User login
For the highly motivated athlete, and often from a parent’s point of view, the return to play after a mild traumatic brain injury (mTBI, or concussion) may affect future scholarship and professional prospects, but it also carries the risk of further injury and permanent disability. Recognition of sport-related mTBI has been described as the most challenging aspect of managing this particular injury.1 Research has shown that patients, athletes,2-7 and health care providers8-10 all lack knowledge regarding some aspect of mTBI, and appropriate education is crucial.
Management of the athlete with mTBI requires both acute and follow-up care, using assessment tools found to be sensitive to detect deficits in cognition, balance, and coordination.
CASE STUDY
An 18-year-old high school football player was tackled during a Saturday afternoon game; on the previous play, he had run 80 yards following an interception. The tackle caused both his ear pads and his chinstrap to break, but he did not lose consciousness. Within two minutes, he was evaluated on the sidelines by the team’s physician assistant and its certified athletic trainer, during which he became nauseated and vomited several times. The player also complained of a new-onset headache and some dizziness. Three weeks earlier, he had been diagnosed with an mTBI; he recovered fully and was medically cleared to play one week later.
On the sidelines immediately after the current injury, the athlete underwent a neurologic examination that yielded no focal neurologic findings. He was transported to the local emergency department (ED) because of the headache and vomiting. The ED provider made a diagnosis of “forehead contusion” and told the patient that he “did not have a brain injury since there was no loss of consciousness.” CT was not ordered, and the athlete was prescribed ibuprofen for his headache.
The following Monday, the athlete was reevaluated by the team PA and the PA’s supervising physician. The athlete reported some residual headache but said the dizziness, nausea, and vomiting had resolved shortly after the injury. His neurologic exam was unremarkable, and although no baseline data were available, results from the Automated Neuropsychological Assessment Metrics (ANAM)11 computerized test demonstrated deficits in reaction time, problem solving, and short-term memory, in comparison with age-matched individuals. CT with contrast performed at that time was negative for hematoma or intracranial swelling.
The athlete was diagnosed with a resolving mTBI and postconcussion syndrome. The consensus was that the vomiting was most likely not a result of the head injury but rather was triggered by the physical exertion of having sprinted 80 yards on the previous play. He was restricted from any exercise and all contact sports until he was asymptomatic, both at rest and during physical activity. The athlete and his mother were informed about the risks of second-impact syndrome.12,13 Follow-up ANAM testing was suggested, but the patient did not return to the office for the test.
mTBI IN THE YOUNGER PATIENT
This case is not an isolated occurrence. In the United States, annual estimates of sport-related traumatic brain injuries, predominantly concussions, range from 1.6 million to 3.8 million.14 According to recent data from injury surveillance systems, concussions sustained by high school athletes represent a greater proportion of sport-related injuries (8.9%) than do those among college athletes (5.8%). Female athletes sustain sport-related mTBI and associated injuries at a higher rate and proportion than do males participating in the same sports.15
Sustaining a sport-related mTBI at an early age is of particular concern: The brain is still developing, and younger patients have an enhanced potential for cumulative effects and prolonged cognitive deficits. Athletes with even one previous mTBI are at increased risk for future mTBI (adjusted risk ratio, 1.4, compared with athletes who have never sustained such an injury).16 Of even greater concern, high school athletes tend to experience delays in cognitive and symptomatic recovery following a concussive injury.17
A growing body of literature has demonstrated difficulties in recognizing and managing mTBIs at all levels of play and within various patient populations.1,18,19 One survey of mTBI evaluation in primary care settings revealed that only 33% of practitioners responsible for sideline coverage used a standard, objective protocol, and an additional 31% used no mTBI guidelines. Among the latter, 71% cited a lack of knowledge, and 16% said they found existing guidelines confusing.10
Another study revealed that hospital discharge instructions for children sustaining an athletic mTBI were inadequate in 69.7% of cases.9 Among these patients, 13% were instructed to return to activity too soon, and 87% were given no instructions at all. The need to better educate athletes, parents, coaches, and health care professionals about the potential seriousness of sport-related mTBI and safe return to the playing field is clear.1,20
This discussion will address new advances in mTBI management and return-to-play decisions for adolescent athletes in the primary care setting.
TERMINOLOGY AND PATHOPHYSIOLOGY
The terminology associated with mTBI has been evolving along with an enhanced understanding of its pathology and etiology. Since more than 90% of sport-related traumatic brain injuries (TBIs) are considered “mild,” the term mTBI is often being used in place of concussion.14 Considering the long-lasting effects of more severe TBI, the contemporary term mTBI more accurately portrays the seriousness of even a seemingly minor injury, often minimized as a “ding” or “bell ringer.” These lay terms do not convey the magnitude and extent of injury sustained and are often thought by nonmedical persons to refer to a different, unrelated injury.6
Since the Concussion in Sport Group18 first met in 2001, several features of sport-related mTBI have been described in an effort to clarify its definition. These include an injury that
(1) is caused by a direct blow to the head or an indirect blow elsewhere in the body that transmits an impulsive force to the head
(2) may cause an immediate and short-lived alteration in neurologic function
(3) may cause neuropathologic changes but typically reflects a functional disturbance rather than a structural injury
(4) is represented by a gradient of clinical symptoms that often resolve sequentially, often without loss of consciousness
(5) is predominantly associated with negative findings on conventional neuroimaging studies (eg, CT, MRI).18
When an athlete sustains an impact to the head, external forces create accelerations and decelerations of the brain within the skull. These forces create the classic coup-contrecoup injury,1 in which the brain impacts the skull at the initial point of contact, with a second point of injury on the directly opposite side of the brain. In some cases, rotational forces occur when the skull is impacted in such a way that the brain rotates about its axis, causing shear and stretch forces on the brain tissue. Either mechanism of injury can trigger a chain of metabolic events in the brain that result in decreased blood flow, increased glucose utilization, and neurotransmitter dysfunction. All of these are thought to contribute to the transient neurologic deficits associated with mTBI.21,22
If a patient who is recovering from mTBI sustains a second head injury before metabolic changes caused by the first injury have fully resolved, a second-impact syndrome (SIS)12,13,16 can develop (see Figure 112,13,16 ). SIS results in massive, rapid brain edema, causing increased intracranial pressure and eventual brain herniation. The majority of cases of SIS are reported in patients younger than 18 and are thought to be the result of altered autoregulation of cerebral blood flow.12 In pediatric athletes, therefore, the proper recognition of mTBI, its effective management, and the return-to-play determination are crucial to decrease the risk of SIS.
INITIAL EVALUATION
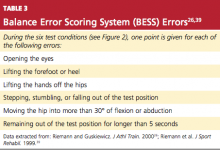
The clinical course and management of mTBI can be separated into two distinct components.23 The first is the initial or acute evaluation, which should occur as close to the time of injury as possible. Evaluation of an acute mTBI centers on the history and physical examination at the time of injury; its focus is to determine whether a neurosurgical emergency exists and what course of treatment is needed. Tools such as the Standard Assessment of Concussion (SAC)24 and Folstein’s Mini–Mental State Examination (MMSE)25 should be used to assess the extent of initial cognitive impairment, while other tools, such as the Balance Error Scoring System26 test for motor impairment, should be combined with the neurologic physical exam to formulate the differential diagnosis.
No two brain injuries are alike. The clinician must key into the mechanism of injury, initial and current symptoms (including headache, confusion, and amnesia),16 and positive and negative neurologic findings. Differentials that must be ruled out immediately include skull fracture, cerebral contusion, and epidural hematomas.
Along with the physical findings associated with mTBI, those suggesting more severe injury may include acute localized swelling, deformity, prolonged loss of consciousness, intractable vomiting, and often multiple positive neurologic exam findings, such as cranial nerve abnormalities and motor weakness. Any one of these findings in the initial evaluation warrants activation of emergency medical services, including immediate transport to an ED equipped to manage a neurosurgical emergency.
