most common cancer in females and the second leading cause of female cancer mortality.6 The fear of this disease causes many women to decline or discontinue ERT, a decision that can profoundly affect their long-term health.7 Hence, the controversy regarding ERT centers on weighing the risk-benefit balance, i.e., trying to select patients who are more likely to benefit from the hormone and less likely to suffer from its adverse effects.
Physicians—particularly gynecologists who devote their care exclusively to women’s health—have longed for the ideal estrogen that will impart all of the benefits without the risks. This ideal estrogen would relieve women of vasomotor and urogenital symptoms and prevent the dire consequences of osteoporosis, accelerated atherosclerosis, neurogenic deficit, and collagen loss from the skin, without increasing the risk of cancer and thromboembolic phenomena. Some experts believe this ideal may be found in the new class of compounds referred to as selective estrogen receptor modulators (SERMs). Although several SERMs with desirable estrogenic properties are available, none is ideal. Nevertheless, this new class of estrogenic substances—with multiple beneficial effects and fewer risks—represents an important pharma-cotherapeutic advance in the care of postmenopausal women.
Tamoxifen
Tamoxifen citrate (Nolvadex; AstraZeneca, Wilmington, Del) has wide clinical applications in the treatment and prevention of breast cancer. In 1980, tamoxifen was approved by the FDA for postmenopausal women with node-positive breast cancer and for premenopausal women with estrogen-receptor (ER) positive advanced breast cancer. In 1990, the FDA extended the approval of tamoxifen to include pre- and postmenopausal women with node-negative, ER-positive breast cancer.
In patients with node-positive breast cancer, the 10-year-survival rate improves from 50% in control subjects to 61% in patients treated with tamoxifen for 5 years. Similar improvements in survival have been reported with tamoxifen therapy in node-negative breast cancer patients. After 5 years of tamoxifen therapy and a median follow-up of 10 years, the reduction of breast cancer recurrence and death with tamoxifen treatment compared with placebo are 47% and 26%, respectively.8
For women with ER-positive tumors (about 70% of all breast cancers), tamoxifen therapy of at least 1 year’s duration results in statistically significant recurrence and survival benefits. These results increase with the duration of treatment up to 5 years. Among ER-positive women treated with tamoxifen for 5 years—the optimal length of therapy—the annual recurrence rate is cut in half and the annual death rate is reduced by 28% (Figure 1).9 Treatment beyond 5 years does not accrue additional benefits and is associated with increased adverse events.10 Tamoxifen has little effect on animal tumors and ER-negative tumors in cell cultures and confers little or no benefit to women with ER-negative tumors.9
In ER-positive tumors, tamoxifen is believed to exert its anti-tumor effects by inhibiting the estrogen-dependent secretion of growth factors and angiogenic factors by the tumor cells, and by inducing programmed death of the tumor cells.9 An understanding of this mechanism, along with the observation that tamoxifen-treated breast cancer patients not only experience a reduction in breast cancer recurrence but also a 47% reduction in contralateral breast cancer, led to the launch of the tamoxifen chemoprevention trials in North America and Europe.
In 1992, the National Surgical Adjuvant Breast and Bowel Project (NSABP) Tamoxifen Breast Cancer Prevention Trial was launched in the United States and Canada. The results of this trial were released early because of the compelling evidence of tamoxifen’s therapeutic efficacy. The drug was found to reduce the incidence of breast cancer by 44%, 51%, and 55% in women aged 35 to 49, 50 to 59, and 60 or older, respectively. Tamoxifen also was found to reduce ductal carcinoma in situ by 50%. Although tamoxifen decreased the overall occurrence of ER-positive tumors by 69%, it did not have a significant impact on the incidence of ER-negative tumors.11
Based on these results, the FDA approved the use of tamoxifen for the primary prevention of breast cancer in high-risk women. It is important to limit the use of tamoxifen to high-risk women because of the potential for serious side effects, which include endometrial cancer, pulmonary embolism, deep vein thrombosis (DVT), and cataract formation.9-11
The issue of whether tamoxifen inhibits the initial development of a tumor or suppresses an occult tumor remains unresolved. It is possible that both mechanisms are involved. This is not merely an academic concern. Questions remain as to whether a suppressed tumor may develop resistance to tamoxifen or even be stimulated by the drug, becoming more virulent during or after discontinuation of therapy. However, given the extensive data and longterm clinical experience and follow-up with tamoxifen, this scenario seems unlikely. In fact, the reduction in the incidence of breast cancer appears to continue for years after the therapy is discontinued.12,13
FIGURE 1Effect of tamoxifen on ER-positive breast cancer
Adapted from: Osborne CK. Tamoxifen in the treatment of breast cancer. N Engl J Med. 1998;339:1609-1618.
