User login
CASE
Mr L., a 57-year-old obese patient (BMI > 40) who had not been to a clinician in a decade, comes to see you after a health fair screening revealed dyslipidemia (LDL cholesterol, 188 mg/dL; HDL cholesterol, 32 mg/dL; total cholesterol, 240 mg/dL; triglycerides, 100 mg/dL). His blood pressure (BP) is 146/90 mm Hg, and his fasting glucose is 101 mg/dL. Labs drawn that day reveal an A1C of 5.9%, alanine aminotransferase (ALT) of 45 U/L, and aspartate aminotransferase (AST) of 62 U/L. In taking his history, you discover that Mr L. also has a notable family history of heart disease.
Mr L. agrees to take a low-dose statin, and you prescribe atorvastatin 10 mg and a thiazide diuretic. You advise the patient to contact you immediately if he develops significant myalgia, jaundice, dark urine, or symptoms of hyperglycemia such as excessive thirst or urination, and to schedule a follow-up visit in eight weeks.
Long recognized as the bedrock of hyperlipidemia therapy, statins achieved even greater prominence when the American College of Cardiology/American Heart Association (ACC/AHA) issued a new cholesterol guideline1 late last year. The ACC and AHA now recommend statins for a wider range of patients, often at a higher starting dose.
Based on the new recommendations, the use of statins is likely to rise.2 (A statin—rosuvastatin—is already the nation’s most widely prescribed medication.2) Thus, it is more important than ever for clinicians to know about the risks associated with statins and to be able to assess the benefits of therapy for individual patients.
A 2013 retrospective cohort study of more than 100,000 patients on statins found that 17% developed adverse effects (AEs). Therapy was withheld, at least temporarily, for 10% of study participants (60% of those experiencing AEs).3 At the same time, the authors of a large meta-analysis (135 randomized controlled trials [RCTs] and > 240,000 patients) reported that AEs associated with statins as a class were uncommon. The meta-analysis also found that the overall discontinuation rate for statin users—5.7%—was not significantly different from that of patients receiving placebo.4
Such discrepancies regarding particular risks, as well as the overall incidence of AEs and discontinuation rates, make the evidence difficult to sort out. We created this update with that in mind.
Continue for symptoms >>
MUSCULOSKELETAL SYMPTOMS ARE MOST COMMON
Musculoskeletal symptoms are the most common AEs reported by patients who are taking statins.5 These range from muscle weakness, fatigue, and pain to (rarely) rhabdomyolysis—a life-threatening condition characterized by severe muscle pain, muscle weakness, a 10-fold increase in creatine kinase (CK), and increased serum creatinine, often with myoglobinuria.5
Patients with myopathy—an umbrella term for any muscle disease—may report stiffness, weakness, tenderness, soreness, cramping, or heaviness. Symptoms are usually symmetrical and often involve the proximal limbs and trunk.6 Studies indicate that exercise increases the risk for statin-induced myalgia—muscle pain or weakness without an increase in CK—and that patients taking statins are more prone to exercise-related injury.7,8
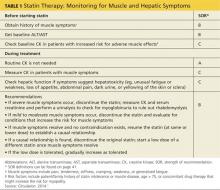
A baseline CK is recommended for patients with an increased risk for muscular disorders.1 Risk factors include a personal or family history of statin intolerance or muscle disease, age older than 75, low levels of vitamin D, and concomitant use of medications that may increase the risk for myopathy (see Table 1).1 Routine monitoring of CK is not recommended, but CK levels should be obtained for those who exhibit muscle symptoms while on statin therapy.1
What the studies show
The incidence of myalgia reported in clinical studies is highly variable, ranging from less than 1% to 20%.1,9,10 The ACC/AHA guideline reports only one additional case of myopathy per 10,000 statin users compared with those on placebo and cites a rhabdomyolysis occurrence rate of less than 0.06% over five years.1
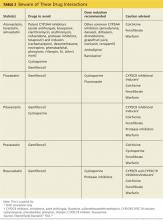
A 2006 systematic review estimated the absolute risk for rhabdomyolysis to be 3.4 per 100,000 person-years, but the incidence was 10 times higher for patients taking both a statin and gemfibrozil.11 (See Table 212,13 for more on drug interactions.) But both the meta-analysis cited earlier4 and a previous systematic review14 (35 RCTs and > 74,000 patients) found that statins as a class do not increase the incidence of myalgia or rhabdomyolysis.
Differences in the way muscular disorders are defined has been suggested as one reason for the discrepancies.10 In addition, many clinical trials exclude patients at higher risk for statin-associated AEs, such as those with renal or hepatic insufficiency, prior muscular complaints, poorly controlled diabetes, or potential drug interactions.1
An FDA advisory. In a safety communication last updated in February 2012, the FDA cautioned against starting patients on the highest dose of simvastatin (80 mg).15 The warning is based on a large study (N = 12,064) that found an increased risk for myopathy (0.9%) and rhabdomyolysis (0.2%) in patients on the
80-mg dose versus those taking 20 mg (0.02% and 0%, respectively).16
With the ACC/AHA now recommending intensive therapy (atorvastatin 40-80 mg or rosuvastatin 20-40 mg) to achieve an LDL reduction greater than 50% for many patients,1 it is important to be aware that this risk is specific to simvastatin. A recent meta-analysis of studies directly comparing patients receiving intensive statin therapy with those on low to moderate doses did not find any increased risk in rhabdomyolysis associated with more intensive therapy when those taking 80-mg simvastatin were excluded.17
The bottom line: Although rhabdomyolysis is rare, its severity—a fatality rate of 10%11—makes it critical to educate patients about the disorder and instruct them to stop taking the statin and call the office immediately if they develop severe muscle pain or weakness.
Recommend CoQ10 for statin-induced myopathy
Although the exact mechanism of statin-induced myopathy is unknown, the most likely explanation is a depletion of coenzyme Q10 (CoQ10), which has negative effects on mitochondrial energy production.18 While studies using CoQ10 to treat this AE have been small and had mixed results, the overall evidence suggests that it decreases the development and/or severity of symptoms.18-20
In fact, CoQ10 supplementation is the only treatment that has shown promise in treating statin-induced muscle symptoms.18-20 Doses of about 100 mg bid have been found to be beneficial and safe; no clinically relevant AEs have been seen with doses lower than 300 mg/d.18,20,21 A large placebo-controlled study is currently evaluating a 600 mg/d dose of CoQ10 in patients with statin-induced myopathy.19
CASE
On his next visit, Mr L. reports a new ache in his left shoulder and upper back, which he describes as mild but annoying. He also tells you his memory seems to be getting worse and that he has developed an odd tingling in his hands. These symptoms began about a month after he started the medications, Mr L. says. He also began a new exercise program, but his BMI is unchanged.
On examination, you find the affected shoulder and upper back modestly and diffusely tender to palpation but with no decline in strength. Mr L.’s BP has fallen to
134/84 mm Hg, and his fasting glucose is 105 mg/dL. Lab tests reveal an LDL of 144 mg/dL and HDL of 36 mg/dL, A1C of 6.1%, ALT of 105 U/L, AST of 61 U/L, and a normal CK.
You recommend 100 mg CoQ10 bid. Because it is available only OTC, you advise the patient to look for a product whose purity and potency have been verified by an external source, such as the US Pharmacopeial Convention. You also prescribe metformin 500 mg bid for insulin resistance, refer the patient to a nutritionist and diabetes specialist, and order tests to evaluate his other symptoms.
Continue for hepatic effects >>
HEPATIC EFFECTS ARE RARE
Historically, statins have been linked to potential hepatotoxicity, with case reports of serum transaminase elevation, cholestasis, hepatitis, and acute liver failure. It is now recognized that hepatic AEs are rare and that statins are not associated with a risk for acute or chronic liver failure.1,11 In patients with coronary heart disease, the incidence of hepatotoxicity with statin use is reported to be less than 1.5% over the course of five years and appears to be dose-dependent.1
In 2012, the FDA revised the labeling for most statins, relaxing its earlier recommendations for monitoring of liver function, clarifying the risk for myopathy, and providing additional information about drug interactions.13
Checking transaminase levels before initiating therapy is recommended by both the ACC/AHA and the FDA.1,13 Routine monitoring is not necessary, the ACC/AHA guideline states, because RCTs have found little evidence of ALT/AST elevation.1 But here, too, evidence varies. An older meta-analysis (13 trials and nearly 50,000 participants) concluded that as a class, statins have no greater risk for transaminase elevations than placebo.22 But the 135-RCT meta-analysis4 found otherwise: Statins did increase the risk for transaminase elevation (odds ratio [OR], 1.51) compared with placebo, with differences associated with particular drugs and higher doses associated with more clinically significant elevations.4 It is important to note, however, that there was significant heterogeneity among the studies and no consistent definition of clinical significance.
The bottom line: Statins have been shown in multiple prospective studies to be safe for patients with chronic liver disease.22,23
STATIN USE AND DIABETES: IS THERE A LINK?
Recent studies have found an increased risk for new-onset type 2 diabetes in statin users, with a greater risk associated with higher-potency statins, including rosuvastatin and atorvastatin.4,24 Although the exact mechanism is not known, statins may modify insulin signaling in peripheral tissues or directly impair insulin secretion.
The ACC/AHA guideline reports an excess rate of diabetes of one per 1,000 patient-years for moderate-intensity therapy and three per 1,000 patient-years for high-intensity therapy.1 The 2013 meta-analysis found that the elevated risk for diabetes was relatively small (OR, 1.09).4 No difference among various statins was found.
In another meta-analysis—this one encompassing 17 RCTs and more than 110,000 patients—no statistically significant difference in the incidence of new-onset diabetes was seen based on either the specific statin being taken or the intensity of therapy (high vs moderate).24
The bottom line: Clinicians should monitor patients taking statins for signs and symptoms of hyperglycemia.