Since 1974, at least 25 different scales have been used to help practitioners evaluating mTBI assign a grade of severity.1 Although grading scales can be helpful to objectify subjective symptoms, they vary considerably, and none has been shown valid, reliable, or sensitive through published research. Furthermore, an assigned grade cannot reliably express the severity of injury or the prognosis for recovery in each case.1,17
DIAGNOSTIC IMAGING
In mTBI management, the sole purpose of diagnostic imaging is to rule out a more severe structural injury, such as intracranial hemorrhage or hematoma. Symptoms commonly associated with mTBI, such as nausea, vomiting, headache, and visual disturbances, are also cardinal signs of a mass effect resulting from both subdural and epidural hematomas. When these symptoms occur in the acute phase of mTBI management, immediate CT without contrast is imperative to rule out skull fracture and intracranial hemorrhage. Note: Negative imaging test results in the presence of mTBI symptoms do not rule out mTBI, a functional injury; they only confirm that no structural pathology exists.1
Clinicians must also be aware that because a subdural hematoma accumulates slowly, positive findings may not be evident on CT or MRI for seven to 14 days after the initial injury. Thus, the sudden return or worsening of mTBI symptoms that were previously resolved or stable warrants evaluation for a slower (and more commonly fatal) chronic subdural hematoma.1 In this emergent case, CT without IV contrast should be performed first to rule out acute hemorrhage or any mass effect. However, because of the lysing effect of clotted blood, CT with IV contrast or MRI is needed to definitively determine dural and gray-matter reactions to an occult bleed.27
At this time, there is no gold standard among imaging techniques to capture the functional disturbances often noted with sport-related mTBI. However, in one recent study of functional MRI (fMRI) use following sport-related mTBI, athletes with evidence of hyperactivation on their initial post-mTBI scan took longer to recover, based on symptom presentation and neurocognitive testing.28 Though currently used for research purposes, fMRI appears to demonstrate measurable metabolic changes in the brain even after symptoms have been resolved. This promising modality may soon provide helpful neurophysiologic data for the clinical assessment and management of sport-related mTBI.
SYMPTOM SURVEILLANCE
The second component of clinical management of mTBI is the follow-up and surveillance of symptoms and neurologic limitations over time. This is essential for making clinical decisions, including the appropriate time to return the athlete safely to sports or work, so as to avoid further injury.
While the acute component of mTBI management relies on the objective nature of physical examination and neuroimaging, follow-up and surveillance are heavily dependent on the subjective symptoms (see Table 11,18), particularly when physical examination and neuroimaging findings are unremarkable.27,29 Therefore, sensitive and specific clinical tools are needed to accurately assess the various elements of cognition and psychological functioning that are most commonly impaired by mTBI.30
COGNITIVE ASSESSMENT
The metabolic changes that occur after cerebral injury have been shown to cause temporary deficits in normal cognition.23,31 Within the first 24 hours of injury, mild to moderate cognitive impairment is noted across all domains, with the greatest deficits occurring in global functioning, memory acquisition, and delayed memory. Deficits in these areas have been shown to resolve within seven to 10 days following the initial injury.31 It is helpful for these cognitive domains to have been clinically evaluated before injury (baseline), as postinjury evaluation can more effectively detect the extent of debilitation caused by mTBI; subsequent reevaluations can be used to monitor the rate of recovery.
Neuropsychological testing has been studied extensively to determine its value and use in the assessment of mTBI.18,32 Currently, two types of testing are used. Traditional neuropsychological testing comprises a battery of pencil-and-paper exercises administered and interpreted by a psychologist to evaluate cognition and identify areas of deficit following mTBI.33 Although these tests produce a wealth of data, they are expensive, they may require a referral, and administering them can take longer than four hours.
The newest form of neuropsychological testing involves computer-based protocols. Though not yet fully validated, these tools require less time to administer than traditional testing and are commonly used in the sports medicine community (see Table 21,11,33-35 ). Computer-based testing, which may be conducted in the school’s computer lab, has the potential to make preseason baseline testing feasible for large numbers of athletes. Other advantages are ease of administration, a time requirement of about 30 minutes, and the availability of multiple versions to control for the effects of practice.1,23
Two approaches have been suggested for effective use of neuropsychological testing in both components of mTBI management1,30:
First, perform baseline testing at the start of the athletic season, before exposure to injury (possibly within the preparticipation physical), then retest the injured athlete once he or she reports being asymptomatic. Return to play may be considered once the injured athlete scores at or above baseline testing.
Second, perform baseline testing at the start of the season, then retest the injured athlete at set time intervals, charting improvement and rate of recovery. This serial assessment can provide a patient recovery curve for the clinician.30
Results from any neuropsychological testing protocol may be more valuable to the trained practitioner (usually a neurophysiologist, although developers of computer-based testing offer training and credentialing) than are subjective symptoms alone. However, clinicians must use these results as only one variable in the return-to-play decision.23 Cognitive testing is most reliable when baseline testing is included, but this may not be feasible in all settings. If baseline data are not available, normative data for the population of interest (eg, high school level) may be used; these are often available from the manufacturer of the computerized cognitive testing platform.
MOTOR CONTROL ASSESSMENT
Immediately following mTBI, a subtle yet significant degree of motor impairment may develop, often lasting beyond the acute phase of injury. This impairment may affect proprioception, fine and gross motor control, reaction time, and postural stability (balance), all of which are necessary and vital components of athletics. Any impairment in motor control will not only have a negative effect on athletic performance; it will undoubtedly increase the likelihood of a second and possibly more severe injury.
In the neurologic physical examination, results from the tests that are traditionally used to assess motor pathways and coordination have been subject to the individual clinician’s skill and interpretation.26 Several new tests have been developed to assess postural stability more objectively, producing data that give the clinician insight into the brain’s ability to organize incoming sensory information and respond appropriately to environmental changes.36 The gold standard for evaluating postural stability has been the force platform system, which measures vertical ground reaction forces as the body’s center of mass moves around a fixed base of support.26,37
This tool detects even minute alterations in the athlete’s postural stability as stress is exerted on the visual, vestibular, and somatosensory modalities; it can quantify the accuracy and timing of the subject’s motor responses. In recent studies of athletes with mTBI, vestibular and visual alterations caused a deficit in mean stability that peaked at 24 hours postinjury and lasted as long as 10 days before returning to baseline scores.36,38
Although performing force platform testing requires very little time, it is ordinarily administered by an otolaryngologist or an audiologist and usually requires a referral. Cost and limited accessibility make it impractical for obtaining baseline testing in entire teams or other large groups.
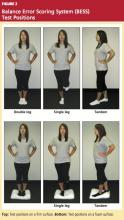
Compared with force platform testing, the Balance Error Scoring System (BESS)26 test has also been shown to produce reliable and valid assessments of postural stability.26,39 This low-cost alternative can be used in the clinic or on the sidelines to identify subtle impairments in postural stability, making baseline assessment for large groups feasible and helping clinicians safely return an injured athlete to play.
The BESS tests the athlete’s balance in three different positions, with eyes closed, on both firm and soft foam surfaces. Postural stability is determined by totaling the number of errors made during six 20-second trials (see Figure 2 and Table 3,26,39 page 21). Comparison studies have shown that athletes who experience mTBI have an increased number of errors on days 1 through 5 postinjury, compared with noninjured controls.36
DETERMINING RETURN TO PLAY
Despite the current emphasis on evidence-based practice and the possible consequences of premature participation in sports, the return-to-play decision often depends more on speculation than quantifiable data. The clinician is challenged to synthesize as much subjective and objective data as are available—patient-reported symptomatology, adjunctive assessment scores, and physical examination findings, and preferably with input from a management team that may include a certified athletic trainer, the team physician, a neurosurgeon, and a neuropsychiatrist.1,17
The student-athlete’s teachers should not be overlooked as observers of baseline classroom performance and possible dysfunction and impairment related to the injury. Parents and coaches may offer additional information, but the possibility of bias must be considered.
Only when the athlete reports that symptoms have fully resolved, it is generally agreed, should return to play even be considered.1,18,19 Then, provided that the athlete has regained at least baseline cognitive function and postural stability, a return-to-play progression can begin (see Table 41,19).
Athletes should be moved slowly through these stages, with ongoing supervision and reevaluation after each stage for possible recurrence of symptoms. On average, this progression takes three to five days.
CONCLUSION
No two mTBIs are alike. The mechanism of injury, the degree of neurologic dysfunction, and the time needed for recovery ultimately have no correlation. No athlete should return to play while mTBI symptoms persist, whether at rest or following exercise. If an athlete sustains an injury resulting in loss of consciousness (whatever the duration) or experiences amnesia, return to play that day should not be considered until further evaluation and/or neuroimaging can be performed.
It is imperative that everyone involved with student athletes, both medically and scholastically, be educated about the signs, symptoms, and management of mTBI to ensure a smooth return to school, sports, and other routine activities.
The CDC has begun to address this issue with several Heads Up tool kits for high school coaches, youth sports supervisors, and medical providers. These are available at www.cdc.gov/ncipc/tbi/TBI.htm.
REFERENCES
1. Guskiewicz KM, Bruce SL, Cantu RC, et al. National Athletic Trainers’ Association Position Statement: Management of Sport-Related Concussion. J Athl Train. 2004; 39(3):280-297.
2. Delaney JS, Abuzeyad F, Correa JA, Foxford R. Recognition and characteristics of concussions in the emergency department population. J Emerg Med. 2005;29(2): 189-197.
3. Kaut KP, DePompei R, Kerr J, Congeni J. Reports of head injury and symptom knowledge among college athletes: implications for assessment and educational intervention. Clin J Sport Med. 2003;13(4):213-221.
4. LaBotz M, Martin MR, Kimura IF, et al. A comparison of a preparticipation evaluation history form and a symptom-based concussion survey in the identification of previous head injury in collegiate athletes. Clin J Sport Med. 2005;15(2):73-78.