Raloxifene
Raloxifene hydrochloride (Evista; Eli Lilly and Company, Indianapolis, Ind) was originally investigated for the treatment of breast cancer and was found to be similar to tamoxifen in its anti-tumor activity. Raloxifene was subsequently studied for its skeletal effects and was approved for osteoporosis prevention in postmenopausal women in December 1997, and for fracture prevention in 1999.
The agent mimics the effects of estrogen on the skeleton and lipids. However, it acts as a complete estrogen antagonist in the breast and the uterus, making it a more desirable SERM in the management of menopausal women with an intact uterus.
The Multiple Outcomes of Raloxifene Evaluation (MORE) trial was a randomized, placebo-controlled, multicenter study involving 7,705 postmenopausal women with osteoporosis at baseline. Although bone metabolism and fractures were the primary endpoints, many other outcomes were evaluated, including uterine and endometrial effects, bleeding, breast cancer incidence, lipid levels, clotting factors, patient tolerance, and adverse events.14-18 The results of these studies support the classification of raloxifene as a SERM with several desirable estrogen-agonistic and -antagonistic effects. Even so, it is far from perfect.
Effects on bone. In the MORE trial, both placebo- and raloxifene-treated postmenopausal women received 500 mg of supplemental calcium and 400 to 600 IU of cholecalciferol (vitamin D) daily. In these postmenopausal women with osteoporosis, a daily dose of 60 mg raloxifene increased bone mineral density (BMD) in the spine by 2.6% and in the femoral neck by 2.1% compared with placebo. The assessment of biochemical bone markers indicated that raloxifene increases BMD by decreasing bone turnover. Indeed, markers of bone resorption and formation—urinary type I collagen Ctelopeptide and serum osteocalcin—were significantly decreased with raloxifene within 3 months of therapy, similar to estrogen.14,19
Raloxifene also reduced vertebral fractures in postmenopausal women with osteoporosis. Vertebral fractures occurred in 10.1% of women randomized to placebo versus 6.6% of women receiving 60 mg raloxifene daily. Although raloxifene decreased the risk of new vertebral fractures regardless of whether a spinal fracture was present at baseline, the absolute percentage decrease in the fracture rate was higher in women who did not have a fracture at baseline.14 This finding underscores the importance of diagnosing and treating osteoporosis before the development of a fracture.
One interesting observation, which is true for raloxifene and other antiresorptive agents, particularly calcitonin, is that the magnitude of the reduction in vertebral fractures is greater than would have been predicted by the modest improvement in BMD observed during therapy. This suggests that antiresorptive agents may influence bone quality, perhaps through its architectural structure, in ways that cannot be ascertained by the assessment of BMD alone. Bone quality refers to skeletal factors that strongly impact the structural properties of bone, independent of the quantity of bone, assessed by BMD. Specific factors that contribute to the structural competence of bone include its microarchitecture, degree of bone mineralization, state of the organic matrix, and rate of bone turnover. The effects of antiresorptive agents on these factors may explain why such modest improvements in BMD—i.e., 2% to 6%—result in reductions in fracture risks in the range of 30% to 50%.
Figure 2 shows the mean changes in BMD and the corresponding reduction in vertebral fractures for the therapeutic agents currently approved for the prevention and treatment of osteoporosis. Despite significant differences in BMD increases, the reductions in vertebral fracture rates are quite comparable. In the MORE trial, there was no difference in the incidence of nontraumatic fractures at sites other than the spine, possibly because of the small number of nonvertebral fractures that occurred during the study period, which yielded insufficient power to detect a difference. A subset of patients in the MORE study is being followed for a longer time to further evaluate the incidence of nonvertebral fractures.