STATINS MAY BE RENOPROTECTIVE
Statin use has been found to be associated with an increased risk for tubular proteinuria—an effect that is both dose- and potency-dependent.25 Nonetheless, it has been suggested that statins may be a rare example of a drug class that is renoprotective in the long term, despite having an increased rate of proteinuria in the short term.25
The evidence? In prospective studies, statin therapy has been shown to slow the progression of kidney disease in diverse patient populations, including renal transplant recipients and those with chronic kidney disease (CKD).26,27
The Kidney Expert Panel of the National Lipid Association (NLA) has concluded that statins do not appear to cause significant proteinuria or acute kidney injury. The panel does not recommend routine monitoring for proteinuria or kidney function in statin users unless otherwise indicated but does recommend a lower dose for patients with CKD.28
The bottom line: Kidney Disease Improving Global Outcomes guidelines recommend that patients who have CKD, but are not on dialysis, be treated with statin therapy. Statins are contraindicated for patients on dialysis, as clinical trials have failed to show significant cardiovascular benefit.29
Continue for the risk of intracerebral hemorrhages >>
INTRACEREBRAL HEMORRHAGE: STATINS INCREASE RECURRENCE RISK
In recent years, there has been considerable concern about a statin-induced increased risk for intracerebral hemorrhage (ICH). In a major prospective study in which patients were put on high-dose statin therapy or placebo after an acute ischemic or hemorrhagic stroke, the overall incidence of a recurrent stroke was significantly lower in the statin group.30 Among those who’d had an ICH, however, the recurrence rate was 73% higher for patients taking statins.
A subanalysis that looked only at patients who’d had a hemorrhagic stroke as their initial event (n = 93) found that the absolute risk for recurrent ICH was 15.6% for patients randomized to atorvastatin versus 4.2% for those on placebo.31 Despite being based on a small subset of the original study group, multivariate analysis indicated the increased risk was statistically significant (hazard ratio [HR], 1.69).
A subsequent decision analysis study based on these results proposed that patients with a history of spontaneous deep ICH would need an exceedingly high 10-year cardiovascular event risk (> 40%) for the benefits of statin therapy to outweigh the risk.32 The risk is particularly high for those with a history of lobar ICH, which has an extremely high recurrence rate. However, subsequent retrospective and observational studies have found that patients who were already on statins when the ICH occurred had less severe strokes and more favorable outcomes, with a lower mortality rate at 90 days post-ICH.33-35
A 2010 ICH guideline from the AHA/American Stroke Association states that there is “insufficient data to recommend restrictions on use of statin agents” for patients who have had an ICH.36
The bottom line: Clinicians should carefully evaluate the anticipated cardiovascular risk for patients who have had a hemorrhagic stroke to determine whether statin therapy would be beneficial.
OTHER SERIOUS ADVERSE EFFECTS: WHICH REPORTS ARE ACCURATE?
Statin use has been associated with a number of other serious AEs. Some reports appear to be accurate; others do not hold up after a close look at the evidence.
Malignancy. A potential link between statins and an increased risk for malignancy has been considered for years. A large trial (N = 5,804) from 2002 found a correlation between pravastatin and an increased risk for new cancer diagnoses compared with placebo (HR, 1.25).37 But a 10-year follow-up did not substantiate this finding, and it is now believed that the original result may have been due to chance.38 Numerous other meta-analyses and systematic reviews have found no link between statin use and malignancy.39-41
Cataracts. Potential ocular effects have been widely studied and debated in recent years. Observational studies reporting an association between statin use and cataracts have had conflicting results, with some showing statins as protective42-45 and others finding an increased risk.46,47 However, a recent propensity-score matched analysis found that statin users do indeed have an increased risk for cataracts.48 The authors concluded that for primary prevention, the risk-benefit equation for statin use should include this added risk.48
In addition, a review of the databases of the National Registry of Drug-Induced Ocular Side Effects, the World Health Organization, and the FDA from 1987 to 2008 indicates that statin therapy may also cause diplopia, ptosis, and ophthalmoplegia.49
Peripheral neuropathy. Despite case reports of statin-induced peripheral neuropathy, the NLA’s Neurology Expert Panel states that statins do not appear to cause this condition. If a patient receiving statin therapy develops peripheral neuropathy, a full work-up for other causes should be initiated before modification of statin therapy is considered, the panel advises.28
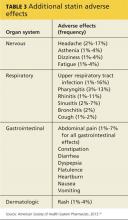
Statins have also been linked to headache and dizziness, respiratory symptoms, gastrointestinal problems, and rash (see Table 3).50
WHICH DRUG? POTENTIAL DIFFERENCES IN STATINS
A meta-analysis with more than 240,000 participants evaluated patients taking seven different statins (atorvastatin, fluvastatin, lovastatin, pravastatin, pitavastatin, rosuvastatin, and simvastatin), looking at AEs of the drugs both collectively and individually.4 As noted earlier, the overall discontinuation rate due to AEs for all statins was 5.7%. Discontinuation rates for each agent were not reported.4
The researchers did report, however, that atorvastatin and rosuvastatin had the highest discontinuation rates; atorvastatin and fluvastatin had the highest incidence of transaminase elevations (OR, 2.6 and 5.2, respectively); and pravastatin and simvastatin appeared to be the best-tolerated and safest statins, with the lowest discontinuation rates. However, higher doses of simvastatin (> 40 mg/d) significantly increased the risk for CK and transaminase elevations (OR, 4.1 and 2.8, respectively),4 as well as the risk for rhabdomyolysis when taken at the highest dose.15,16
Continue for safety concerns >>
ARE STATINS SAFE FOR THESE PATIENTS?
When considering statin therapy, there are some patient populations that warrant particular concern:
Women of childbearing age. Statins are contraindicated in women who are pregnant or breastfeeding1 and should not be initiated in women who are trying to conceive.
Children and adolescents (ages 8-18 years). Statins have been shown to be safe and effective for children and adolescents with familial hyperlipidemia. No effect on growth or maturation has been seen.51 As with adults, however, higher statin doses and the use of concomitant interacting drugs increase the risk for AEs.
Asians. The new ACC/AHA guideline suggests taking Asian ancestry into consideration when prescribing statins because Asians may be more sensitive to medications metabolized by the CYP450 system.1 However, there are no reports of an increased risk for AEs in Asian patients on statins.52
Patient factors that increase risk
Risk factors for statin-induced AEs include1
• Multiple and/or serious comorbidities (eg, hypothyroidism, impaired renal or hepatic function, rheumatic disorders)
• Unexplained ALT elevation more than 3x the upper limit of normal
• History of prior statin intolerance or concomitant use of drugs that affect statin metabolism
• Age older than 75
• Preexisting muscle disorders
• Low vitamin D levels.
If a patient who would clearly benefit from statin therapy develops an AE requiring discontinuation, a retrial—with the same drug or a different statin—is generally recommended once the symptoms resolve.1
CASE
The risk for elevated serum transaminases, insulin resistance, cognitive impairment, and neuropathy associated with statin use is minimal, and further evaluation revealed that Mr L.’s recent symptoms had other causes. The elevated transaminases were due to fatty liver disease, the cognitive impairment was secondary to sleep apnea (both linked to his obesity), and the tingling in his hands was the result of carpal tunnel syndrome caused by his exercise regimen.
When he returns in six months, Mr L. reports that he has been working with both a nutritionist and an athletic trainer. He has sustained a 15-lb weight loss. He is still taking atorvastatin 10 mg; after he began taking CoQ10, his muscle pain resolved. The patient’s cholesterol and transaminase levels are normal, and the cognitive impairment and peripheral neuropathy he reported at his last visit have improved significantly.
REFERENCES
1. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation. 2014;129:S1-S45.
2. Lowes R. Top 100 selling drugs through September reported. Medscape Med News. WebMD, LLC. 2013. www.medscape.com/viewarti cle/813571#3. Accessed October 19, 2014.
3. Zhang H, Plutzky J, Skentzos S, et al. Discontinuation of statins in routine care settings: a cohort study. Ann Intern Med. 2013;158:526-534.
4. Naci H, Brugts J, Ades T. Comparative tolerability and harms of individual statins: a study-level network meta-analysis of 246,955 participants from 135 randomized, controlled trials. Circ Cardiovasc Qual Outcomes. 2013;6:390-399.
5. Pasternak RC, Smith SC Jr, Bairey-Merz CN, et al; American College of Cardiology; American Heart Association; National Heart, Lung and Blood Institute. ACC/AHA/NHLBI clinical advisory on the use and safety of statins. Circulation. 2002;106:1024-1028.
6. Eckel RH. Approach to the patient who is intolerant of statin therapy.
J Clin Endocrinol Metab. 2010;95:2015-2022.
7. Parker BA, Thompson PD. Effect of statins on skeletal muscle: exercise, myopathy, and muscle outcomes. Exerc Sport Sci Rev. 2012;40:188-194.
8. Mansi I, Frei CR, Pugh MJ, et al. Statins and musculoskeletal conditions, arthropathies, and injuries. JAMA Intern Med. 2013;173:1-10.
9. Bruckert E, Hayem G, Dejager S, et al. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients—the PRIMO study. Cardiovasc Drugs Ther. 2005;19:403-414.
10. Fernandez G, Spatz ES, Jablecki C, et al. Statin myopathy: a common dilemma not reflected in clinical trials. Cleve Clin J Med. 2011;78:
393-403.
11. Law M, Rudnicka AR. Statin safety: a systematic review. Am J Cardiol. 2006;97:52C-60C.
12. Elsevier/Gold Standard. Gold Standard Drug Database. www.goldstand ard.com/product/gold-standard-drug-database/. Accessed October 19, 2014.
13. FDA. FDA drug safety communication: important safety label changes to cholesterol-lowering statin drugs. www.fda.gov/drugs/drugsafety/ucm293101.htm. Accessed October 19,2014.
14. Kashani A, Phillips CO, Foody JM, et al. Risks associated with statin therapy: a systematic overview of randomized clinical trials. Circulation. 2006;114:2788-2797.
15. FDA. FDA drug safety communication: ongoing safety review of high-dose Zocor (simvastatin) and increased risk of muscle injury. www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm204882.htm. Updated February 15, 2012. Accessed October 19, 2014.