5. McCrea M, Hammeke T, Olsen G, et al. Unreported concussion in high school football players: implications for prevention. Clin J Sport Med. 2004;14(1):13-17.
6. Valovich McLeod TC, Bay RC, Heil J, McVeigh SD. Identification of sport and recreational activity concussion history through the preparticipation screening and a symptom survey in young athletes. Clin J Sport Med. 2008;18(3):235-240.
7. Williamson IJ, Goodman D. Converging evidence for the under-reporting of concussions in youth ice hockey. Br J Sports Med. 2006;40(2):128-132.
8. Bazarian JJ, Veenema T, Brayer AF, Lee E. Knowledge of concussion guidelines among practitioners caring for children. Clin Pediatr (Phila). 2001;40(4):207-212.
9. Genuardi FJ, King WD. Inappropriate discharge instructions for youth athletes hospitalized for concussion. Pediatrics. 1995;95(2):216-218.
10. Pleacher MD, Dexter WW. Concussion management by primary care providers. Br J Sports Med. 2006;40(1):e2.
11. Levinson DM, Reeves DL. Monitoring recovery from traumatic brain injury using automated neuropsychological assessment metrics (ANAM V1.0). Arch Clin Neuropsychol. 1997;12(2):155-166.
12. Cantu RC. Second-impact syndrome. Clin Sports Med. 1998;17(1):37-44.
13. McCrory PR, Berkovic SF. Second impact syndrome. Neurology. 1998;50(3):677-683.
14. Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil. 2006;21(5):375-378.
15. Gessel LM, Fields SK, Collins CL, et al. Concussions among United States high school and collegiate athletes. J Athl Train. 2007;42(4):495-503.
16. Guskiewicz KM, McCrea M, Marshall SW, et al. Cumulative effects associated with recurrent concussion in collegiate football players: the NCAA Concussion Study. JAMA. 2003;290(19):2549-2555.
17. Field M, Collins MW, Lovell MR, Maroon J. Does age play a role in recovery from sports-related concussion? A comparison of high school and collegiate athletes. J Pediatr. 2003;142(5):546-553.
18. Aubry M, Cantu RC, Dvorak J, et al. Summary and agreement statement of the First International Conference on Concussion in Sport, Vienna 2001: recommendations for the improvement of safety and health of athletes who may suffer concussive injuries. Br J Sports Med. 2002;36(1):6-10.
19. McCrory P, Johnston K, Meeuwisse W, et al. Summary and agreement statement of the 2nd International Conference on Concussion in Sport, Prague 2004. Clin J Sport Med. 2005;15(2):48-55.
20. Centers for Disease Control and Prevention. Nonfatal traumatic brain injuries from sports and recreation activities—United States, 2001-2005. MMWR Morb Mortal Wkly Rep. 2007;56(29):733-737.
21. Giza CC, Hovda DA. Ionic and metabolic consequences of concussion. In: Cantu RC, ed. Neurologic Athletic Head and Spine Injuries. Philadelphia, PA: WB Saunders Co; 2000:80-100.
22. Giza CC, Hovda DA. The neurometabolic cascade of concussion. J Athl Train. 2001;36(3):228-235.
23. Guskiewicz KM, Cantu RC. The concussion puzzle: evaluation of the sport-related concussion. Am J Med Sports. 2004;6:13-21.
24. McCrea M, Randolph C, Kelly JP. The Standardized Assessment of Concussion (SAC): Manual for Administration, Scoring and Interpretation. 2nd ed. Waukesha, WI: CNS Inc; 2000.
25. Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198.
26. Riemann BL, Guskiewicz KM. Effects of mild head injury on postural stability as measured through clinical balance testing. J Athl Train. 2000;35(1):19-25.
27. Borg J, Holm L, Cassidy JD, et al. Diagnostic procedures in mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med. 2004;43(43 Suppl):61-75.
28. Lovell MR, Pardini JE, Welling J, et al. Functional brain abnormalities are related to clinical recovery and time to return-to-play in athletes. Neurosurgery. 2007;61(2): 352-359.
29. Chan RC. Base rate of post-concussion symptoms among normal people and its neuropsychological correlates. Clin Rehabil. 2001;15(3):266-273.
30. Randolph C, McCrea M, Barr WB. Is neuropsychological testing useful in the management of sport-related concussion? J Athl Train. 2005;40(3):139-152.
31. Belanger HG, Vanderploeg RD. The neuropsychological impact of sports-related concussion: a meta-analysis. J Int Neuropsychol Soc. 2005;11(4):345-357.
32. Guskiewicz KM, Bruce SL, Cantu RC, et al; National Athletic Trainers’ Association. Research based recommendations on management of sport related concussion: summary of the National Athletic Trainers’ Association position statement. Br J Sports Med. 2006;40(1):6-10.
33. Collie A, Darby D, Maruff P. Computerised cognitive assessment of athletes with sports related head injury. Br J Sports Med. 2001;35(5):297-302.
34. Erlanger D, Saliba E, Barth J, et al. Monitoring resolution of postconcussion symptoms in athletes: preliminary results of a Web-based neuropsychological test protocol. J Athl Train. 2001;36(3):280-287.
35. Schatz P, Pardini JE, Lovell MR, et al. Sensitivity and specificity of the ImPACT Test Battery for concussion in athletes. Arch Clin Neuropsychol. 2006;21(1):91-99.
36. Guskiewicz KM, Ross SE, Marshall SW. Postural stability and neuropsychological deficits after concussion in collegiate athletes. J Athl Train. 2001;36(3):263-273.
37. Guskiewicz KM. Postural stability assessment following concussion: one piece of the puzzle. Clin J Sport Med. 2001;11(3):182-189.
38. Peterson CL, Ferrara MS, Mrazik M, et al. Evaluation of neuropsychological domain scores and postural stability following cerebral concussion in sports. Clin J Sport Med. 2003;13(4):230-237.
39. Riemann BL, Guskiewicz KM, Shields EW. Relationship between clinical and forceplate measure of postural stability. J Sport Rehabil. 1999;8:71-82.
For the highly motivated athlete, and often from a parent’s point of view, the return to play after a mild traumatic brain injury (mTBI, or concussion) may affect future scholarship and professional prospects, but it also carries the risk of further injury and permanent disability. Recognition of sport-related mTBI has been described as the most challenging aspect of managing this particular injury.1 Research has shown that patients, athletes,2-7 and health care providers8-10 all lack knowledge regarding some aspect of mTBI, and appropriate education is crucial.
Management of the athlete with mTBI requires both acute and follow-up care, using assessment tools found to be sensitive to detect deficits in cognition, balance, and coordination.
CASE STUDY
An 18-year-old high school football player was tackled during a Saturday afternoon game; on the previous play, he had run 80 yards following an interception. The tackle caused both his ear pads and his chinstrap to break, but he did not lose consciousness. Within two minutes, he was evaluated on the sidelines by the team’s physician assistant and its certified athletic trainer, during which he became nauseated and vomited several times. The player also complained of a new-onset headache and some dizziness. Three weeks earlier, he had been diagnosed with an mTBI; he recovered fully and was medically cleared to play one week later.
On the sidelines immediately after the current injury, the athlete underwent a neurologic examination that yielded no focal neurologic findings. He was transported to the local emergency department (ED) because of the headache and vomiting. The ED provider made a diagnosis of “forehead contusion” and told the patient that he “did not have a brain injury since there was no loss of consciousness.” CT was not ordered, and the athlete was prescribed ibuprofen for his headache.
The following Monday, the athlete was reevaluated by the team PA and the PA’s supervising physician. The athlete reported some residual headache but said the dizziness, nausea, and vomiting had resolved shortly after the injury. His neurologic exam was unremarkable, and although no baseline data were available, results from the Automated Neuropsychological Assessment Metrics (ANAM)11 computerized test demonstrated deficits in reaction time, problem solving, and short-term memory, in comparison with age-matched individuals. CT with contrast performed at that time was negative for hematoma or intracranial swelling.
