User login
The authors report no financial relationships relevant to this article.
CASE: Premature treatment for osteopenia
A 57-year-old woman presents to establish care and discuss concerns related to menopause and osteoporosis management. She is G2P2, healthy, 5 ft 6 in, and 130 lb. She underwent natural menopause at age 51; her vasomotor symptoms have been mild, and she has not used hormone therapy. Upon annual physical examination at age 52, her former physician referred her for a mammogram, bone-density assessment, and colonoscopy. Osteopenia (femoral neck T-score, –1.8) was noted on dual-energy x-ray absorptiometry (DXA), and alendronate 70 mg per week was started.
Today, she reports 6 months of worsening vaginal dryness. In addition, she mentions concerns about taking alendronate based on recent news reports describing serious potential adverse effects.
This patient is taking osteoporosis medication unnecessarily
BMD screening was not indicated in this patient at age 52. Schnatz and colleagues found that 41% of 612 women who had been referred for and underwent DXA screening did not meet the criteria for such screening, according to the 2006 Osteoporosis Position Statement of The North American Menopause Society.1 In addition, nearly 18% of those women who underwent DXA screening without the proper indications for it were being treated for osteopenia or osteoporosis with a bisphosphonate, raloxifene, or calcitonin therapy.
These groups should be screened for BMD2–4:
- women aged 65 and older
- women younger than age 65 who are at high risk for fracture
- adults with fracture after age 50
- adults with a medical condition or who are taking a medication that is associated with low bone mass.
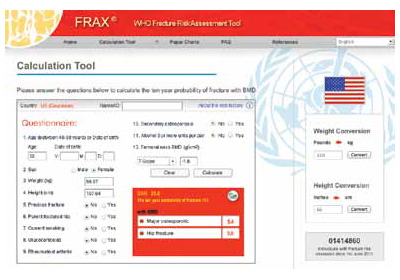
Bone-health treatment was not indicated in this patient at age 52. The World Health Organization’s fracture risk assessment tool (FRAX) indicated that the patient’s risk of having a major osteoporotic fracture in the next 10 years was 5.4%, and her calculated risk of hip fracture in the next 10 years was 0.5% (FIGURE). Relying on both the patient’s T-score and FRAX results, alendronate therapy should not have been initiated.
These groups meet the criteria for treatment with a prescription medication according to FRAX results4:
- >20% risk of major osteoporotic fracture in next 10 years
- >3% risk of hip fracture in the next 10 years.
FRAX calculation tool
What now?
This patient is at low risk for osteoporosis. With an estimated rate of bone loss of 0.5% per year, over the next 8 years (from age 57 to age 65), she is unlikely to have osteoporosis at age 65. Therefore, her next BMD screening should be when she reaches age 65, according to current screening guidelines.4
Treatment holiday. Although bisphosphonate side effects, including esophageal irritation and osteonecrosis of the jaw and atypical femur fracture, are rare, it makes no sense for this patient to continue her bisphosphonate since its use was not indicated in the first place. Since bisphosphonates have been shown to accumulate in bone, their treatment effect can last up to 2 years after discontinuing therapy. Ceasing treatment for 1 to 2 years, after 3 to 5 years of continuous treatment, is a good option to minimize drug exposure and the risk of complications while preserving bone density and fracture risk reduction in patients at low risk for fracture. In patients at high risk for fracture, the 1- to 2-year drug holiday should occur after 10 years of bisphosphonate treatment.5,6
Your patient’s lifestyle can help her avoid fracture. Tell her.
Counsel your patients to exercise (30 to 45 minutes per day of weight-bearing exercise), limit their alcohol intake, and ensure adequate calcium and vitamin D intake. Food sources, rather than supplement sources, of calcium are better for bone health. The total daily calcium and vitamin D intake for women should be7-10:
- Calcium: Age older than 50 years = 1200 mg/d
- Vitamin D: Until age 70 = 600 IU/d; after age 70 = 800 IU/d.
We want to hear from you! Tell us what you think.
1. Schnatz PF, Marakovitz KA, Dubois M, O’Sullivan DM. Osteoporosis screening and treatment guidelines: are they being followed? Menopause. 2011;18(10):1072-1078.
2. North American Menopause Society. Management of osteoporosis in postmenopausal women: 2010 position statement. Menopause. 2010;17(1):25-54.
3. US Preventive Services Task Force. Screening for osteoporosis: recommendation statement. http://www.uspreventiveservicestaskforce.org/uspstf10/osteoporosis/osteors.htm. Published January 18 2011. Accessed December 6, 2012.
4. National Osteoporosis Foundation. Clinician’s Guide to Prevention and Treatment of Osteoporosis. http://www.nof.org/files/nof/public/content/file/344/upload/159.pdf. Revised January 2010. Accessed December 6 2012.