16. Bowman L, Armitage J, Bulbulia R, et al; SEARCH Study Collaborative Group. Study of the effectiveness of additional reductions in cholesterol and homocysteine (SEARCH): characteristics of a randomized trial among 12064 myocardial infarction survivors. Am J Heart. 2007;154:815-823.
17. Mills EJ, O’Regan C, Eyawo O, et al. Intensive statin therapy compared with moderate dosing for prevention of cardiovascular events: a meta-analysis of >40,000 patients. Euro Heart J. 2011;32:1409-1415.
18. Bookstaver DA, Burkhalter NA, Hatzigeorgiou C. Effect of coenzyme Q10 supplementation on statin-induced myalgias. Am J Cardiol. 2012;110:
526-529.
19. Parker BA, Gregory SM, Lorson L, et al. A randomized trial of coenzyme Q10 in patients with statin myopathy: rationale and study design. J Clin Lipidol. 2013;7:187-193.
20. Fedacko J, Pella D, Fedackova P, et al. Coenzyme Q(10) and selenium in statin-associated myopathy treatment. Can J Physiol Pharmacol. 2013;91:165-170.
21. Jellin JM, Gregory PJ, et al. Natural Medicines Comprehensive Database. www.naturaldatabase.com.libproxy.uwyo.edu. Accessed October 19, 2014.
22. de Denus S, Spinler SA, Miller K, et al. Statins and liver toxicity: a meta-analysis. Pharmacotherapy. 2004;24:584-591.
23. Lewis JH. Clinical perspective: statins and the liver—harmful or helpful? Dig Dis Sci. 2012;57:1754-1763.
24. Navarese EP, Buffon A, Andreotti F, et al. Meta-analysis of impact of different types and doses of statins on new-onset diabetes mellitus. Am J Cardiol. 2013;111:1123-1130.
25. Agarwal R. Effects of statins on renal function. Am J Cardiol. 2006;97:748-755.
26. Fried LF, Orchard TJ, Lasiske BL. Effect of lipid reduction on the progression of renal disease: a meta-analysis. Kidney Int. 2001;59:260-269.
27. Fellström B, Holdaas H, Jardine AG, et al; Assessment of Lescol in Renal Transportation Study Investigators. Effect of fluvastatin on renal end points in the Assessment of Lescol in Renal Transplant (ALERT) Trial. Kidney Int. 2004;66:1549-1555.
28. McKenney JM, Davidson MH, Jacobson TA, et al; National Lipid Association Statin Safety Assessment Task Force. Final conclusions and recommendations of the National Lipid Association Statin Safety Assessment Task Force. Am J Cardiol. 2006;97:89C-94C.
29. KDIGO Clinical Practice Guideline for Lipid Management in Chronic Kidney Disease. Kidney Int. 2013;3(suppl):S259-S305.
30. Goldstein LB, Amarenco P, Szarek M, et al; SPARCL Investigators. Hemorrhagic stroke in the Stroke Prevention by Aggressive Reduction in Cholesterol Levels study. Neurology. 2008;70(24 pt 2):2364-2370.
31. Goldstein LB, Amarenco P, Lamonte M, et al; SPARCL investigators. Relative effects of statin therapy on stroke and cardiovascular events in men and women: secondary analysis of the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Study. Stroke. 2008;39:
2444-2448.
32. Westover MB, Bianchi MT, Eckman MH, et al. Statin use following intracerebral hemorrhage: a decision analysis. Arch Neurol. 2011;68:573-579.
33. Biffi A, Devan WJ, Anderson CD, et al. Statin use and outcome after intracerebral hemorrhage: case-control study and meta-analysis. Neurology. 2011;76:1581-1588.
34. Dowlatshahi D, Demchuck AM, Fang J, et al; Registry of the Canadian Stroke Network. Association of statins and statin discontinuation with poor outcome and survival after intracerebral hemorrhage. Stroke. 2012;43:1518-1523.
35. Bustamante A, Montaner J. Statin therapy should not be discontinued in patients with intracerebral hemorrhage. Stroke. 2013;44:2060-2061.
36. Morgenstern LB, Hemphill JC 3rd, Anderson C, et al; American Heart Association Stroke Council and Council on Cardiovascular Nursing. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2010;41:2108-2129.
37. Shepherd J, Blauw GJ, Murphy MB, et al; PROSPER study group. PROspective Study of Pravastatin in the Elderly at Risk. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360:1623-1630.
38. Jukema JW, Cannon CP, de Craen AJ, et al. The controversies of statin therapy: weighing the evidence. J Am Coll Cardiol. 2012;60:875-881.
39. Alberton M, Wu P, Druyts E, et al. Adverse events associated with individual statin treatments for cardiovascular disease: an indirect comparison meta-analysis. QJM. 2012;105:145-157.
40. Baigent C, Blackwell L, Emberson J, et al; Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670-1681.
41. Emberson JR, Kearney PM, Blackwell L, et al; Cholesterol Treatment Trialists’ (CTT) Collaboration. Lack of effect of lowering LDL cholesterol on cancer: meta-analysis of individual data from 175,000 people in 27 randomised trials of statin therapy. PLoS One. 2012;7:e29849.
42. Klein BE, Klein R, Lee KE, et al. Statin use and incident nuclear cataract. JAMA. 2006;295:2752-2758.
43. Fong DS, Poon KY. Recent statin use and cataract surgery. Am J Ophthalmol. 2012;153:222-228.e1.
44. Chodick G, Heymann AD, Flash S, et al. Persistence with statins and incident cataract: a population-based historical cohort study. Ann Epidemiol. 2010;20:136-142.
45. Tan JS, Mitchell P, Rochtchina E, et al. Statin use and the long-term risk of incident cataract: the Blue Mountains Eye Study. Am J Ophthalmol. 2007;143:687-689.
46. Machan CM, Hrynchak PK, Irving EL. Age-related cataract is associated with type 2 diabetes and statin use. Optom Vis Sci. 2012;89:1165-1171.
47. Hippisley-Cox J, Coupland C. Unintended effects of statins in men and women in England and Wales: population based cohort study using the QResearch database. BMJ. 2010;340:c2197.
48. Leuschen J, Mortensen EM, Frei CR, et al. Association of statin use with cataracts: a propensity score-matched analysis. JAMA Ophthalmol. 2013;131:1427-1434.
49. Fraunfelder FW, Richards AB. Diplopia, blepharoptosis, and ophthalmoplegia and 3-hydroxy-3-methyl-glutaryl-CoA reductase inhibitor use. Ophthalmology. 2008;115:2282-2285.
50. AHFS Drug Information 2013. Bethesda, MD: American Society of Health-System Pharmacists; 2013.
51. Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents; National Heart, Lung, and Blood Institute. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics. 2011;128(suppl 5):S213-S256.
52. Liao JK. Safety and efficacy of statins in Asians. Am J Cardiol. 2007;99:410-414.
CASE
Mr L., a 57-year-old obese patient (BMI > 40) who had not been to a clinician in a decade, comes to see you after a health fair screening revealed dyslipidemia (LDL cholesterol, 188 mg/dL; HDL cholesterol, 32 mg/dL; total cholesterol, 240 mg/dL; triglycerides, 100 mg/dL). His blood pressure (BP) is 146/90 mm Hg, and his fasting glucose is 101 mg/dL. Labs drawn that day reveal an A1C of 5.9%, alanine aminotransferase (ALT) of 45 U/L, and aspartate aminotransferase (AST) of 62 U/L. In taking his history, you discover that Mr L. also has a notable family history of heart disease.
Mr L. agrees to take a low-dose statin, and you prescribe atorvastatin 10 mg and a thiazide diuretic. You advise the patient to contact you immediately if he develops significant myalgia, jaundice, dark urine, or symptoms of hyperglycemia such as excessive thirst or urination, and to schedule a follow-up visit in eight weeks.
Long recognized as the bedrock of hyperlipidemia therapy, statins achieved even greater prominence when the American College of Cardiology/American Heart Association (ACC/AHA) issued a new cholesterol guideline1 late last year. The ACC and AHA now recommend statins for a wider range of patients, often at a higher starting dose.
Based on the new recommendations, the use of statins is likely to rise.2 (A statin—rosuvastatin—is already the nation’s most widely prescribed medication.2) Thus, it is more important than ever for clinicians to know about the risks associated with statins and to be able to assess the benefits of therapy for individual patients.
A 2013 retrospective cohort study of more than 100,000 patients on statins found that 17% developed adverse effects (AEs). Therapy was withheld, at least temporarily, for 10% of study participants (60% of those experiencing AEs).3 At the same time, the authors of a large meta-analysis (135 randomized controlled trials [RCTs] and > 240,000 patients) reported that AEs associated with statins as a class were uncommon. The meta-analysis also found that the overall discontinuation rate for statin users—5.7%—was not significantly different from that of patients receiving placebo.4
Such discrepancies regarding particular risks, as well as the overall incidence of AEs and discontinuation rates, make the evidence difficult to sort out. We created this update with that in mind.