The athlete was diagnosed with a resolving mTBI and postconcussion syndrome. The consensus was that the vomiting was most likely not a result of the head injury but rather was triggered by the physical exertion of having sprinted 80 yards on the previous play. He was restricted from any exercise and all contact sports until he was asymptomatic, both at rest and during physical activity. The athlete and his mother were informed about the risks of second-impact syndrome.12,13 Follow-up ANAM testing was suggested, but the patient did not return to the office for the test.
mTBI IN THE YOUNGER PATIENT
This case is not an isolated occurrence. In the United States, annual estimates of sport-related traumatic brain injuries, predominantly concussions, range from 1.6 million to 3.8 million.14 According to recent data from injury surveillance systems, concussions sustained by high school athletes represent a greater proportion of sport-related injuries (8.9%) than do those among college athletes (5.8%). Female athletes sustain sport-related mTBI and associated injuries at a higher rate and proportion than do males participating in the same sports.15
Sustaining a sport-related mTBI at an early age is of particular concern: The brain is still developing, and younger patients have an enhanced potential for cumulative effects and prolonged cognitive deficits. Athletes with even one previous mTBI are at increased risk for future mTBI (adjusted risk ratio, 1.4, compared with athletes who have never sustained such an injury).16 Of even greater concern, high school athletes tend to experience delays in cognitive and symptomatic recovery following a concussive injury.17
A growing body of literature has demonstrated difficulties in recognizing and managing mTBIs at all levels of play and within various patient populations.1,18,19 One survey of mTBI evaluation in primary care settings revealed that only 33% of practitioners responsible for sideline coverage used a standard, objective protocol, and an additional 31% used no mTBI guidelines. Among the latter, 71% cited a lack of knowledge, and 16% said they found existing guidelines confusing.10
Another study revealed that hospital discharge instructions for children sustaining an athletic mTBI were inadequate in 69.7% of cases.9 Among these patients, 13% were instructed to return to activity too soon, and 87% were given no instructions at all. The need to better educate athletes, parents, coaches, and health care professionals about the potential seriousness of sport-related mTBI and safe return to the playing field is clear.1,20
This discussion will address new advances in mTBI management and return-to-play decisions for adolescent athletes in the primary care setting.
TERMINOLOGY AND PATHOPHYSIOLOGY
The terminology associated with mTBI has been evolving along with an enhanced understanding of its pathology and etiology. Since more than 90% of sport-related traumatic brain injuries (TBIs) are considered “mild,” the term mTBI is often being used in place of concussion.14 Considering the long-lasting effects of more severe TBI, the contemporary term mTBI more accurately portrays the seriousness of even a seemingly minor injury, often minimized as a “ding” or “bell ringer.” These lay terms do not convey the magnitude and extent of injury sustained and are often thought by nonmedical persons to refer to a different, unrelated injury.6
Since the Concussion in Sport Group18 first met in 2001, several features of sport-related mTBI have been described in an effort to clarify its definition. These include an injury that
(1) is caused by a direct blow to the head or an indirect blow elsewhere in the body that transmits an impulsive force to the head
(2) may cause an immediate and short-lived alteration in neurologic function
(3) may cause neuropathologic changes but typically reflects a functional disturbance rather than a structural injury
(4) is represented by a gradient of clinical symptoms that often resolve sequentially, often without loss of consciousness
(5) is predominantly associated with negative findings on conventional neuroimaging studies (eg, CT, MRI).18
When an athlete sustains an impact to the head, external forces create accelerations and decelerations of the brain within the skull. These forces create the classic coup-contrecoup injury,1 in which the brain impacts the skull at the initial point of contact, with a second point of injury on the directly opposite side of the brain. In some cases, rotational forces occur when the skull is impacted in such a way that the brain rotates about its axis, causing shear and stretch forces on the brain tissue. Either mechanism of injury can trigger a chain of metabolic events in the brain that result in decreased blood flow, increased glucose utilization, and neurotransmitter dysfunction. All of these are thought to contribute to the transient neurologic deficits associated with mTBI.21,22
If a patient who is recovering from mTBI sustains a second head injury before metabolic changes caused by the first injury have fully resolved, a second-impact syndrome (SIS)12,13,16 can develop (see Figure 112,13,16 ). SIS results in massive, rapid brain edema, causing increased intracranial pressure and eventual brain herniation. The majority of cases of SIS are reported in patients younger than 18 and are thought to be the result of altered autoregulation of cerebral blood flow.12 In pediatric athletes, therefore, the proper recognition of mTBI, its effective management, and the return-to-play determination are crucial to decrease the risk of SIS.
INITIAL EVALUATION
The clinical course and management of mTBI can be separated into two distinct components.23 The first is the initial or acute evaluation, which should occur as close to the time of injury as possible. Evaluation of an acute mTBI centers on the history and physical examination at the time of injury; its focus is to determine whether a neurosurgical emergency exists and what course of treatment is needed. Tools such as the Standard Assessment of Concussion (SAC)24 and Folstein’s Mini–Mental State Examination (MMSE)25 should be used to assess the extent of initial cognitive impairment, while other tools, such as the Balance Error Scoring System26 test for motor impairment, should be combined with the neurologic physical exam to formulate the differential diagnosis.
No two brain injuries are alike. The clinician must key into the mechanism of injury, initial and current symptoms (including headache, confusion, and amnesia),16 and positive and negative neurologic findings. Differentials that must be ruled out immediately include skull fracture, cerebral contusion, and epidural hematomas.
Along with the physical findings associated with mTBI, those suggesting more severe injury may include acute localized swelling, deformity, prolonged loss of consciousness, intractable vomiting, and often multiple positive neurologic exam findings, such as cranial nerve abnormalities and motor weakness. Any one of these findings in the initial evaluation warrants activation of emergency medical services, including immediate transport to an ED equipped to manage a neurosurgical emergency.
Since 1974, at least 25 different scales have been used to help practitioners evaluating mTBI assign a grade of severity.1 Although grading scales can be helpful to objectify subjective symptoms, they vary considerably, and none has been shown valid, reliable, or sensitive through published research. Furthermore, an assigned grade cannot reliably express the severity of injury or the prognosis for recovery in each case.1,17
DIAGNOSTIC IMAGING
In mTBI management, the sole purpose of diagnostic imaging is to rule out a more severe structural injury, such as intracranial hemorrhage or hematoma. Symptoms commonly associated with mTBI, such as nausea, vomiting, headache, and visual disturbances, are also cardinal signs of a mass effect resulting from both subdural and epidural hematomas. When these symptoms occur in the acute phase of mTBI management, immediate CT without contrast is imperative to rule out skull fracture and intracranial hemorrhage. Note: Negative imaging test results in the presence of mTBI symptoms do not rule out mTBI, a functional injury; they only confirm that no structural pathology exists.1
Clinicians must also be aware that because a subdural hematoma accumulates slowly, positive findings may not be evident on CT or MRI for seven to 14 days after the initial injury. Thus, the sudden return or worsening of mTBI symptoms that were previously resolved or stable warrants evaluation for a slower (and more commonly fatal) chronic subdural hematoma.1 In this emergent case, CT without IV contrast should be performed first to rule out acute hemorrhage or any mass effect. However, because of the lysing effect of clotted blood, CT with IV contrast or MRI is needed to definitively determine dural and gray-matter reactions to an occult bleed.27
At this time, there is no gold standard among imaging techniques to capture the functional disturbances often noted with sport-related mTBI. However, in one recent study of functional MRI (fMRI) use following sport-related mTBI, athletes with evidence of hyperactivation on their initial post-mTBI scan took longer to recover, based on symptom presentation and neurocognitive testing.28 Though currently used for research purposes, fMRI appears to demonstrate measurable metabolic changes in the brain even after symptoms have been resolved. This promising modality may soon provide helpful neurophysiologic data for the clinical assessment and management of sport-related mTBI.
SYMPTOM SURVEILLANCE
The second component of clinical management of mTBI is the follow-up and surveillance of symptoms and neurologic limitations over time. This is essential for making clinical decisions, including the appropriate time to return the athlete safely to sports or work, so as to avoid further injury.
While the acute component of mTBI management relies on the objective nature of physical examination and neuroimaging, follow-up and surveillance are heavily dependent on the subjective symptoms (see Table 11,18), particularly when physical examination and neuroimaging findings are unremarkable.27,29 Therefore, sensitive and specific clinical tools are needed to accurately assess the various elements of cognition and psychological functioning that are most commonly impaired by mTBI.30
COGNITIVE ASSESSMENT
The metabolic changes that occur after cerebral injury have been shown to cause temporary deficits in normal cognition.23,31 Within the first 24 hours of injury, mild to moderate cognitive impairment is noted across all domains, with the greatest deficits occurring in global functioning, memory acquisition, and delayed memory. Deficits in these areas have been shown to resolve within seven to 10 days following the initial injury.31 It is helpful for these cognitive domains to have been clinically evaluated before injury (baseline), as postinjury evaluation can more effectively detect the extent of debilitation caused by mTBI; subsequent reevaluations can be used to monitor the rate of recovery.
Neuropsychological testing has been studied extensively to determine its value and use in the assessment of mTBI.18,32 Currently, two types of testing are used. Traditional neuropsychological testing comprises a battery of pencil-and-paper exercises administered and interpreted by a psychologist to evaluate cognition and identify areas of deficit following mTBI.33 Although these tests produce a wealth of data, they are expensive, they may require a referral, and administering them can take longer than four hours.