Effects on cardiovascular health. Raloxifene’s effects on total and low-density lipoprotein (LDL) cholesterol are similar to those of estrogen, with decreases of 11% and 6%, respectively. Unlike estrogen, which increases the circulating levels of both high-density lipoprotein (HDL) cholesterol and triglycerides, raloxifene causes an insignificant increase in HDL and a slight decrease in triglyceride levels. Serum levels of lipoprotein(a) are reduced much more with ERT (16.3%) than raloxifene therapy (4.1%), whereas fibrinogen levels are reduced more with raloxifene (12.2%) than ERT (2.8%).15
The effects of raloxifene on 2 additional independent risk factors for cardiovascular disease were recently reported. Elevated levels of C-reactive protein, a circulating marker of inflammation, are associated with a significantly greater risk of myocardial infarction. Also, elevated levels of homocysteine predict a greater risk of coronary artery disease. While ERT significantly increases circulating levels of C-reactive protein (by as much as 84%), raloxifene lacks a significant effect. Like estrogen, raloxifene significantly lowers homocysteine levels by 6% to 8%.16
The changes in serum levels of the several markers of cardiovascular health are summarized for both ERT and raloxifene in Table 1. Overall, raloxifene therapy is associated with favorable changes in the serum levels of several of these markers, suggesting that the SERM may substantially reduce the risk of heart disease in postmenopausal women. However, conclusive proof would require a clinical trial with cardiovascular events as the definitive endpoints. Such an investigation is currently being carried out in the Raloxifene Use for the Heart (RUTH) trial. The results should indicate whether these favorable biochemical effects are indeed associated with a reduction in the incidence of cardiovascular disease.
Effects on the endometrium. Unlike tamoxifen and toremifene (Fareston; Schering-Plough, Kenilworth, NJ), which stimulate endometrial proliferation and increase the risk of endometrial cancer, raloxifene has no stimulatory effects on either the endometrium or the myometrium.17,18 In postmenopausal women receiving raloxifene, the incidence of vaginal bleeding is the same as in women on placebo. In the MORE trial, raloxifene did not increase the risk of endometrial cancer, endometrial hyperplasia, or uterine bleeding over a follow-up period of 40 months.20 Several other studies have evaluated the endometrial response to raloxifene—compared with placebo or ERT—for periods ranging from 12 to 24 months. These studies have consistently reported that raloxifene has no stimulatory effects on the uterus of postmenopausal women.17,18,21 Thus, it seems clear that postmenopausal patients on raloxifene need not worry about an increased risk of polyps, endometrial proliferation, hyperplasia, or cancer. Their endometrial thickness and uterine volume will not increase, nor will they experience a greater incidence of vaginal bleeding than untreated women.5,17,18,20
In postmenopausal women, the administration of raloxifene does not require the addition of a progestin or routine endometrial monitoring by biopsy or ultrasonography. Any bleeding during raloxifene therapy should therefore be promptly evaluated, since it is unlikely to be related to the SERM.
Effects on the breast. In contrast to ERT, raloxifene does not increase the frequency of breast pain or tenderness in postmenopausal women. In addition, recent data suggest that raloxifene may reduce the risk of breast cancer.
Like tamoxifen, raloxifene inhibits the growth of mammary tumors in rodents and the growth of the human MCF-7 breast cancer cell line in vitro.9,22 The latest follow-up data from the MORE trial indicate that 4 years of raloxifene therapy reduces the risk of all breast cancers by 72% compared with placebo. At the 4-year mark of the MORE trial, a total of 79 breast cancer cases occurred—4.7 cases per 1,000 patient-years in the placebo group compared with 1.3 cases per 1,000 patient-years in the raloxifene group. The protective effect was essentially restricted to ER-positive cancers. As in previous observations, women with the highest serum estrogen levels had the highest BMD and the highest incidence of breast cancer. These same women experienced the greatest reduction in breast cancer risk with raloxifene therapy.
Because raloxifene has not yet been studied directly as a prophylactic, it cannot be recommended for the prevention of breast cancer. Several other issues regarding raloxifene and breast cancer prevention also need to be addressed. For example, the efficacy rates of raloxifene in women at increased risk for breast cancer and in younger postmenopausal women are unknown. We also lack data regarding the optimal duration of raloxifene therapy for maximal, sustained protection against breast cancer.