Continue for symptoms >>
MUSCULOSKELETAL SYMPTOMS ARE MOST COMMON
Musculoskeletal symptoms are the most common AEs reported by patients who are taking statins.5 These range from muscle weakness, fatigue, and pain to (rarely) rhabdomyolysis—a life-threatening condition characterized by severe muscle pain, muscle weakness, a 10-fold increase in creatine kinase (CK), and increased serum creatinine, often with myoglobinuria.5
Patients with myopathy—an umbrella term for any muscle disease—may report stiffness, weakness, tenderness, soreness, cramping, or heaviness. Symptoms are usually symmetrical and often involve the proximal limbs and trunk.6 Studies indicate that exercise increases the risk for statin-induced myalgia—muscle pain or weakness without an increase in CK—and that patients taking statins are more prone to exercise-related injury.7,8
A baseline CK is recommended for patients with an increased risk for muscular disorders.1 Risk factors include a personal or family history of statin intolerance or muscle disease, age older than 75, low levels of vitamin D, and concomitant use of medications that may increase the risk for myopathy (see Table 1).1 Routine monitoring of CK is not recommended, but CK levels should be obtained for those who exhibit muscle symptoms while on statin therapy.1
What the studies show
The incidence of myalgia reported in clinical studies is highly variable, ranging from less than 1% to 20%.1,9,10 The ACC/AHA guideline reports only one additional case of myopathy per 10,000 statin users compared with those on placebo and cites a rhabdomyolysis occurrence rate of less than 0.06% over five years.1
A 2006 systematic review estimated the absolute risk for rhabdomyolysis to be 3.4 per 100,000 person-years, but the incidence was 10 times higher for patients taking both a statin and gemfibrozil.11 (See Table 212,13 for more on drug interactions.) But both the meta-analysis cited earlier4 and a previous systematic review14 (35 RCTs and > 74,000 patients) found that statins as a class do not increase the incidence of myalgia or rhabdomyolysis.
Differences in the way muscular disorders are defined has been suggested as one reason for the discrepancies.10 In addition, many clinical trials exclude patients at higher risk for statin-associated AEs, such as those with renal or hepatic insufficiency, prior muscular complaints, poorly controlled diabetes, or potential drug interactions.1
An FDA advisory. In a safety communication last updated in February 2012, the FDA cautioned against starting patients on the highest dose of simvastatin (80 mg).15 The warning is based on a large study (N = 12,064) that found an increased risk for myopathy (0.9%) and rhabdomyolysis (0.2%) in patients on the
80-mg dose versus those taking 20 mg (0.02% and 0%, respectively).16
With the ACC/AHA now recommending intensive therapy (atorvastatin 40-80 mg or rosuvastatin 20-40 mg) to achieve an LDL reduction greater than 50% for many patients,1 it is important to be aware that this risk is specific to simvastatin. A recent meta-analysis of studies directly comparing patients receiving intensive statin therapy with those on low to moderate doses did not find any increased risk in rhabdomyolysis associated with more intensive therapy when those taking 80-mg simvastatin were excluded.17
The bottom line: Although rhabdomyolysis is rare, its severity—a fatality rate of 10%11—makes it critical to educate patients about the disorder and instruct them to stop taking the statin and call the office immediately if they develop severe muscle pain or weakness.
Recommend CoQ10 for statin-induced myopathy
Although the exact mechanism of statin-induced myopathy is unknown, the most likely explanation is a depletion of coenzyme Q10 (CoQ10), which has negative effects on mitochondrial energy production.18 While studies using CoQ10 to treat this AE have been small and had mixed results, the overall evidence suggests that it decreases the development and/or severity of symptoms.18-20
In fact, CoQ10 supplementation is the only treatment that has shown promise in treating statin-induced muscle symptoms.18-20 Doses of about 100 mg bid have been found to be beneficial and safe; no clinically relevant AEs have been seen with doses lower than 300 mg/d.18,20,21 A large placebo-controlled study is currently evaluating a 600 mg/d dose of CoQ10 in patients with statin-induced myopathy.19
CASE
On his next visit, Mr L. reports a new ache in his left shoulder and upper back, which he describes as mild but annoying. He also tells you his memory seems to be getting worse and that he has developed an odd tingling in his hands. These symptoms began about a month after he started the medications, Mr L. says. He also began a new exercise program, but his BMI is unchanged.
On examination, you find the affected shoulder and upper back modestly and diffusely tender to palpation but with no decline in strength. Mr L.’s BP has fallen to
134/84 mm Hg, and his fasting glucose is 105 mg/dL. Lab tests reveal an LDL of 144 mg/dL and HDL of 36 mg/dL, A1C of 6.1%, ALT of 105 U/L, AST of 61 U/L, and a normal CK.
You recommend 100 mg CoQ10 bid. Because it is available only OTC, you advise the patient to look for a product whose purity and potency have been verified by an external source, such as the US Pharmacopeial Convention. You also prescribe metformin 500 mg bid for insulin resistance, refer the patient to a nutritionist and diabetes specialist, and order tests to evaluate his other symptoms.
Continue for hepatic effects >>
HEPATIC EFFECTS ARE RARE
Historically, statins have been linked to potential hepatotoxicity, with case reports of serum transaminase elevation, cholestasis, hepatitis, and acute liver failure. It is now recognized that hepatic AEs are rare and that statins are not associated with a risk for acute or chronic liver failure.1,11 In patients with coronary heart disease, the incidence of hepatotoxicity with statin use is reported to be less than 1.5% over the course of five years and appears to be dose-dependent.1
In 2012, the FDA revised the labeling for most statins, relaxing its earlier recommendations for monitoring of liver function, clarifying the risk for myopathy, and providing additional information about drug interactions.13
Checking transaminase levels before initiating therapy is recommended by both the ACC/AHA and the FDA.1,13 Routine monitoring is not necessary, the ACC/AHA guideline states, because RCTs have found little evidence of ALT/AST elevation.1 But here, too, evidence varies. An older meta-analysis (13 trials and nearly 50,000 participants) concluded that as a class, statins have no greater risk for transaminase elevations than placebo.22 But the 135-RCT meta-analysis4 found otherwise: Statins did increase the risk for transaminase elevation (odds ratio [OR], 1.51) compared with placebo, with differences associated with particular drugs and higher doses associated with more clinically significant elevations.4 It is important to note, however, that there was significant heterogeneity among the studies and no consistent definition of clinical significance.
The bottom line: Statins have been shown in multiple prospective studies to be safe for patients with chronic liver disease.22,23
STATIN USE AND DIABETES: IS THERE A LINK?
Recent studies have found an increased risk for new-onset type 2 diabetes in statin users, with a greater risk associated with higher-potency statins, including rosuvastatin and atorvastatin.4,24 Although the exact mechanism is not known, statins may modify insulin signaling in peripheral tissues or directly impair insulin secretion.
The ACC/AHA guideline reports an excess rate of diabetes of one per 1,000 patient-years for moderate-intensity therapy and three per 1,000 patient-years for high-intensity therapy.1 The 2013 meta-analysis found that the elevated risk for diabetes was relatively small (OR, 1.09).4 No difference among various statins was found.
In another meta-analysis—this one encompassing 17 RCTs and more than 110,000 patients—no statistically significant difference in the incidence of new-onset diabetes was seen based on either the specific statin being taken or the intensity of therapy (high vs moderate).24
The bottom line: Clinicians should monitor patients taking statins for signs and symptoms of hyperglycemia.
STATINS MAY BE RENOPROTECTIVE
Statin use has been found to be associated with an increased risk for tubular proteinuria—an effect that is both dose- and potency-dependent.25 Nonetheless, it has been suggested that statins may be a rare example of a drug class that is renoprotective in the long term, despite having an increased rate of proteinuria in the short term.25
The evidence? In prospective studies, statin therapy has been shown to slow the progression of kidney disease in diverse patient populations, including renal transplant recipients and those with chronic kidney disease (CKD).26,27
The Kidney Expert Panel of the National Lipid Association (NLA) has concluded that statins do not appear to cause significant proteinuria or acute kidney injury. The panel does not recommend routine monitoring for proteinuria or kidney function in statin users unless otherwise indicated but does recommend a lower dose for patients with CKD.28
The bottom line: Kidney Disease Improving Global Outcomes guidelines recommend that patients who have CKD, but are not on dialysis, be treated with statin therapy. Statins are contraindicated for patients on dialysis, as clinical trials have failed to show significant cardiovascular benefit.29
Continue for the risk of intracerebral hemorrhages >>
INTRACEREBRAL HEMORRHAGE: STATINS INCREASE RECURRENCE RISK
In recent years, there has been considerable concern about a statin-induced increased risk for intracerebral hemorrhage (ICH). In a major prospective study in which patients were put on high-dose statin therapy or placebo after an acute ischemic or hemorrhagic stroke, the overall incidence of a recurrent stroke was significantly lower in the statin group.30 Among those who’d had an ICH, however, the recurrence rate was 73% higher for patients taking statins.
A subanalysis that looked only at patients who’d had a hemorrhagic stroke as their initial event (n = 93) found that the absolute risk for recurrent ICH was 15.6% for patients randomized to atorvastatin versus 4.2% for those on placebo.31 Despite being based on a small subset of the original study group, multivariate analysis indicated the increased risk was statistically significant (hazard ratio [HR], 1.69).
A subsequent decision analysis study based on these results proposed that patients with a history of spontaneous deep ICH would need an exceedingly high 10-year cardiovascular event risk (> 40%) for the benefits of statin therapy to outweigh the risk.32 The risk is particularly high for those with a history of lobar ICH, which has an extremely high recurrence rate. However, subsequent retrospective and observational studies have found that patients who were already on statins when the ICH occurred had less severe strokes and more favorable outcomes, with a lower mortality rate at 90 days post-ICH.33-35
A 2010 ICH guideline from the AHA/American Stroke Association states that there is “insufficient data to recommend restrictions on use of statin agents” for patients who have had an ICH.36
The bottom line: Clinicians should carefully evaluate the anticipated cardiovascular risk for patients who have had a hemorrhagic stroke to determine whether statin therapy would be beneficial.
OTHER SERIOUS ADVERSE EFFECTS: WHICH REPORTS ARE ACCURATE?
Statin use has been associated with a number of other serious AEs. Some reports appear to be accurate; others do not hold up after a close look at the evidence.