The newest form of neuropsychological testing involves computer-based protocols. Though not yet fully validated, these tools require less time to administer than traditional testing and are commonly used in the sports medicine community (see Table 21,11,33-35 ). Computer-based testing, which may be conducted in the school’s computer lab, has the potential to make preseason baseline testing feasible for large numbers of athletes. Other advantages are ease of administration, a time requirement of about 30 minutes, and the availability of multiple versions to control for the effects of practice.1,23
Two approaches have been suggested for effective use of neuropsychological testing in both components of mTBI management1,30:
First, perform baseline testing at the start of the athletic season, before exposure to injury (possibly within the preparticipation physical), then retest the injured athlete once he or she reports being asymptomatic. Return to play may be considered once the injured athlete scores at or above baseline testing.
Second, perform baseline testing at the start of the season, then retest the injured athlete at set time intervals, charting improvement and rate of recovery. This serial assessment can provide a patient recovery curve for the clinician.30
Results from any neuropsychological testing protocol may be more valuable to the trained practitioner (usually a neurophysiologist, although developers of computer-based testing offer training and credentialing) than are subjective symptoms alone. However, clinicians must use these results as only one variable in the return-to-play decision.23 Cognitive testing is most reliable when baseline testing is included, but this may not be feasible in all settings. If baseline data are not available, normative data for the population of interest (eg, high school level) may be used; these are often available from the manufacturer of the computerized cognitive testing platform.
MOTOR CONTROL ASSESSMENT
Immediately following mTBI, a subtle yet significant degree of motor impairment may develop, often lasting beyond the acute phase of injury. This impairment may affect proprioception, fine and gross motor control, reaction time, and postural stability (balance), all of which are necessary and vital components of athletics. Any impairment in motor control will not only have a negative effect on athletic performance; it will undoubtedly increase the likelihood of a second and possibly more severe injury.
In the neurologic physical examination, results from the tests that are traditionally used to assess motor pathways and coordination have been subject to the individual clinician’s skill and interpretation.26 Several new tests have been developed to assess postural stability more objectively, producing data that give the clinician insight into the brain’s ability to organize incoming sensory information and respond appropriately to environmental changes.36 The gold standard for evaluating postural stability has been the force platform system, which measures vertical ground reaction forces as the body’s center of mass moves around a fixed base of support.26,37
This tool detects even minute alterations in the athlete’s postural stability as stress is exerted on the visual, vestibular, and somatosensory modalities; it can quantify the accuracy and timing of the subject’s motor responses. In recent studies of athletes with mTBI, vestibular and visual alterations caused a deficit in mean stability that peaked at 24 hours postinjury and lasted as long as 10 days before returning to baseline scores.36,38
Although performing force platform testing requires very little time, it is ordinarily administered by an otolaryngologist or an audiologist and usually requires a referral. Cost and limited accessibility make it impractical for obtaining baseline testing in entire teams or other large groups.
Compared with force platform testing, the Balance Error Scoring System (BESS)26 test has also been shown to produce reliable and valid assessments of postural stability.26,39 This low-cost alternative can be used in the clinic or on the sidelines to identify subtle impairments in postural stability, making baseline assessment for large groups feasible and helping clinicians safely return an injured athlete to play.
The BESS tests the athlete’s balance in three different positions, with eyes closed, on both firm and soft foam surfaces. Postural stability is determined by totaling the number of errors made during six 20-second trials (see Figure 2 and Table 3,26,39 page 21). Comparison studies have shown that athletes who experience mTBI have an increased number of errors on days 1 through 5 postinjury, compared with noninjured controls.36
DETERMINING RETURN TO PLAY
Despite the current emphasis on evidence-based practice and the possible consequences of premature participation in sports, the return-to-play decision often depends more on speculation than quantifiable data. The clinician is challenged to synthesize as much subjective and objective data as are available—patient-reported symptomatology, adjunctive assessment scores, and physical examination findings, and preferably with input from a management team that may include a certified athletic trainer, the team physician, a neurosurgeon, and a neuropsychiatrist.1,17
The student-athlete’s teachers should not be overlooked as observers of baseline classroom performance and possible dysfunction and impairment related to the injury. Parents and coaches may offer additional information, but the possibility of bias must be considered.
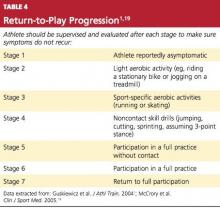
Only when the athlete reports that symptoms have fully resolved, it is generally agreed, should return to play even be considered.1,18,19 Then, provided that the athlete has regained at least baseline cognitive function and postural stability, a return-to-play progression can begin (see Table 41,19).
Athletes should be moved slowly through these stages, with ongoing supervision and reevaluation after each stage for possible recurrence of symptoms. On average, this progression takes three to five days.
CONCLUSION
No two mTBIs are alike. The mechanism of injury, the degree of neurologic dysfunction, and the time needed for recovery ultimately have no correlation. No athlete should return to play while mTBI symptoms persist, whether at rest or following exercise. If an athlete sustains an injury resulting in loss of consciousness (whatever the duration) or experiences amnesia, return to play that day should not be considered until further evaluation and/or neuroimaging can be performed.
It is imperative that everyone involved with student athletes, both medically and scholastically, be educated about the signs, symptoms, and management of mTBI to ensure a smooth return to school, sports, and other routine activities.
The CDC has begun to address this issue with several Heads Up tool kits for high school coaches, youth sports supervisors, and medical providers. These are available at www.cdc.gov/ncipc/tbi/TBI.htm.
REFERENCES
1. Guskiewicz KM, Bruce SL, Cantu RC, et al. National Athletic Trainers’ Association Position Statement: Management of Sport-Related Concussion. J Athl Train. 2004; 39(3):280-297.
2. Delaney JS, Abuzeyad F, Correa JA, Foxford R. Recognition and characteristics of concussions in the emergency department population. J Emerg Med. 2005;29(2): 189-197.
3. Kaut KP, DePompei R, Kerr J, Congeni J. Reports of head injury and symptom knowledge among college athletes: implications for assessment and educational intervention. Clin J Sport Med. 2003;13(4):213-221.
4. LaBotz M, Martin MR, Kimura IF, et al. A comparison of a preparticipation evaluation history form and a symptom-based concussion survey in the identification of previous head injury in collegiate athletes. Clin J Sport Med. 2005;15(2):73-78.
5. McCrea M, Hammeke T, Olsen G, et al. Unreported concussion in high school football players: implications for prevention. Clin J Sport Med. 2004;14(1):13-17.
6. Valovich McLeod TC, Bay RC, Heil J, McVeigh SD. Identification of sport and recreational activity concussion history through the preparticipation screening and a symptom survey in young athletes. Clin J Sport Med. 2008;18(3):235-240.
7. Williamson IJ, Goodman D. Converging evidence for the under-reporting of concussions in youth ice hockey. Br J Sports Med. 2006;40(2):128-132.
8. Bazarian JJ, Veenema T, Brayer AF, Lee E. Knowledge of concussion guidelines among practitioners caring for children. Clin Pediatr (Phila). 2001;40(4):207-212.
9. Genuardi FJ, King WD. Inappropriate discharge instructions for youth athletes hospitalized for concussion. Pediatrics. 1995;95(2):216-218.
10. Pleacher MD, Dexter WW. Concussion management by primary care providers. Br J Sports Med. 2006;40(1):e2.
11. Levinson DM, Reeves DL. Monitoring recovery from traumatic brain injury using automated neuropsychological assessment metrics (ANAM V1.0). Arch Clin Neuropsychol. 1997;12(2):155-166.
12. Cantu RC. Second-impact syndrome. Clin Sports Med. 1998;17(1):37-44.
13. McCrory PR, Berkovic SF. Second impact syndrome. Neurology. 1998;50(3):677-683.
14. Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil. 2006;21(5):375-378.
15. Gessel LM, Fields SK, Collins CL, et al. Concussions among United States high school and collegiate athletes. J Athl Train. 2007;42(4):495-503.
16. Guskiewicz KM, McCrea M, Marshall SW, et al. Cumulative effects associated with recurrent concussion in collegiate football players: the NCAA Concussion Study. JAMA. 2003;290(19):2549-2555.
17. Field M, Collins MW, Lovell MR, Maroon J. Does age play a role in recovery from sports-related concussion? A comparison of high school and collegiate athletes. J Pediatr. 2003;142(5):546-553.
18. Aubry M, Cantu RC, Dvorak J, et al. Summary and agreement statement of the First International Conference on Concussion in Sport, Vienna 2001: recommendations for the improvement of safety and health of athletes who may suffer concussive injuries. Br J Sports Med. 2002;36(1):6-10.
19. McCrory P, Johnston K, Meeuwisse W, et al. Summary and agreement statement of the 2nd International Conference on Concussion in Sport, Prague 2004. Clin J Sport Med. 2005;15(2):48-55.
20. Centers for Disease Control and Prevention. Nonfatal traumatic brain injuries from sports and recreation activities—United States, 2001-2005. MMWR Morb Mortal Wkly Rep. 2007;56(29):733-737.
21. Giza CC, Hovda DA. Ionic and metabolic consequences of concussion. In: Cantu RC, ed. Neurologic Athletic Head and Spine Injuries. Philadelphia, PA: WB Saunders Co; 2000:80-100.