These issues are expected to be resolved at the completion of the Study of Tamoxifen and Raloxifene (STAR) trial. This randomized, double-blind comparison of tamoxifen and raloxifene is the largest breast-cancer prevention study ever conducted, involving more than 300 institutions throughout the U.S., Canada, and Puerto Rico and approximately 22,000 postmenopausal women. Participants include women 35 to 59 years of age who face an increased risk of breast cancer and women 60 and older with no additional risk factors. These women are given either 20 mg tamoxifen daily or 60 mg raloxifene daily for 5 years. The study, which was launched in 1999 and is expected to continue for 5 to 10 years, should provide definitive data regarding the role of these SERMs in the prevention of breast cancer and in preventing disease in general in postmenopausal women.
Adverse effects. Although raloxifene exerts several desirable estrogen-agonist and -antagonist effects, it also exerts several undesirable effects (Table 2), which may preclude its administration in a large segment of postmenopausal women. Perhaps its most worrisome side effect is the increased risk of venous thromboembolic events, which has been reported to be approximately 3 times the risk incurred by women on placebo.20,22 (This level of risk is similar to that reported for estrogen use.21) Raloxifene does not improve vasomotor symptoms or vaginal dryness. Leg cramps have been reported in 6% of women on raloxifene versus 2% of women on placebo.23
Raloxifene’s effects on the central nervous system (CNS) are largely unknown and seem to differ from those of estrogen. In an in vitro system using cultured rat neurons derived from areas of the brain important for memory, raloxifene was neuroprotective at low concentrations but neurotoxic at high concentrations. Other animal studies have reported that raloxifene, like estrogen, may have a beneficial effect on cholinergic transmission within the brain and induce neurite outgrowth in ER-positive rat neuronal cell lines.12,24 Because it does not decrease the incidence of hot flushes, some experts believe that raloxifene may exert anti-estrogenic effects on the CNS. However there are few clinical studies of the effects of raloxifene on the CNS in women. The few that exist indicate neither beneficial nor adverse effectson cognition, mood, or memory.25 No clinical data on raloxifene and the risk of Alzheimer’s disease are currently available.
FIGURE 2Effect of bone therapy on vertebral fracture rates
RLX = raloxifene, AL = alendronate, RIS = risedronate, CCTN = calcitonin, PBO = placeboTABLE 1
Effects of raloxifene and ERT on markers of cardiovascular disease risk in healthy postmenopausal women
| Marker | Raloxifene | ERT |
|---|---|---|
| LDL cholesterol | -12 | -14 |
| HDL cholesterol | 0 | +10 |
| HDL2 | +15 | +33 |
| Triglycerides | -4 | +20 |
| Lipoprotein(a) | -7 | -19 |
| Fibrinogen | -10 | -1 |
| Homocysteine | -8 | -6.6 |
| C-reactive protein | -4 | +84.1 |
| Data are reported as percent change compared with placebo except for homocysteine and C-reactive protein, which are reported as percent change from baseline. | ||
| Source: Walsh B, et al. Effects of raloxifene on serum lipids and coagulation factors in healthy postmenopausal women. JAMA. 1998;279:1445-1451. Walsh B, et al. The effects of hormone replacement therapy and raloxifene on C-reactive protein and homocysteine in healthy postmenopausal women: a randomized controlled trial. J Clin Endocrinol Metab. 2000;85:214-218. | ||
TABLE 2
Adverse events reported by postmenopausal women in controlled trials of raloxifene
| Adverse event | Raloxifene | Placebo | P value |
|---|---|---|---|
| Vasomotor symptoms | 9.7% | 6.4% | .01 |
| Leg cramps | 7% | 3.7% | <.001 |
| Influenza syndrome | 13.4% | 11.4% | <.001 |
| Peripheral edema | 5.2% | 4.4% | <.01 |
| Endometrial cavity fluid | 8.1% | 5.7% | .02 |
| Venous thromboembolism | 1% | 0.3% | <.001 |
| Source: Ettinger B, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. JAMA. 1999;282:637-644. Nilsen J, et al. Raloxifene induces neurite outgrowth in estrogen receptor positive PC12 cells. Menopause. 1998;5:211-216. | |||
Patient selection
The candidates for tamoxifen are clearly defined. These include ER-positive breast-cancer patients as well as pre- and postmenopausal women at significant risk for the disease. There currently is no other indication for tamoxifen therapy in the U.S. In other countries, the drug is used—as clomiphene citrate is in the U.S.—to induce ovulation. Because of the risk of venous thromboem-bolism, it is important to limit the use of tamoxifen for the prevention of breast cancer to women at significant risk for the disease.