Malignancy. A potential link between statins and an increased risk for malignancy has been considered for years. A large trial (N = 5,804) from 2002 found a correlation between pravastatin and an increased risk for new cancer diagnoses compared with placebo (HR, 1.25).37 But a 10-year follow-up did not substantiate this finding, and it is now believed that the original result may have been due to chance.38 Numerous other meta-analyses and systematic reviews have found no link between statin use and malignancy.39-41
Cataracts. Potential ocular effects have been widely studied and debated in recent years. Observational studies reporting an association between statin use and cataracts have had conflicting results, with some showing statins as protective42-45 and others finding an increased risk.46,47 However, a recent propensity-score matched analysis found that statin users do indeed have an increased risk for cataracts.48 The authors concluded that for primary prevention, the risk-benefit equation for statin use should include this added risk.48
In addition, a review of the databases of the National Registry of Drug-Induced Ocular Side Effects, the World Health Organization, and the FDA from 1987 to 2008 indicates that statin therapy may also cause diplopia, ptosis, and ophthalmoplegia.49
Peripheral neuropathy. Despite case reports of statin-induced peripheral neuropathy, the NLA’s Neurology Expert Panel states that statins do not appear to cause this condition. If a patient receiving statin therapy develops peripheral neuropathy, a full work-up for other causes should be initiated before modification of statin therapy is considered, the panel advises.28
Statins have also been linked to headache and dizziness, respiratory symptoms, gastrointestinal problems, and rash (see Table 3).50
WHICH DRUG? POTENTIAL DIFFERENCES IN STATINS
A meta-analysis with more than 240,000 participants evaluated patients taking seven different statins (atorvastatin, fluvastatin, lovastatin, pravastatin, pitavastatin, rosuvastatin, and simvastatin), looking at AEs of the drugs both collectively and individually.4 As noted earlier, the overall discontinuation rate due to AEs for all statins was 5.7%. Discontinuation rates for each agent were not reported.4
The researchers did report, however, that atorvastatin and rosuvastatin had the highest discontinuation rates; atorvastatin and fluvastatin had the highest incidence of transaminase elevations (OR, 2.6 and 5.2, respectively); and pravastatin and simvastatin appeared to be the best-tolerated and safest statins, with the lowest discontinuation rates. However, higher doses of simvastatin (> 40 mg/d) significantly increased the risk for CK and transaminase elevations (OR, 4.1 and 2.8, respectively),4 as well as the risk for rhabdomyolysis when taken at the highest dose.15,16
Continue for safety concerns >>
ARE STATINS SAFE FOR THESE PATIENTS?
When considering statin therapy, there are some patient populations that warrant particular concern:
Women of childbearing age. Statins are contraindicated in women who are pregnant or breastfeeding1 and should not be initiated in women who are trying to conceive.
Children and adolescents (ages 8-18 years). Statins have been shown to be safe and effective for children and adolescents with familial hyperlipidemia. No effect on growth or maturation has been seen.51 As with adults, however, higher statin doses and the use of concomitant interacting drugs increase the risk for AEs.
Asians. The new ACC/AHA guideline suggests taking Asian ancestry into consideration when prescribing statins because Asians may be more sensitive to medications metabolized by the CYP450 system.1 However, there are no reports of an increased risk for AEs in Asian patients on statins.52
Patient factors that increase risk
Risk factors for statin-induced AEs include1
• Multiple and/or serious comorbidities (eg, hypothyroidism, impaired renal or hepatic function, rheumatic disorders)
• Unexplained ALT elevation more than 3x the upper limit of normal
• History of prior statin intolerance or concomitant use of drugs that affect statin metabolism
• Age older than 75
• Preexisting muscle disorders
• Low vitamin D levels.
If a patient who would clearly benefit from statin therapy develops an AE requiring discontinuation, a retrial—with the same drug or a different statin—is generally recommended once the symptoms resolve.1
CASE
The risk for elevated serum transaminases, insulin resistance, cognitive impairment, and neuropathy associated with statin use is minimal, and further evaluation revealed that Mr L.’s recent symptoms had other causes. The elevated transaminases were due to fatty liver disease, the cognitive impairment was secondary to sleep apnea (both linked to his obesity), and the tingling in his hands was the result of carpal tunnel syndrome caused by his exercise regimen.
When he returns in six months, Mr L. reports that he has been working with both a nutritionist and an athletic trainer. He has sustained a 15-lb weight loss. He is still taking atorvastatin 10 mg; after he began taking CoQ10, his muscle pain resolved. The patient’s cholesterol and transaminase levels are normal, and the cognitive impairment and peripheral neuropathy he reported at his last visit have improved significantly.
REFERENCES
1. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation. 2014;129:S1-S45.
2. Lowes R. Top 100 selling drugs through September reported. Medscape Med News. WebMD, LLC. 2013. www.medscape.com/viewarti cle/813571#3. Accessed October 19, 2014.
3. Zhang H, Plutzky J, Skentzos S, et al. Discontinuation of statins in routine care settings: a cohort study. Ann Intern Med. 2013;158:526-534.
4. Naci H, Brugts J, Ades T. Comparative tolerability and harms of individual statins: a study-level network meta-analysis of 246,955 participants from 135 randomized, controlled trials. Circ Cardiovasc Qual Outcomes. 2013;6:390-399.
5. Pasternak RC, Smith SC Jr, Bairey-Merz CN, et al; American College of Cardiology; American Heart Association; National Heart, Lung and Blood Institute. ACC/AHA/NHLBI clinical advisory on the use and safety of statins. Circulation. 2002;106:1024-1028.
6. Eckel RH. Approach to the patient who is intolerant of statin therapy.
J Clin Endocrinol Metab. 2010;95:2015-2022.
7. Parker BA, Thompson PD. Effect of statins on skeletal muscle: exercise, myopathy, and muscle outcomes. Exerc Sport Sci Rev. 2012;40:188-194.
8. Mansi I, Frei CR, Pugh MJ, et al. Statins and musculoskeletal conditions, arthropathies, and injuries. JAMA Intern Med. 2013;173:1-10.
9. Bruckert E, Hayem G, Dejager S, et al. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients—the PRIMO study. Cardiovasc Drugs Ther. 2005;19:403-414.
10. Fernandez G, Spatz ES, Jablecki C, et al. Statin myopathy: a common dilemma not reflected in clinical trials. Cleve Clin J Med. 2011;78:
393-403.
11. Law M, Rudnicka AR. Statin safety: a systematic review. Am J Cardiol. 2006;97:52C-60C.
12. Elsevier/Gold Standard. Gold Standard Drug Database. www.goldstand ard.com/product/gold-standard-drug-database/. Accessed October 19, 2014.
13. FDA. FDA drug safety communication: important safety label changes to cholesterol-lowering statin drugs. www.fda.gov/drugs/drugsafety/ucm293101.htm. Accessed October 19,2014.
14. Kashani A, Phillips CO, Foody JM, et al. Risks associated with statin therapy: a systematic overview of randomized clinical trials. Circulation. 2006;114:2788-2797.
15. FDA. FDA drug safety communication: ongoing safety review of high-dose Zocor (simvastatin) and increased risk of muscle injury. www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm204882.htm. Updated February 15, 2012. Accessed October 19, 2014.
16. Bowman L, Armitage J, Bulbulia R, et al; SEARCH Study Collaborative Group. Study of the effectiveness of additional reductions in cholesterol and homocysteine (SEARCH): characteristics of a randomized trial among 12064 myocardial infarction survivors. Am J Heart. 2007;154:815-823.
17. Mills EJ, O’Regan C, Eyawo O, et al. Intensive statin therapy compared with moderate dosing for prevention of cardiovascular events: a meta-analysis of >40,000 patients. Euro Heart J. 2011;32:1409-1415.
18. Bookstaver DA, Burkhalter NA, Hatzigeorgiou C. Effect of coenzyme Q10 supplementation on statin-induced myalgias. Am J Cardiol. 2012;110:
526-529.
19. Parker BA, Gregory SM, Lorson L, et al. A randomized trial of coenzyme Q10 in patients with statin myopathy: rationale and study design. J Clin Lipidol. 2013;7:187-193.
20. Fedacko J, Pella D, Fedackova P, et al. Coenzyme Q(10) and selenium in statin-associated myopathy treatment. Can J Physiol Pharmacol. 2013;91:165-170.
21. Jellin JM, Gregory PJ, et al. Natural Medicines Comprehensive Database. www.naturaldatabase.com.libproxy.uwyo.edu. Accessed October 19, 2014.
22. de Denus S, Spinler SA, Miller K, et al. Statins and liver toxicity: a meta-analysis. Pharmacotherapy. 2004;24:584-591.
23. Lewis JH. Clinical perspective: statins and the liver—harmful or helpful? Dig Dis Sci. 2012;57:1754-1763.
24. Navarese EP, Buffon A, Andreotti F, et al. Meta-analysis of impact of different types and doses of statins on new-onset diabetes mellitus. Am J Cardiol. 2013;111:1123-1130.
25. Agarwal R. Effects of statins on renal function. Am J Cardiol. 2006;97:748-755.
26. Fried LF, Orchard TJ, Lasiske BL. Effect of lipid reduction on the progression of renal disease: a meta-analysis. Kidney Int. 2001;59:260-269.
27. Fellström B, Holdaas H, Jardine AG, et al; Assessment of Lescol in Renal Transportation Study Investigators. Effect of fluvastatin on renal end points in the Assessment of Lescol in Renal Transplant (ALERT) Trial. Kidney Int. 2004;66:1549-1555.
28. McKenney JM, Davidson MH, Jacobson TA, et al; National Lipid Association Statin Safety Assessment Task Force. Final conclusions and recommendations of the National Lipid Association Statin Safety Assessment Task Force. Am J Cardiol. 2006;97:89C-94C.
29. KDIGO Clinical Practice Guideline for Lipid Management in Chronic Kidney Disease. Kidney Int. 2013;3(suppl):S259-S305.
30. Goldstein LB, Amarenco P, Szarek M, et al; SPARCL Investigators. Hemorrhagic stroke in the Stroke Prevention by Aggressive Reduction in Cholesterol Levels study. Neurology. 2008;70(24 pt 2):2364-2370.
31. Goldstein LB, Amarenco P, Lamonte M, et al; SPARCL investigators. Relative effects of statin therapy on stroke and cardiovascular events in men and women: secondary analysis of the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Study. Stroke. 2008;39:
2444-2448.
32. Westover MB, Bianchi MT, Eckman MH, et al. Statin use following intracerebral hemorrhage: a decision analysis. Arch Neurol. 2011;68:573-579.