22. Giza CC, Hovda DA. The neurometabolic cascade of concussion. J Athl Train. 2001;36(3):228-235.
23. Guskiewicz KM, Cantu RC. The concussion puzzle: evaluation of the sport-related concussion. Am J Med Sports. 2004;6:13-21.
24. McCrea M, Randolph C, Kelly JP. The Standardized Assessment of Concussion (SAC): Manual for Administration, Scoring and Interpretation. 2nd ed. Waukesha, WI: CNS Inc; 2000.
25. Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198.
26. Riemann BL, Guskiewicz KM. Effects of mild head injury on postural stability as measured through clinical balance testing. J Athl Train. 2000;35(1):19-25.
27. Borg J, Holm L, Cassidy JD, et al. Diagnostic procedures in mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med. 2004;43(43 Suppl):61-75.
28. Lovell MR, Pardini JE, Welling J, et al. Functional brain abnormalities are related to clinical recovery and time to return-to-play in athletes. Neurosurgery. 2007;61(2): 352-359.
29. Chan RC. Base rate of post-concussion symptoms among normal people and its neuropsychological correlates. Clin Rehabil. 2001;15(3):266-273.
30. Randolph C, McCrea M, Barr WB. Is neuropsychological testing useful in the management of sport-related concussion? J Athl Train. 2005;40(3):139-152.
31. Belanger HG, Vanderploeg RD. The neuropsychological impact of sports-related concussion: a meta-analysis. J Int Neuropsychol Soc. 2005;11(4):345-357.
32. Guskiewicz KM, Bruce SL, Cantu RC, et al; National Athletic Trainers’ Association. Research based recommendations on management of sport related concussion: summary of the National Athletic Trainers’ Association position statement. Br J Sports Med. 2006;40(1):6-10.
33. Collie A, Darby D, Maruff P. Computerised cognitive assessment of athletes with sports related head injury. Br J Sports Med. 2001;35(5):297-302.
34. Erlanger D, Saliba E, Barth J, et al. Monitoring resolution of postconcussion symptoms in athletes: preliminary results of a Web-based neuropsychological test protocol. J Athl Train. 2001;36(3):280-287.
35. Schatz P, Pardini JE, Lovell MR, et al. Sensitivity and specificity of the ImPACT Test Battery for concussion in athletes. Arch Clin Neuropsychol. 2006;21(1):91-99.
36. Guskiewicz KM, Ross SE, Marshall SW. Postural stability and neuropsychological deficits after concussion in collegiate athletes. J Athl Train. 2001;36(3):263-273.
37. Guskiewicz KM. Postural stability assessment following concussion: one piece of the puzzle. Clin J Sport Med. 2001;11(3):182-189.
38. Peterson CL, Ferrara MS, Mrazik M, et al. Evaluation of neuropsychological domain scores and postural stability following cerebral concussion in sports. Clin J Sport Med. 2003;13(4):230-237.
39. Riemann BL, Guskiewicz KM, Shields EW. Relationship between clinical and forceplate measure of postural stability. J Sport Rehabil. 1999;8:71-82.
For the highly motivated athlete, and often from a parent’s point of view, the return to play after a mild traumatic brain injury (mTBI, or concussion) may affect future scholarship and professional prospects, but it also carries the risk of further injury and permanent disability. Recognition of sport-related mTBI has been described as the most challenging aspect of managing this particular injury.1 Research has shown that patients, athletes,2-7 and health care providers8-10 all lack knowledge regarding some aspect of mTBI, and appropriate education is crucial.
Management of the athlete with mTBI requires both acute and follow-up care, using assessment tools found to be sensitive to detect deficits in cognition, balance, and coordination.
CASE STUDY
An 18-year-old high school football player was tackled during a Saturday afternoon game; on the previous play, he had run 80 yards following an interception. The tackle caused both his ear pads and his chinstrap to break, but he did not lose consciousness. Within two minutes, he was evaluated on the sidelines by the team’s physician assistant and its certified athletic trainer, during which he became nauseated and vomited several times. The player also complained of a new-onset headache and some dizziness. Three weeks earlier, he had been diagnosed with an mTBI; he recovered fully and was medically cleared to play one week later.
On the sidelines immediately after the current injury, the athlete underwent a neurologic examination that yielded no focal neurologic findings. He was transported to the local emergency department (ED) because of the headache and vomiting. The ED provider made a diagnosis of “forehead contusion” and told the patient that he “did not have a brain injury since there was no loss of consciousness.” CT was not ordered, and the athlete was prescribed ibuprofen for his headache.
The following Monday, the athlete was reevaluated by the team PA and the PA’s supervising physician. The athlete reported some residual headache but said the dizziness, nausea, and vomiting had resolved shortly after the injury. His neurologic exam was unremarkable, and although no baseline data were available, results from the Automated Neuropsychological Assessment Metrics (ANAM)11 computerized test demonstrated deficits in reaction time, problem solving, and short-term memory, in comparison with age-matched individuals. CT with contrast performed at that time was negative for hematoma or intracranial swelling.
The athlete was diagnosed with a resolving mTBI and postconcussion syndrome. The consensus was that the vomiting was most likely not a result of the head injury but rather was triggered by the physical exertion of having sprinted 80 yards on the previous play. He was restricted from any exercise and all contact sports until he was asymptomatic, both at rest and during physical activity. The athlete and his mother were informed about the risks of second-impact syndrome.12,13 Follow-up ANAM testing was suggested, but the patient did not return to the office for the test.
mTBI IN THE YOUNGER PATIENT
This case is not an isolated occurrence. In the United States, annual estimates of sport-related traumatic brain injuries, predominantly concussions, range from 1.6 million to 3.8 million.14 According to recent data from injury surveillance systems, concussions sustained by high school athletes represent a greater proportion of sport-related injuries (8.9%) than do those among college athletes (5.8%). Female athletes sustain sport-related mTBI and associated injuries at a higher rate and proportion than do males participating in the same sports.15
Sustaining a sport-related mTBI at an early age is of particular concern: The brain is still developing, and younger patients have an enhanced potential for cumulative effects and prolonged cognitive deficits. Athletes with even one previous mTBI are at increased risk for future mTBI (adjusted risk ratio, 1.4, compared with athletes who have never sustained such an injury).16 Of even greater concern, high school athletes tend to experience delays in cognitive and symptomatic recovery following a concussive injury.17
A growing body of literature has demonstrated difficulties in recognizing and managing mTBIs at all levels of play and within various patient populations.1,18,19 One survey of mTBI evaluation in primary care settings revealed that only 33% of practitioners responsible for sideline coverage used a standard, objective protocol, and an additional 31% used no mTBI guidelines. Among the latter, 71% cited a lack of knowledge, and 16% said they found existing guidelines confusing.10
Another study revealed that hospital discharge instructions for children sustaining an athletic mTBI were inadequate in 69.7% of cases.9 Among these patients, 13% were instructed to return to activity too soon, and 87% were given no instructions at all. The need to better educate athletes, parents, coaches, and health care professionals about the potential seriousness of sport-related mTBI and safe return to the playing field is clear.1,20
This discussion will address new advances in mTBI management and return-to-play decisions for adolescent athletes in the primary care setting.
TERMINOLOGY AND PATHOPHYSIOLOGY
The terminology associated with mTBI has been evolving along with an enhanced understanding of its pathology and etiology. Since more than 90% of sport-related traumatic brain injuries (TBIs) are considered “mild,” the term mTBI is often being used in place of concussion.14 Considering the long-lasting effects of more severe TBI, the contemporary term mTBI more accurately portrays the seriousness of even a seemingly minor injury, often minimized as a “ding” or “bell ringer.” These lay terms do not convey the magnitude and extent of injury sustained and are often thought by nonmedical persons to refer to a different, unrelated injury.6
Since the Concussion in Sport Group18 first met in 2001, several features of sport-related mTBI have been described in an effort to clarify its definition. These include an injury that
(1) is caused by a direct blow to the head or an indirect blow elsewhere in the body that transmits an impulsive force to the head
(2) may cause an immediate and short-lived alteration in neurologic function
(3) may cause neuropathologic changes but typically reflects a functional disturbance rather than a structural injury
(4) is represented by a gradient of clinical symptoms that often resolve sequentially, often without loss of consciousness
(5) is predominantly associated with negative findings on conventional neuroimaging studies (eg, CT, MRI).18
When an athlete sustains an impact to the head, external forces create accelerations and decelerations of the brain within the skull. These forces create the classic coup-contrecoup injury,1 in which the brain impacts the skull at the initial point of contact, with a second point of injury on the directly opposite side of the brain. In some cases, rotational forces occur when the skull is impacted in such a way that the brain rotates about its axis, causing shear and stretch forces on the brain tissue. Either mechanism of injury can trigger a chain of metabolic events in the brain that result in decreased blood flow, increased glucose utilization, and neurotransmitter dysfunction. All of these are thought to contribute to the transient neurologic deficits associated with mTBI.21,22
If a patient who is recovering from mTBI sustains a second head injury before metabolic changes caused by the first injury have fully resolved, a second-impact syndrome (SIS)12,13,16 can develop (see Figure 112,13,16 ). SIS results in massive, rapid brain edema, causing increased intracranial pressure and eventual brain herniation. The majority of cases of SIS are reported in patients younger than 18 and are thought to be the result of altered autoregulation of cerebral blood flow.12 In pediatric athletes, therefore, the proper recognition of mTBI, its effective management, and the return-to-play determination are crucial to decrease the risk of SIS.