The best candidates for raloxifene are not as easily defined because several other therapeutic options are available for the same indications. For example, for the prevention and treatment of osteoporosis, options include the bisphosphonates (alendronate and risedronate), calcitonin, ERT, and raloxifene. However, only ERT and raloxifene have other beneficial effects that may be important to postmenopausal women (Table 3). Consequently, to prevent or treat osteoporosis in a postmenopausal woman with vasomotor symptoms and/or urogenital atrophic changes, the best option appears to be ERT, which not only protects against bone loss but relieves menopausal symptoms as well. A similar woman without menopausal symptoms who is concerned about or at risk for breast cancer might best be treated with raloxifene, which not only confers bone protection but may diminish breast cancer risk. However, if the same woman has a history of venous thromboembolism, the bisphosphonates or calcitonin might be better options, since both ERT and raloxifene would expose her to an unnecessarily high risk of a thromboembolic event.
The ideal candidate for raloxifene appears to be a postmenopausal woman with osteopenia or osteoporosis who has no increased risk of thromboembolism and few or no vasomotor symptoms. Raloxifene would be especially suitable for such a candidate if she has experienced any bleeding on ERT or fears an increased risk of breast cancer. This would include women who have been on ERT for 5 years or more, since the use of ERT for more than 5 years significantly increases the risk of breast cancer.4
In my practice, the women who are happiest with raloxifene tend to be older (4 or more years past menopause), concerned about osteoporosis and cardiovascular symptoms, and very glad to be free of vaginal bleeding while on the drug. Since the 2 most common reasons for discontinuing ERT—vaginal bleeding and the fear of breast cancer—are non-issues when raloxifene is given, compliance is likely to be high. The few cases of vaginal dryness that occur usually can be treated with lubricants, small doses of vaginal estrogen tablets, or the vaginal estrogen ring.
TABLE 3
Treatment options for osteoporosis: benefits and side effects
| Agent | Benefits | Side effects |
|---|---|---|
| ERT | Effective, safe, multidimensional.* Eases vasomotor and urogenital symptoms, improves mood, protects cardiovascular health | Bleeding, blood clots, breast cancer |
| Raloxifene | Effective, safe, multidimensional. Improves lipid profile. No bleeding or breast cancer risk | Blood clots. No relief of hot flushes or vaginal dryness |
| Alendronate, risedronate | Effective, safe, multidimensional | Difficult to take. Esophageal and gastric irritation, bleeding |
| Calcitonin | Effective, safe, unidimensional. Analgesic effects in patients with compression fractures | Insignificant |
| *The term “multidimensional” suggests that the agent affects more than 1 organ system. | ||
Conclusion
Thanks to a dramatic increase in life expectancy, American women can expect to live 30 or more years beyond the average age of menopause. With aging, the risk of health problems increases progressively. Several of these problems are specifically related to or augmented by estrogen deficiency. Despite several health benefits of ERT, its use remains very low (less than 30% in postmenopausal women) because of side effects and concerns about safety, bleeding, and breast cancer.7
SERMs—particularly raloxifene—provide many of estrogen’s positive effects on bone metabolism and lipids and other markers of cardiovascular disease without increasing the risk of bleeding or breast cancer. In fact, recent data suggest that raloxifene not only fails to increase the risk of breast cancer but actually may be protective against the disease.
SERMs do not necessarily replace older therapies such as ERT, but they do enhance our ability to modify and improve therapies, individualize regimens, and boost compliance. This is especially important with long-term preventive therapy, which requires a high level of acceptance and commitment by both patient and physician. Because of their multiple beneficial effects and favorable long-term risk-benefit profile, SERMs represent an important therapeutic advance in the field of women’s health.