33. Biffi A, Devan WJ, Anderson CD, et al. Statin use and outcome after intracerebral hemorrhage: case-control study and meta-analysis. Neurology. 2011;76:1581-1588.
34. Dowlatshahi D, Demchuck AM, Fang J, et al; Registry of the Canadian Stroke Network. Association of statins and statin discontinuation with poor outcome and survival after intracerebral hemorrhage. Stroke. 2012;43:1518-1523.
35. Bustamante A, Montaner J. Statin therapy should not be discontinued in patients with intracerebral hemorrhage. Stroke. 2013;44:2060-2061.
36. Morgenstern LB, Hemphill JC 3rd, Anderson C, et al; American Heart Association Stroke Council and Council on Cardiovascular Nursing. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2010;41:2108-2129.
37. Shepherd J, Blauw GJ, Murphy MB, et al; PROSPER study group. PROspective Study of Pravastatin in the Elderly at Risk. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360:1623-1630.
38. Jukema JW, Cannon CP, de Craen AJ, et al. The controversies of statin therapy: weighing the evidence. J Am Coll Cardiol. 2012;60:875-881.
39. Alberton M, Wu P, Druyts E, et al. Adverse events associated with individual statin treatments for cardiovascular disease: an indirect comparison meta-analysis. QJM. 2012;105:145-157.
40. Baigent C, Blackwell L, Emberson J, et al; Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670-1681.
41. Emberson JR, Kearney PM, Blackwell L, et al; Cholesterol Treatment Trialists’ (CTT) Collaboration. Lack of effect of lowering LDL cholesterol on cancer: meta-analysis of individual data from 175,000 people in 27 randomised trials of statin therapy. PLoS One. 2012;7:e29849.
42. Klein BE, Klein R, Lee KE, et al. Statin use and incident nuclear cataract. JAMA. 2006;295:2752-2758.
43. Fong DS, Poon KY. Recent statin use and cataract surgery. Am J Ophthalmol. 2012;153:222-228.e1.
44. Chodick G, Heymann AD, Flash S, et al. Persistence with statins and incident cataract: a population-based historical cohort study. Ann Epidemiol. 2010;20:136-142.
45. Tan JS, Mitchell P, Rochtchina E, et al. Statin use and the long-term risk of incident cataract: the Blue Mountains Eye Study. Am J Ophthalmol. 2007;143:687-689.
46. Machan CM, Hrynchak PK, Irving EL. Age-related cataract is associated with type 2 diabetes and statin use. Optom Vis Sci. 2012;89:1165-1171.
47. Hippisley-Cox J, Coupland C. Unintended effects of statins in men and women in England and Wales: population based cohort study using the QResearch database. BMJ. 2010;340:c2197.
48. Leuschen J, Mortensen EM, Frei CR, et al. Association of statin use with cataracts: a propensity score-matched analysis. JAMA Ophthalmol. 2013;131:1427-1434.
49. Fraunfelder FW, Richards AB. Diplopia, blepharoptosis, and ophthalmoplegia and 3-hydroxy-3-methyl-glutaryl-CoA reductase inhibitor use. Ophthalmology. 2008;115:2282-2285.
50. AHFS Drug Information 2013. Bethesda, MD: American Society of Health-System Pharmacists; 2013.
51. Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents; National Heart, Lung, and Blood Institute. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics. 2011;128(suppl 5):S213-S256.
52. Liao JK. Safety and efficacy of statins in Asians. Am J Cardiol. 2007;99:410-414.
CASE
Mr L., a 57-year-old obese patient (BMI > 40) who had not been to a clinician in a decade, comes to see you after a health fair screening revealed dyslipidemia (LDL cholesterol, 188 mg/dL; HDL cholesterol, 32 mg/dL; total cholesterol, 240 mg/dL; triglycerides, 100 mg/dL). His blood pressure (BP) is 146/90 mm Hg, and his fasting glucose is 101 mg/dL. Labs drawn that day reveal an A1C of 5.9%, alanine aminotransferase (ALT) of 45 U/L, and aspartate aminotransferase (AST) of 62 U/L. In taking his history, you discover that Mr L. also has a notable family history of heart disease.
Mr L. agrees to take a low-dose statin, and you prescribe atorvastatin 10 mg and a thiazide diuretic. You advise the patient to contact you immediately if he develops significant myalgia, jaundice, dark urine, or symptoms of hyperglycemia such as excessive thirst or urination, and to schedule a follow-up visit in eight weeks.
Long recognized as the bedrock of hyperlipidemia therapy, statins achieved even greater prominence when the American College of Cardiology/American Heart Association (ACC/AHA) issued a new cholesterol guideline1 late last year. The ACC and AHA now recommend statins for a wider range of patients, often at a higher starting dose.
Based on the new recommendations, the use of statins is likely to rise.2 (A statin—rosuvastatin—is already the nation’s most widely prescribed medication.2) Thus, it is more important than ever for clinicians to know about the risks associated with statins and to be able to assess the benefits of therapy for individual patients.
A 2013 retrospective cohort study of more than 100,000 patients on statins found that 17% developed adverse effects (AEs). Therapy was withheld, at least temporarily, for 10% of study participants (60% of those experiencing AEs).3 At the same time, the authors of a large meta-analysis (135 randomized controlled trials [RCTs] and > 240,000 patients) reported that AEs associated with statins as a class were uncommon. The meta-analysis also found that the overall discontinuation rate for statin users—5.7%—was not significantly different from that of patients receiving placebo.4
Such discrepancies regarding particular risks, as well as the overall incidence of AEs and discontinuation rates, make the evidence difficult to sort out. We created this update with that in mind.
Continue for symptoms >>
MUSCULOSKELETAL SYMPTOMS ARE MOST COMMON
Musculoskeletal symptoms are the most common AEs reported by patients who are taking statins.5 These range from muscle weakness, fatigue, and pain to (rarely) rhabdomyolysis—a life-threatening condition characterized by severe muscle pain, muscle weakness, a 10-fold increase in creatine kinase (CK), and increased serum creatinine, often with myoglobinuria.5
Patients with myopathy—an umbrella term for any muscle disease—may report stiffness, weakness, tenderness, soreness, cramping, or heaviness. Symptoms are usually symmetrical and often involve the proximal limbs and trunk.6 Studies indicate that exercise increases the risk for statin-induced myalgia—muscle pain or weakness without an increase in CK—and that patients taking statins are more prone to exercise-related injury.7,8
A baseline CK is recommended for patients with an increased risk for muscular disorders.1 Risk factors include a personal or family history of statin intolerance or muscle disease, age older than 75, low levels of vitamin D, and concomitant use of medications that may increase the risk for myopathy (see Table 1).1 Routine monitoring of CK is not recommended, but CK levels should be obtained for those who exhibit muscle symptoms while on statin therapy.1
What the studies show
The incidence of myalgia reported in clinical studies is highly variable, ranging from less than 1% to 20%.1,9,10 The ACC/AHA guideline reports only one additional case of myopathy per 10,000 statin users compared with those on placebo and cites a rhabdomyolysis occurrence rate of less than 0.06% over five years.1
A 2006 systematic review estimated the absolute risk for rhabdomyolysis to be 3.4 per 100,000 person-years, but the incidence was 10 times higher for patients taking both a statin and gemfibrozil.11 (See Table 212,13 for more on drug interactions.) But both the meta-analysis cited earlier4 and a previous systematic review14 (35 RCTs and > 74,000 patients) found that statins as a class do not increase the incidence of myalgia or rhabdomyolysis.
Differences in the way muscular disorders are defined has been suggested as one reason for the discrepancies.10 In addition, many clinical trials exclude patients at higher risk for statin-associated AEs, such as those with renal or hepatic insufficiency, prior muscular complaints, poorly controlled diabetes, or potential drug interactions.1
An FDA advisory. In a safety communication last updated in February 2012, the FDA cautioned against starting patients on the highest dose of simvastatin (80 mg).15 The warning is based on a large study (N = 12,064) that found an increased risk for myopathy (0.9%) and rhabdomyolysis (0.2%) in patients on the
80-mg dose versus those taking 20 mg (0.02% and 0%, respectively).16
With the ACC/AHA now recommending intensive therapy (atorvastatin 40-80 mg or rosuvastatin 20-40 mg) to achieve an LDL reduction greater than 50% for many patients,1 it is important to be aware that this risk is specific to simvastatin. A recent meta-analysis of studies directly comparing patients receiving intensive statin therapy with those on low to moderate doses did not find any increased risk in rhabdomyolysis associated with more intensive therapy when those taking 80-mg simvastatin were excluded.17
The bottom line: Although rhabdomyolysis is rare, its severity—a fatality rate of 10%11—makes it critical to educate patients about the disorder and instruct them to stop taking the statin and call the office immediately if they develop severe muscle pain or weakness.
Recommend CoQ10 for statin-induced myopathy
Although the exact mechanism of statin-induced myopathy is unknown, the most likely explanation is a depletion of coenzyme Q10 (CoQ10), which has negative effects on mitochondrial energy production.18 While studies using CoQ10 to treat this AE have been small and had mixed results, the overall evidence suggests that it decreases the development and/or severity of symptoms.18-20
In fact, CoQ10 supplementation is the only treatment that has shown promise in treating statin-induced muscle symptoms.18-20 Doses of about 100 mg bid have been found to be beneficial and safe; no clinically relevant AEs have been seen with doses lower than 300 mg/d.18,20,21 A large placebo-controlled study is currently evaluating a 600 mg/d dose of CoQ10 in patients with statin-induced myopathy.19
CASE
On his next visit, Mr L. reports a new ache in his left shoulder and upper back, which he describes as mild but annoying. He also tells you his memory seems to be getting worse and that he has developed an odd tingling in his hands. These symptoms began about a month after he started the medications, Mr L. says. He also began a new exercise program, but his BMI is unchanged.