INITIAL EVALUATION
The clinical course and management of mTBI can be separated into two distinct components.23 The first is the initial or acute evaluation, which should occur as close to the time of injury as possible. Evaluation of an acute mTBI centers on the history and physical examination at the time of injury; its focus is to determine whether a neurosurgical emergency exists and what course of treatment is needed. Tools such as the Standard Assessment of Concussion (SAC)24 and Folstein’s Mini–Mental State Examination (MMSE)25 should be used to assess the extent of initial cognitive impairment, while other tools, such as the Balance Error Scoring System26 test for motor impairment, should be combined with the neurologic physical exam to formulate the differential diagnosis.
No two brain injuries are alike. The clinician must key into the mechanism of injury, initial and current symptoms (including headache, confusion, and amnesia),16 and positive and negative neurologic findings. Differentials that must be ruled out immediately include skull fracture, cerebral contusion, and epidural hematomas.
Along with the physical findings associated with mTBI, those suggesting more severe injury may include acute localized swelling, deformity, prolonged loss of consciousness, intractable vomiting, and often multiple positive neurologic exam findings, such as cranial nerve abnormalities and motor weakness. Any one of these findings in the initial evaluation warrants activation of emergency medical services, including immediate transport to an ED equipped to manage a neurosurgical emergency.
Since 1974, at least 25 different scales have been used to help practitioners evaluating mTBI assign a grade of severity.1 Although grading scales can be helpful to objectify subjective symptoms, they vary considerably, and none has been shown valid, reliable, or sensitive through published research. Furthermore, an assigned grade cannot reliably express the severity of injury or the prognosis for recovery in each case.1,17
DIAGNOSTIC IMAGING
In mTBI management, the sole purpose of diagnostic imaging is to rule out a more severe structural injury, such as intracranial hemorrhage or hematoma. Symptoms commonly associated with mTBI, such as nausea, vomiting, headache, and visual disturbances, are also cardinal signs of a mass effect resulting from both subdural and epidural hematomas. When these symptoms occur in the acute phase of mTBI management, immediate CT without contrast is imperative to rule out skull fracture and intracranial hemorrhage. Note: Negative imaging test results in the presence of mTBI symptoms do not rule out mTBI, a functional injury; they only confirm that no structural pathology exists.1
Clinicians must also be aware that because a subdural hematoma accumulates slowly, positive findings may not be evident on CT or MRI for seven to 14 days after the initial injury. Thus, the sudden return or worsening of mTBI symptoms that were previously resolved or stable warrants evaluation for a slower (and more commonly fatal) chronic subdural hematoma.1 In this emergent case, CT without IV contrast should be performed first to rule out acute hemorrhage or any mass effect. However, because of the lysing effect of clotted blood, CT with IV contrast or MRI is needed to definitively determine dural and gray-matter reactions to an occult bleed.27
At this time, there is no gold standard among imaging techniques to capture the functional disturbances often noted with sport-related mTBI. However, in one recent study of functional MRI (fMRI) use following sport-related mTBI, athletes with evidence of hyperactivation on their initial post-mTBI scan took longer to recover, based on symptom presentation and neurocognitive testing.28 Though currently used for research purposes, fMRI appears to demonstrate measurable metabolic changes in the brain even after symptoms have been resolved. This promising modality may soon provide helpful neurophysiologic data for the clinical assessment and management of sport-related mTBI.
SYMPTOM SURVEILLANCE
The second component of clinical management of mTBI is the follow-up and surveillance of symptoms and neurologic limitations over time. This is essential for making clinical decisions, including the appropriate time to return the athlete safely to sports or work, so as to avoid further injury.
While the acute component of mTBI management relies on the objective nature of physical examination and neuroimaging, follow-up and surveillance are heavily dependent on the subjective symptoms (see Table 11,18), particularly when physical examination and neuroimaging findings are unremarkable.27,29 Therefore, sensitive and specific clinical tools are needed to accurately assess the various elements of cognition and psychological functioning that are most commonly impaired by mTBI.30
COGNITIVE ASSESSMENT
The metabolic changes that occur after cerebral injury have been shown to cause temporary deficits in normal cognition.23,31 Within the first 24 hours of injury, mild to moderate cognitive impairment is noted across all domains, with the greatest deficits occurring in global functioning, memory acquisition, and delayed memory. Deficits in these areas have been shown to resolve within seven to 10 days following the initial injury.31 It is helpful for these cognitive domains to have been clinically evaluated before injury (baseline), as postinjury evaluation can more effectively detect the extent of debilitation caused by mTBI; subsequent reevaluations can be used to monitor the rate of recovery.
Neuropsychological testing has been studied extensively to determine its value and use in the assessment of mTBI.18,32 Currently, two types of testing are used. Traditional neuropsychological testing comprises a battery of pencil-and-paper exercises administered and interpreted by a psychologist to evaluate cognition and identify areas of deficit following mTBI.33 Although these tests produce a wealth of data, they are expensive, they may require a referral, and administering them can take longer than four hours.
The newest form of neuropsychological testing involves computer-based protocols. Though not yet fully validated, these tools require less time to administer than traditional testing and are commonly used in the sports medicine community (see Table 21,11,33-35 ). Computer-based testing, which may be conducted in the school’s computer lab, has the potential to make preseason baseline testing feasible for large numbers of athletes. Other advantages are ease of administration, a time requirement of about 30 minutes, and the availability of multiple versions to control for the effects of practice.1,23
Two approaches have been suggested for effective use of neuropsychological testing in both components of mTBI management1,30:
First, perform baseline testing at the start of the athletic season, before exposure to injury (possibly within the preparticipation physical), then retest the injured athlete once he or she reports being asymptomatic. Return to play may be considered once the injured athlete scores at or above baseline testing.
Second, perform baseline testing at the start of the season, then retest the injured athlete at set time intervals, charting improvement and rate of recovery. This serial assessment can provide a patient recovery curve for the clinician.30
Results from any neuropsychological testing protocol may be more valuable to the trained practitioner (usually a neurophysiologist, although developers of computer-based testing offer training and credentialing) than are subjective symptoms alone. However, clinicians must use these results as only one variable in the return-to-play decision.23 Cognitive testing is most reliable when baseline testing is included, but this may not be feasible in all settings. If baseline data are not available, normative data for the population of interest (eg, high school level) may be used; these are often available from the manufacturer of the computerized cognitive testing platform.
MOTOR CONTROL ASSESSMENT
Immediately following mTBI, a subtle yet significant degree of motor impairment may develop, often lasting beyond the acute phase of injury. This impairment may affect proprioception, fine and gross motor control, reaction time, and postural stability (balance), all of which are necessary and vital components of athletics. Any impairment in motor control will not only have a negative effect on athletic performance; it will undoubtedly increase the likelihood of a second and possibly more severe injury.
In the neurologic physical examination, results from the tests that are traditionally used to assess motor pathways and coordination have been subject to the individual clinician’s skill and interpretation.26 Several new tests have been developed to assess postural stability more objectively, producing data that give the clinician insight into the brain’s ability to organize incoming sensory information and respond appropriately to environmental changes.36 The gold standard for evaluating postural stability has been the force platform system, which measures vertical ground reaction forces as the body’s center of mass moves around a fixed base of support.26,37
This tool detects even minute alterations in the athlete’s postural stability as stress is exerted on the visual, vestibular, and somatosensory modalities; it can quantify the accuracy and timing of the subject’s motor responses. In recent studies of athletes with mTBI, vestibular and visual alterations caused a deficit in mean stability that peaked at 24 hours postinjury and lasted as long as 10 days before returning to baseline scores.36,38
Although performing force platform testing requires very little time, it is ordinarily administered by an otolaryngologist or an audiologist and usually requires a referral. Cost and limited accessibility make it impractical for obtaining baseline testing in entire teams or other large groups.
Compared with force platform testing, the Balance Error Scoring System (BESS)26 test has also been shown to produce reliable and valid assessments of postural stability.26,39 This low-cost alternative can be used in the clinic or on the sidelines to identify subtle impairments in postural stability, making baseline assessment for large groups feasible and helping clinicians safely return an injured athlete to play.