Dr. Luciano reports that he serves on the speakers’ bureaus for Eli Lilly and Co., Wyeth Labs, and Pharmacia, and that he receives research grants from Pharmacia.
1. Grady D, Rubin SM, Petitti DB, et al. Hormone therapy to prevent disease and prolong life in menopausal women. Ann Intern Med. 1992;117(12):1016-1037.
2. Sudhir K, Komesaroff PA. Cardiovascular actions of estrogen in men. J Clin Endocrinol Metab. 1999;84:3411-3415.
3. Barrett-Connors E, Grady D. Hormone replacement therapy, heart disease and other considerations. Annu Rev Public Health. 1998;19:55-72.
4. Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormone replacement therapy: collaborative reanalysis of data from 51 epidemiologic studies of 52,705 women with breast cancer and 108,411 women without breast cancer. Lancet. 1997;350:1047-1059.
5. Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA. 1998;280:605-613.
6. Cancer Facts and Figures—1997. Atlanta: American Cancer Society; 1997.
7. Berman RS, et al. Risk factors associated with women’s compliance with estrogen replacement therapy. J Women’s Health. 1997;6:219-226.
8. Early Breast Cancer Trialists’ Collaborative Group. Tamoxifen for early breast cancer: an overview of the randomized trials. Lancet. 1998;351:1451-1467.
9. Osborne CK. Tamoxifen in the treatment of breast cancer. N Engl J Med. 1998;339:1609-1618.
10. Fisher B, et al. Five versus more than five years of tamoxifen therapy for breast cancer patients with negative lymph nodes and estrogen-positive tumors. J Natl Cancer Inst. 1996;88:1529-1542.
11. Fisher B, et al. Tamoxifen for the prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 1998;90:1371-1388.
12. Baker VL, Leitman D, Jaffe RB. Selective estrogen receptor modulators in reproductive medicine and biology. Obstet Gynecol Survey. 2000;55(7 Suppl 2):S21-S47.
13. Early Breast Cancer Collaborative Trial Group. Tamoxifen for early breast cancer: an overview of the randomized trials. Lancet. 1998;351:1451-1467.
14. Ettinger B, Black D, Mitlak B, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. JAMA. 1999;282:637-644.
15. Walsh B, Kuller L, Wild R, et al. Effects of raloxifene on serum lipids and coagulation factors in healthy postmenopausal women. JAMA. 1998;279:1445-1451.
16. Walsh B, Paul S, Wild R, et al. The effects of hormone replacement therapy and raloxifene on C-reactive protein and homocysteine in healthy postmenopausal women: a randomized controlled trial. J Clin Endocrinol Metab. 2000;85:214-218.
17. Goldstein SR, Scheele WH, Rajagopalan SK, et al. A 12-month comparative study of raloxifene, estrogen and placebo on the postmenopausal endometrium. Obstet Gynecol. 2000;95:95-103.
18. Cohen FJ, Watts S, Shah A, et al. Uterine effects of 3-year raloxifene therapy in postmenopausal women younger than age 60. Obstet Gynecol. 2000;95:104-110.
19. Delmas P, Bjarnason N, Mitlak B, et al. Effects of raloxifene on bone mineral density, serum cholesterol concentrations, and uterine endometrium in postmenopausal women. N Engl J Med. 1997;337:1641-1647.
20. Cummings S, Eckert S, Krueger K, et al. The effect of raloxifene on risk of breast cancer in postmenopausal women. JAMA. 1999;281:2189-2197.
21. Daly E, Vessey MP, Hawkins MM, et al. Risk of venous thromboembolism in users of hormone replacement therapy. Lancet. 1996;348:977-980.
22. Bryant H, Glasebrook A, Yang N, et al. A pharmacologic review of raloxifene. J Bone Miner Metab. 1995;13:75-78.
23. Davies G, et al. Adverse events reported by postmenopausal women in controlled trials with raloxifene. Obstet Gynecol. 1999;93:558-565.
24. Nilsen J, Mor G, Naftolin F. Raloxifene induces neurite outgrowth in estrogen receptor positive PC12 cells. Menopause. 1998;5:211-216.