On examination, you find the affected shoulder and upper back modestly and diffusely tender to palpation but with no decline in strength. Mr L.’s BP has fallen to
134/84 mm Hg, and his fasting glucose is 105 mg/dL. Lab tests reveal an LDL of 144 mg/dL and HDL of 36 mg/dL, A1C of 6.1%, ALT of 105 U/L, AST of 61 U/L, and a normal CK.
You recommend 100 mg CoQ10 bid. Because it is available only OTC, you advise the patient to look for a product whose purity and potency have been verified by an external source, such as the US Pharmacopeial Convention. You also prescribe metformin 500 mg bid for insulin resistance, refer the patient to a nutritionist and diabetes specialist, and order tests to evaluate his other symptoms.
Continue for hepatic effects >>
HEPATIC EFFECTS ARE RARE
Historically, statins have been linked to potential hepatotoxicity, with case reports of serum transaminase elevation, cholestasis, hepatitis, and acute liver failure. It is now recognized that hepatic AEs are rare and that statins are not associated with a risk for acute or chronic liver failure.1,11 In patients with coronary heart disease, the incidence of hepatotoxicity with statin use is reported to be less than 1.5% over the course of five years and appears to be dose-dependent.1
In 2012, the FDA revised the labeling for most statins, relaxing its earlier recommendations for monitoring of liver function, clarifying the risk for myopathy, and providing additional information about drug interactions.13
Checking transaminase levels before initiating therapy is recommended by both the ACC/AHA and the FDA.1,13 Routine monitoring is not necessary, the ACC/AHA guideline states, because RCTs have found little evidence of ALT/AST elevation.1 But here, too, evidence varies. An older meta-analysis (13 trials and nearly 50,000 participants) concluded that as a class, statins have no greater risk for transaminase elevations than placebo.22 But the 135-RCT meta-analysis4 found otherwise: Statins did increase the risk for transaminase elevation (odds ratio [OR], 1.51) compared with placebo, with differences associated with particular drugs and higher doses associated with more clinically significant elevations.4 It is important to note, however, that there was significant heterogeneity among the studies and no consistent definition of clinical significance.
The bottom line: Statins have been shown in multiple prospective studies to be safe for patients with chronic liver disease.22,23
STATIN USE AND DIABETES: IS THERE A LINK?
Recent studies have found an increased risk for new-onset type 2 diabetes in statin users, with a greater risk associated with higher-potency statins, including rosuvastatin and atorvastatin.4,24 Although the exact mechanism is not known, statins may modify insulin signaling in peripheral tissues or directly impair insulin secretion.
The ACC/AHA guideline reports an excess rate of diabetes of one per 1,000 patient-years for moderate-intensity therapy and three per 1,000 patient-years for high-intensity therapy.1 The 2013 meta-analysis found that the elevated risk for diabetes was relatively small (OR, 1.09).4 No difference among various statins was found.
In another meta-analysis—this one encompassing 17 RCTs and more than 110,000 patients—no statistically significant difference in the incidence of new-onset diabetes was seen based on either the specific statin being taken or the intensity of therapy (high vs moderate).24
The bottom line: Clinicians should monitor patients taking statins for signs and symptoms of hyperglycemia.
STATINS MAY BE RENOPROTECTIVE
Statin use has been found to be associated with an increased risk for tubular proteinuria—an effect that is both dose- and potency-dependent.25 Nonetheless, it has been suggested that statins may be a rare example of a drug class that is renoprotective in the long term, despite having an increased rate of proteinuria in the short term.25
The evidence? In prospective studies, statin therapy has been shown to slow the progression of kidney disease in diverse patient populations, including renal transplant recipients and those with chronic kidney disease (CKD).26,27
The Kidney Expert Panel of the National Lipid Association (NLA) has concluded that statins do not appear to cause significant proteinuria or acute kidney injury. The panel does not recommend routine monitoring for proteinuria or kidney function in statin users unless otherwise indicated but does recommend a lower dose for patients with CKD.28
The bottom line: Kidney Disease Improving Global Outcomes guidelines recommend that patients who have CKD, but are not on dialysis, be treated with statin therapy. Statins are contraindicated for patients on dialysis, as clinical trials have failed to show significant cardiovascular benefit.29
Continue for the risk of intracerebral hemorrhages >>
INTRACEREBRAL HEMORRHAGE: STATINS INCREASE RECURRENCE RISK
In recent years, there has been considerable concern about a statin-induced increased risk for intracerebral hemorrhage (ICH). In a major prospective study in which patients were put on high-dose statin therapy or placebo after an acute ischemic or hemorrhagic stroke, the overall incidence of a recurrent stroke was significantly lower in the statin group.30 Among those who’d had an ICH, however, the recurrence rate was 73% higher for patients taking statins.
A subanalysis that looked only at patients who’d had a hemorrhagic stroke as their initial event (n = 93) found that the absolute risk for recurrent ICH was 15.6% for patients randomized to atorvastatin versus 4.2% for those on placebo.31 Despite being based on a small subset of the original study group, multivariate analysis indicated the increased risk was statistically significant (hazard ratio [HR], 1.69).
A subsequent decision analysis study based on these results proposed that patients with a history of spontaneous deep ICH would need an exceedingly high 10-year cardiovascular event risk (> 40%) for the benefits of statin therapy to outweigh the risk.32 The risk is particularly high for those with a history of lobar ICH, which has an extremely high recurrence rate. However, subsequent retrospective and observational studies have found that patients who were already on statins when the ICH occurred had less severe strokes and more favorable outcomes, with a lower mortality rate at 90 days post-ICH.33-35
A 2010 ICH guideline from the AHA/American Stroke Association states that there is “insufficient data to recommend restrictions on use of statin agents” for patients who have had an ICH.36
The bottom line: Clinicians should carefully evaluate the anticipated cardiovascular risk for patients who have had a hemorrhagic stroke to determine whether statin therapy would be beneficial.
OTHER SERIOUS ADVERSE EFFECTS: WHICH REPORTS ARE ACCURATE?
Statin use has been associated with a number of other serious AEs. Some reports appear to be accurate; others do not hold up after a close look at the evidence.
Malignancy. A potential link between statins and an increased risk for malignancy has been considered for years. A large trial (N = 5,804) from 2002 found a correlation between pravastatin and an increased risk for new cancer diagnoses compared with placebo (HR, 1.25).37 But a 10-year follow-up did not substantiate this finding, and it is now believed that the original result may have been due to chance.38 Numerous other meta-analyses and systematic reviews have found no link between statin use and malignancy.39-41
Cataracts. Potential ocular effects have been widely studied and debated in recent years. Observational studies reporting an association between statin use and cataracts have had conflicting results, with some showing statins as protective42-45 and others finding an increased risk.46,47 However, a recent propensity-score matched analysis found that statin users do indeed have an increased risk for cataracts.48 The authors concluded that for primary prevention, the risk-benefit equation for statin use should include this added risk.48
In addition, a review of the databases of the National Registry of Drug-Induced Ocular Side Effects, the World Health Organization, and the FDA from 1987 to 2008 indicates that statin therapy may also cause diplopia, ptosis, and ophthalmoplegia.49
Peripheral neuropathy. Despite case reports of statin-induced peripheral neuropathy, the NLA’s Neurology Expert Panel states that statins do not appear to cause this condition. If a patient receiving statin therapy develops peripheral neuropathy, a full work-up for other causes should be initiated before modification of statin therapy is considered, the panel advises.28
Statins have also been linked to headache and dizziness, respiratory symptoms, gastrointestinal problems, and rash (see Table 3).50
WHICH DRUG? POTENTIAL DIFFERENCES IN STATINS
A meta-analysis with more than 240,000 participants evaluated patients taking seven different statins (atorvastatin, fluvastatin, lovastatin, pravastatin, pitavastatin, rosuvastatin, and simvastatin), looking at AEs of the drugs both collectively and individually.4 As noted earlier, the overall discontinuation rate due to AEs for all statins was 5.7%. Discontinuation rates for each agent were not reported.4
The researchers did report, however, that atorvastatin and rosuvastatin had the highest discontinuation rates; atorvastatin and fluvastatin had the highest incidence of transaminase elevations (OR, 2.6 and 5.2, respectively); and pravastatin and simvastatin appeared to be the best-tolerated and safest statins, with the lowest discontinuation rates. However, higher doses of simvastatin (> 40 mg/d) significantly increased the risk for CK and transaminase elevations (OR, 4.1 and 2.8, respectively),4 as well as the risk for rhabdomyolysis when taken at the highest dose.15,16
Continue for safety concerns >>
ARE STATINS SAFE FOR THESE PATIENTS?
When considering statin therapy, there are some patient populations that warrant particular concern:
Women of childbearing age. Statins are contraindicated in women who are pregnant or breastfeeding1 and should not be initiated in women who are trying to conceive.
Children and adolescents (ages 8-18 years). Statins have been shown to be safe and effective for children and adolescents with familial hyperlipidemia. No effect on growth or maturation has been seen.51 As with adults, however, higher statin doses and the use of concomitant interacting drugs increase the risk for AEs.
Asians. The new ACC/AHA guideline suggests taking Asian ancestry into consideration when prescribing statins because Asians may be more sensitive to medications metabolized by the CYP450 system.1 However, there are no reports of an increased risk for AEs in Asian patients on statins.52
Patient factors that increase risk
Risk factors for statin-induced AEs include1
• Multiple and/or serious comorbidities (eg, hypothyroidism, impaired renal or hepatic function, rheumatic disorders)
• Unexplained ALT elevation more than 3x the upper limit of normal
• History of prior statin intolerance or concomitant use of drugs that affect statin metabolism
• Age older than 75
• Preexisting muscle disorders
• Low vitamin D levels.
If a patient who would clearly benefit from statin therapy develops an AE requiring discontinuation, a retrial—with the same drug or a different statin—is generally recommended once the symptoms resolve.1
CASE
The risk for elevated serum transaminases, insulin resistance, cognitive impairment, and neuropathy associated with statin use is minimal, and further evaluation revealed that Mr L.’s recent symptoms had other causes. The elevated transaminases were due to fatty liver disease, the cognitive impairment was secondary to sleep apnea (both linked to his obesity), and the tingling in his hands was the result of carpal tunnel syndrome caused by his exercise regimen.