The BESS tests the athlete’s balance in three different positions, with eyes closed, on both firm and soft foam surfaces. Postural stability is determined by totaling the number of errors made during six 20-second trials (see Figure 2 and Table 3,26,39 page 21). Comparison studies have shown that athletes who experience mTBI have an increased number of errors on days 1 through 5 postinjury, compared with noninjured controls.36
DETERMINING RETURN TO PLAY
Despite the current emphasis on evidence-based practice and the possible consequences of premature participation in sports, the return-to-play decision often depends more on speculation than quantifiable data. The clinician is challenged to synthesize as much subjective and objective data as are available—patient-reported symptomatology, adjunctive assessment scores, and physical examination findings, and preferably with input from a management team that may include a certified athletic trainer, the team physician, a neurosurgeon, and a neuropsychiatrist.1,17
The student-athlete’s teachers should not be overlooked as observers of baseline classroom performance and possible dysfunction and impairment related to the injury. Parents and coaches may offer additional information, but the possibility of bias must be considered.
Only when the athlete reports that symptoms have fully resolved, it is generally agreed, should return to play even be considered.1,18,19 Then, provided that the athlete has regained at least baseline cognitive function and postural stability, a return-to-play progression can begin (see Table 41,19).
Athletes should be moved slowly through these stages, with ongoing supervision and reevaluation after each stage for possible recurrence of symptoms. On average, this progression takes three to five days.
CONCLUSION
No two mTBIs are alike. The mechanism of injury, the degree of neurologic dysfunction, and the time needed for recovery ultimately have no correlation. No athlete should return to play while mTBI symptoms persist, whether at rest or following exercise. If an athlete sustains an injury resulting in loss of consciousness (whatever the duration) or experiences amnesia, return to play that day should not be considered until further evaluation and/or neuroimaging can be performed.
It is imperative that everyone involved with student athletes, both medically and scholastically, be educated about the signs, symptoms, and management of mTBI to ensure a smooth return to school, sports, and other routine activities.
The CDC has begun to address this issue with several Heads Up tool kits for high school coaches, youth sports supervisors, and medical providers. These are available at www.cdc.gov/ncipc/tbi/TBI.htm.
REFERENCES
1. Guskiewicz KM, Bruce SL, Cantu RC, et al. National Athletic Trainers’ Association Position Statement: Management of Sport-Related Concussion. J Athl Train. 2004; 39(3):280-297.
2. Delaney JS, Abuzeyad F, Correa JA, Foxford R. Recognition and characteristics of concussions in the emergency department population. J Emerg Med. 2005;29(2): 189-197.
3. Kaut KP, DePompei R, Kerr J, Congeni J. Reports of head injury and symptom knowledge among college athletes: implications for assessment and educational intervention. Clin J Sport Med. 2003;13(4):213-221.
4. LaBotz M, Martin MR, Kimura IF, et al. A comparison of a preparticipation evaluation history form and a symptom-based concussion survey in the identification of previous head injury in collegiate athletes. Clin J Sport Med. 2005;15(2):73-78.
5. McCrea M, Hammeke T, Olsen G, et al. Unreported concussion in high school football players: implications for prevention. Clin J Sport Med. 2004;14(1):13-17.
6. Valovich McLeod TC, Bay RC, Heil J, McVeigh SD. Identification of sport and recreational activity concussion history through the preparticipation screening and a symptom survey in young athletes. Clin J Sport Med. 2008;18(3):235-240.
7. Williamson IJ, Goodman D. Converging evidence for the under-reporting of concussions in youth ice hockey. Br J Sports Med. 2006;40(2):128-132.
8. Bazarian JJ, Veenema T, Brayer AF, Lee E. Knowledge of concussion guidelines among practitioners caring for children. Clin Pediatr (Phila). 2001;40(4):207-212.
9. Genuardi FJ, King WD. Inappropriate discharge instructions for youth athletes hospitalized for concussion. Pediatrics. 1995;95(2):216-218.
10. Pleacher MD, Dexter WW. Concussion management by primary care providers. Br J Sports Med. 2006;40(1):e2.
11. Levinson DM, Reeves DL. Monitoring recovery from traumatic brain injury using automated neuropsychological assessment metrics (ANAM V1.0). Arch Clin Neuropsychol. 1997;12(2):155-166.
12. Cantu RC. Second-impact syndrome. Clin Sports Med. 1998;17(1):37-44.
13. McCrory PR, Berkovic SF. Second impact syndrome. Neurology. 1998;50(3):677-683.
14. Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil. 2006;21(5):375-378.
15. Gessel LM, Fields SK, Collins CL, et al. Concussions among United States high school and collegiate athletes. J Athl Train. 2007;42(4):495-503.
16. Guskiewicz KM, McCrea M, Marshall SW, et al. Cumulative effects associated with recurrent concussion in collegiate football players: the NCAA Concussion Study. JAMA. 2003;290(19):2549-2555.
17. Field M, Collins MW, Lovell MR, Maroon J. Does age play a role in recovery from sports-related concussion? A comparison of high school and collegiate athletes. J Pediatr. 2003;142(5):546-553.
18. Aubry M, Cantu RC, Dvorak J, et al. Summary and agreement statement of the First International Conference on Concussion in Sport, Vienna 2001: recommendations for the improvement of safety and health of athletes who may suffer concussive injuries. Br J Sports Med. 2002;36(1):6-10.
19. McCrory P, Johnston K, Meeuwisse W, et al. Summary and agreement statement of the 2nd International Conference on Concussion in Sport, Prague 2004. Clin J Sport Med. 2005;15(2):48-55.
20. Centers for Disease Control and Prevention. Nonfatal traumatic brain injuries from sports and recreation activities—United States, 2001-2005. MMWR Morb Mortal Wkly Rep. 2007;56(29):733-737.
21. Giza CC, Hovda DA. Ionic and metabolic consequences of concussion. In: Cantu RC, ed. Neurologic Athletic Head and Spine Injuries. Philadelphia, PA: WB Saunders Co; 2000:80-100.
22. Giza CC, Hovda DA. The neurometabolic cascade of concussion. J Athl Train. 2001;36(3):228-235.
23. Guskiewicz KM, Cantu RC. The concussion puzzle: evaluation of the sport-related concussion. Am J Med Sports. 2004;6:13-21.
24. McCrea M, Randolph C, Kelly JP. The Standardized Assessment of Concussion (SAC): Manual for Administration, Scoring and Interpretation. 2nd ed. Waukesha, WI: CNS Inc; 2000.
25. Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198.
26. Riemann BL, Guskiewicz KM. Effects of mild head injury on postural stability as measured through clinical balance testing. J Athl Train. 2000;35(1):19-25.
27. Borg J, Holm L, Cassidy JD, et al. Diagnostic procedures in mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med. 2004;43(43 Suppl):61-75.
28. Lovell MR, Pardini JE, Welling J, et al. Functional brain abnormalities are related to clinical recovery and time to return-to-play in athletes. Neurosurgery. 2007;61(2): 352-359.
29. Chan RC. Base rate of post-concussion symptoms among normal people and its neuropsychological correlates. Clin Rehabil. 2001;15(3):266-273.
30. Randolph C, McCrea M, Barr WB. Is neuropsychological testing useful in the management of sport-related concussion? J Athl Train. 2005;40(3):139-152.
31. Belanger HG, Vanderploeg RD. The neuropsychological impact of sports-related concussion: a meta-analysis. J Int Neuropsychol Soc. 2005;11(4):345-357.
32. Guskiewicz KM, Bruce SL, Cantu RC, et al; National Athletic Trainers’ Association. Research based recommendations on management of sport related concussion: summary of the National Athletic Trainers’ Association position statement. Br J Sports Med. 2006;40(1):6-10.
33. Collie A, Darby D, Maruff P. Computerised cognitive assessment of athletes with sports related head injury. Br J Sports Med. 2001;35(5):297-302.
34. Erlanger D, Saliba E, Barth J, et al. Monitoring resolution of postconcussion symptoms in athletes: preliminary results of a Web-based neuropsychological test protocol. J Athl Train. 2001;36(3):280-287.
35. Schatz P, Pardini JE, Lovell MR, et al. Sensitivity and specificity of the ImPACT Test Battery for concussion in athletes. Arch Clin Neuropsychol. 2006;21(1):91-99.
36. Guskiewicz KM, Ross SE, Marshall SW. Postural stability and neuropsychological deficits after concussion in collegiate athletes. J Athl Train. 2001;36(3):263-273.
37. Guskiewicz KM. Postural stability assessment following concussion: one piece of the puzzle. Clin J Sport Med. 2001;11(3):182-189.
38. Peterson CL, Ferrara MS, Mrazik M, et al. Evaluation of neuropsychological domain scores and postural stability following cerebral concussion in sports. Clin J Sport Med. 2003;13(4):230-237.
39. Riemann BL, Guskiewicz KM, Shields EW. Relationship between clinical and forceplate measure of postural stability. J Sport Rehabil. 1999;8:71-82.