25. Evista [package insert]. Indianapolis, Ind: Eli Lilly and Company; 1997.
1. Grady D, Rubin SM, Petitti DB, et al. Hormone therapy to prevent disease and prolong life in menopausal women. Ann Intern Med. 1992;117(12):1016-1037.
2. Sudhir K, Komesaroff PA. Cardiovascular actions of estrogen in men. J Clin Endocrinol Metab. 1999;84:3411-3415.
3. Barrett-Connors E, Grady D. Hormone replacement therapy, heart disease and other considerations. Annu Rev Public Health. 1998;19:55-72.
4. Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormone replacement therapy: collaborative reanalysis of data from 51 epidemiologic studies of 52,705 women with breast cancer and 108,411 women without breast cancer. Lancet. 1997;350:1047-1059.
5. Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA. 1998;280:605-613.
6. Cancer Facts and Figures—1997. Atlanta: American Cancer Society; 1997.
7. Berman RS, et al. Risk factors associated with women’s compliance with estrogen replacement therapy. J Women’s Health. 1997;6:219-226.
8. Early Breast Cancer Trialists’ Collaborative Group. Tamoxifen for early breast cancer: an overview of the randomized trials. Lancet. 1998;351:1451-1467.
9. Osborne CK. Tamoxifen in the treatment of breast cancer. N Engl J Med. 1998;339:1609-1618.
10. Fisher B, et al. Five versus more than five years of tamoxifen therapy for breast cancer patients with negative lymph nodes and estrogen-positive tumors. J Natl Cancer Inst. 1996;88:1529-1542.
11. Fisher B, et al. Tamoxifen for the prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 1998;90:1371-1388.
12. Baker VL, Leitman D, Jaffe RB. Selective estrogen receptor modulators in reproductive medicine and biology. Obstet Gynecol Survey. 2000;55(7 Suppl 2):S21-S47.
13. Early Breast Cancer Collaborative Trial Group. Tamoxifen for early breast cancer: an overview of the randomized trials. Lancet. 1998;351:1451-1467.
14. Ettinger B, Black D, Mitlak B, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. JAMA. 1999;282:637-644.
15. Walsh B, Kuller L, Wild R, et al. Effects of raloxifene on serum lipids and coagulation factors in healthy postmenopausal women. JAMA. 1998;279:1445-1451.
16. Walsh B, Paul S, Wild R, et al. The effects of hormone replacement therapy and raloxifene on C-reactive protein and homocysteine in healthy postmenopausal women: a randomized controlled trial. J Clin Endocrinol Metab. 2000;85:214-218.
17. Goldstein SR, Scheele WH, Rajagopalan SK, et al. A 12-month comparative study of raloxifene, estrogen and placebo on the postmenopausal endometrium. Obstet Gynecol. 2000;95:95-103.
18. Cohen FJ, Watts S, Shah A, et al. Uterine effects of 3-year raloxifene therapy in postmenopausal women younger than age 60. Obstet Gynecol. 2000;95:104-110.
19. Delmas P, Bjarnason N, Mitlak B, et al. Effects of raloxifene on bone mineral density, serum cholesterol concentrations, and uterine endometrium in postmenopausal women. N Engl J Med. 1997;337:1641-1647.
20. Cummings S, Eckert S, Krueger K, et al. The effect of raloxifene on risk of breast cancer in postmenopausal women. JAMA. 1999;281:2189-2197.
21. Daly E, Vessey MP, Hawkins MM, et al. Risk of venous thromboembolism in users of hormone replacement therapy. Lancet. 1996;348:977-980.
22. Bryant H, Glasebrook A, Yang N, et al. A pharmacologic review of raloxifene. J Bone Miner Metab. 1995;13:75-78.
23. Davies G, et al. Adverse events reported by postmenopausal women in controlled trials with raloxifene. Obstet Gynecol. 1999;93:558-565.
24. Nilsen J, Mor G, Naftolin F. Raloxifene induces neurite outgrowth in estrogen receptor positive PC12 cells. Menopause. 1998;5:211-216.
25. Evista [package insert]. Indianapolis, Ind: Eli Lilly and Company; 1997.