When he returns in six months, Mr L. reports that he has been working with both a nutritionist and an athletic trainer. He has sustained a 15-lb weight loss. He is still taking atorvastatin 10 mg; after he began taking CoQ10, his muscle pain resolved. The patient’s cholesterol and transaminase levels are normal, and the cognitive impairment and peripheral neuropathy he reported at his last visit have improved significantly.
REFERENCES
1. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation. 2014;129:S1-S45.
2. Lowes R. Top 100 selling drugs through September reported. Medscape Med News. WebMD, LLC. 2013. www.medscape.com/viewarti cle/813571#3. Accessed October 19, 2014.
3. Zhang H, Plutzky J, Skentzos S, et al. Discontinuation of statins in routine care settings: a cohort study. Ann Intern Med. 2013;158:526-534.
4. Naci H, Brugts J, Ades T. Comparative tolerability and harms of individual statins: a study-level network meta-analysis of 246,955 participants from 135 randomized, controlled trials. Circ Cardiovasc Qual Outcomes. 2013;6:390-399.
5. Pasternak RC, Smith SC Jr, Bairey-Merz CN, et al; American College of Cardiology; American Heart Association; National Heart, Lung and Blood Institute. ACC/AHA/NHLBI clinical advisory on the use and safety of statins. Circulation. 2002;106:1024-1028.
6. Eckel RH. Approach to the patient who is intolerant of statin therapy.
J Clin Endocrinol Metab. 2010;95:2015-2022.
7. Parker BA, Thompson PD. Effect of statins on skeletal muscle: exercise, myopathy, and muscle outcomes. Exerc Sport Sci Rev. 2012;40:188-194.
8. Mansi I, Frei CR, Pugh MJ, et al. Statins and musculoskeletal conditions, arthropathies, and injuries. JAMA Intern Med. 2013;173:1-10.
9. Bruckert E, Hayem G, Dejager S, et al. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients—the PRIMO study. Cardiovasc Drugs Ther. 2005;19:403-414.
10. Fernandez G, Spatz ES, Jablecki C, et al. Statin myopathy: a common dilemma not reflected in clinical trials. Cleve Clin J Med. 2011;78:
393-403.
11. Law M, Rudnicka AR. Statin safety: a systematic review. Am J Cardiol. 2006;97:52C-60C.
12. Elsevier/Gold Standard. Gold Standard Drug Database. www.goldstand ard.com/product/gold-standard-drug-database/. Accessed October 19, 2014.
13. FDA. FDA drug safety communication: important safety label changes to cholesterol-lowering statin drugs. www.fda.gov/drugs/drugsafety/ucm293101.htm. Accessed October 19,2014.
14. Kashani A, Phillips CO, Foody JM, et al. Risks associated with statin therapy: a systematic overview of randomized clinical trials. Circulation. 2006;114:2788-2797.
15. FDA. FDA drug safety communication: ongoing safety review of high-dose Zocor (simvastatin) and increased risk of muscle injury. www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm204882.htm. Updated February 15, 2012. Accessed October 19, 2014.
16. Bowman L, Armitage J, Bulbulia R, et al; SEARCH Study Collaborative Group. Study of the effectiveness of additional reductions in cholesterol and homocysteine (SEARCH): characteristics of a randomized trial among 12064 myocardial infarction survivors. Am J Heart. 2007;154:815-823.
17. Mills EJ, O’Regan C, Eyawo O, et al. Intensive statin therapy compared with moderate dosing for prevention of cardiovascular events: a meta-analysis of >40,000 patients. Euro Heart J. 2011;32:1409-1415.
18. Bookstaver DA, Burkhalter NA, Hatzigeorgiou C. Effect of coenzyme Q10 supplementation on statin-induced myalgias. Am J Cardiol. 2012;110:
526-529.
19. Parker BA, Gregory SM, Lorson L, et al. A randomized trial of coenzyme Q10 in patients with statin myopathy: rationale and study design. J Clin Lipidol. 2013;7:187-193.
20. Fedacko J, Pella D, Fedackova P, et al. Coenzyme Q(10) and selenium in statin-associated myopathy treatment. Can J Physiol Pharmacol. 2013;91:165-170.
21. Jellin JM, Gregory PJ, et al. Natural Medicines Comprehensive Database. www.naturaldatabase.com.libproxy.uwyo.edu. Accessed October 19, 2014.
22. de Denus S, Spinler SA, Miller K, et al. Statins and liver toxicity: a meta-analysis. Pharmacotherapy. 2004;24:584-591.
23. Lewis JH. Clinical perspective: statins and the liver—harmful or helpful? Dig Dis Sci. 2012;57:1754-1763.
24. Navarese EP, Buffon A, Andreotti F, et al. Meta-analysis of impact of different types and doses of statins on new-onset diabetes mellitus. Am J Cardiol. 2013;111:1123-1130.
25. Agarwal R. Effects of statins on renal function. Am J Cardiol. 2006;97:748-755.
26. Fried LF, Orchard TJ, Lasiske BL. Effect of lipid reduction on the progression of renal disease: a meta-analysis. Kidney Int. 2001;59:260-269.
27. Fellström B, Holdaas H, Jardine AG, et al; Assessment of Lescol in Renal Transportation Study Investigators. Effect of fluvastatin on renal end points in the Assessment of Lescol in Renal Transplant (ALERT) Trial. Kidney Int. 2004;66:1549-1555.
28. McKenney JM, Davidson MH, Jacobson TA, et al; National Lipid Association Statin Safety Assessment Task Force. Final conclusions and recommendations of the National Lipid Association Statin Safety Assessment Task Force. Am J Cardiol. 2006;97:89C-94C.
29. KDIGO Clinical Practice Guideline for Lipid Management in Chronic Kidney Disease. Kidney Int. 2013;3(suppl):S259-S305.
30. Goldstein LB, Amarenco P, Szarek M, et al; SPARCL Investigators. Hemorrhagic stroke in the Stroke Prevention by Aggressive Reduction in Cholesterol Levels study. Neurology. 2008;70(24 pt 2):2364-2370.
31. Goldstein LB, Amarenco P, Lamonte M, et al; SPARCL investigators. Relative effects of statin therapy on stroke and cardiovascular events in men and women: secondary analysis of the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Study. Stroke. 2008;39:
2444-2448.
32. Westover MB, Bianchi MT, Eckman MH, et al. Statin use following intracerebral hemorrhage: a decision analysis. Arch Neurol. 2011;68:573-579.
33. Biffi A, Devan WJ, Anderson CD, et al. Statin use and outcome after intracerebral hemorrhage: case-control study and meta-analysis. Neurology. 2011;76:1581-1588.
34. Dowlatshahi D, Demchuck AM, Fang J, et al; Registry of the Canadian Stroke Network. Association of statins and statin discontinuation with poor outcome and survival after intracerebral hemorrhage. Stroke. 2012;43:1518-1523.
35. Bustamante A, Montaner J. Statin therapy should not be discontinued in patients with intracerebral hemorrhage. Stroke. 2013;44:2060-2061.
36. Morgenstern LB, Hemphill JC 3rd, Anderson C, et al; American Heart Association Stroke Council and Council on Cardiovascular Nursing. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2010;41:2108-2129.
37. Shepherd J, Blauw GJ, Murphy MB, et al; PROSPER study group. PROspective Study of Pravastatin in the Elderly at Risk. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360:1623-1630.
38. Jukema JW, Cannon CP, de Craen AJ, et al. The controversies of statin therapy: weighing the evidence. J Am Coll Cardiol. 2012;60:875-881.
39. Alberton M, Wu P, Druyts E, et al. Adverse events associated with individual statin treatments for cardiovascular disease: an indirect comparison meta-analysis. QJM. 2012;105:145-157.
40. Baigent C, Blackwell L, Emberson J, et al; Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670-1681.
41. Emberson JR, Kearney PM, Blackwell L, et al; Cholesterol Treatment Trialists’ (CTT) Collaboration. Lack of effect of lowering LDL cholesterol on cancer: meta-analysis of individual data from 175,000 people in 27 randomised trials of statin therapy. PLoS One. 2012;7:e29849.
42. Klein BE, Klein R, Lee KE, et al. Statin use and incident nuclear cataract. JAMA. 2006;295:2752-2758.
43. Fong DS, Poon KY. Recent statin use and cataract surgery. Am J Ophthalmol. 2012;153:222-228.e1.
44. Chodick G, Heymann AD, Flash S, et al. Persistence with statins and incident cataract: a population-based historical cohort study. Ann Epidemiol. 2010;20:136-142.
45. Tan JS, Mitchell P, Rochtchina E, et al. Statin use and the long-term risk of incident cataract: the Blue Mountains Eye Study. Am J Ophthalmol. 2007;143:687-689.
46. Machan CM, Hrynchak PK, Irving EL. Age-related cataract is associated with type 2 diabetes and statin use. Optom Vis Sci. 2012;89:1165-1171.
47. Hippisley-Cox J, Coupland C. Unintended effects of statins in men and women in England and Wales: population based cohort study using the QResearch database. BMJ. 2010;340:c2197.
48. Leuschen J, Mortensen EM, Frei CR, et al. Association of statin use with cataracts: a propensity score-matched analysis. JAMA Ophthalmol. 2013;131:1427-1434.
49. Fraunfelder FW, Richards AB. Diplopia, blepharoptosis, and ophthalmoplegia and 3-hydroxy-3-methyl-glutaryl-CoA reductase inhibitor use. Ophthalmology. 2008;115:2282-2285.
50. AHFS Drug Information 2013. Bethesda, MD: American Society of Health-System Pharmacists; 2013.
51. Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents; National Heart, Lung, and Blood Institute. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics. 2011;128(suppl 5):S213-S256.
52. Liao JK. Safety and efficacy of statins in Asians. Am J Cardiol. 2007;99:410-414.