5. Watts NB, Diab D. Long-term use of bisphosphonates in osteoporosis. J Clin Endocrinol Metab. 2010;95(4):1555-1565.
6. Black DM, Bauer DC, Schwartz AV, Cummings ST, Rosen CJ. Continuing bisphosphonate treatment for osteoporosis—for whom and for how long? N Engl J Med. 2012;366(22):2051-2053.
7. Manson JE, Bassuk SS. NAMS Practice Pearl: Calcium supplements: Do they help or harm? North American Menopause Society. http://www.menopause.org/publications/clinical-practice-materials/practice-pearls. Published September 6 2012. Accessed December 6, 2012.
8. Jackson RD, LaCrois AZ, Gass M, et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354(7):669-683.
9. Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96(1):53-58.
10. Ross AC, Taylor CL, Yaktine AL, Del Valle HB. eds; Committee to Review Dietary Reference Intakes for Vitamin D and Calcium, Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D. Washington DC: The National Academies Press; 2011.
The authors report no financial relationships relevant to this article.
CASE: Premature treatment for osteopenia
A 57-year-old woman presents to establish care and discuss concerns related to menopause and osteoporosis management. She is G2P2, healthy, 5 ft 6 in, and 130 lb. She underwent natural menopause at age 51; her vasomotor symptoms have been mild, and she has not used hormone therapy. Upon annual physical examination at age 52, her former physician referred her for a mammogram, bone-density assessment, and colonoscopy. Osteopenia (femoral neck T-score, –1.8) was noted on dual-energy x-ray absorptiometry (DXA), and alendronate 70 mg per week was started.
Today, she reports 6 months of worsening vaginal dryness. In addition, she mentions concerns about taking alendronate based on recent news reports describing serious potential adverse effects.
This patient is taking osteoporosis medication unnecessarily
BMD screening was not indicated in this patient at age 52. Schnatz and colleagues found that 41% of 612 women who had been referred for and underwent DXA screening did not meet the criteria for such screening, according to the 2006 Osteoporosis Position Statement of The North American Menopause Society.1 In addition, nearly 18% of those women who underwent DXA screening without the proper indications for it were being treated for osteopenia or osteoporosis with a bisphosphonate, raloxifene, or calcitonin therapy.
These groups should be screened for BMD2–4:
- women aged 65 and older
- women younger than age 65 who are at high risk for fracture
- adults with fracture after age 50
- adults with a medical condition or who are taking a medication that is associated with low bone mass.
Bone-health treatment was not indicated in this patient at age 52. The World Health Organization’s fracture risk assessment tool (FRAX) indicated that the patient’s risk of having a major osteoporotic fracture in the next 10 years was 5.4%, and her calculated risk of hip fracture in the next 10 years was 0.5% (FIGURE). Relying on both the patient’s T-score and FRAX results, alendronate therapy should not have been initiated.
These groups meet the criteria for treatment with a prescription medication according to FRAX results4:
- >20% risk of major osteoporotic fracture in next 10 years
- >3% risk of hip fracture in the next 10 years.

FRAX calculation tool
What now?
This patient is at low risk for osteoporosis. With an estimated rate of bone loss of 0.5% per year, over the next 8 years (from age 57 to age 65), she is unlikely to have osteoporosis at age 65. Therefore, her next BMD screening should be when she reaches age 65, according to current screening guidelines.4
Treatment holiday. Although bisphosphonate side effects, including esophageal irritation and osteonecrosis of the jaw and atypical femur fracture, are rare, it makes no sense for this patient to continue her bisphosphonate since its use was not indicated in the first place. Since bisphosphonates have been shown to accumulate in bone, their treatment effect can last up to 2 years after discontinuing therapy. Ceasing treatment for 1 to 2 years, after 3 to 5 years of continuous treatment, is a good option to minimize drug exposure and the risk of complications while preserving bone density and fracture risk reduction in patients at low risk for fracture. In patients at high risk for fracture, the 1- to 2-year drug holiday should occur after 10 years of bisphosphonate treatment.5,6
Your patient’s lifestyle can help her avoid fracture. Tell her.
Counsel your patients to exercise (30 to 45 minutes per day of weight-bearing exercise), limit their alcohol intake, and ensure adequate calcium and vitamin D intake. Food sources, rather than supplement sources, of calcium are better for bone health. The total daily calcium and vitamin D intake for women should be7-10:
- Calcium: Age older than 50 years = 1200 mg/d
- Vitamin D: Until age 70 = 600 IU/d; after age 70 = 800 IU/d.
We want to hear from you! Tell us what you think.
The authors report no financial relationships relevant to this article.
CASE: Premature treatment for osteopenia
A 57-year-old woman presents to establish care and discuss concerns related to menopause and osteoporosis management. She is G2P2, healthy, 5 ft 6 in, and 130 lb. She underwent natural menopause at age 51; her vasomotor symptoms have been mild, and she has not used hormone therapy. Upon annual physical examination at age 52, her former physician referred her for a mammogram, bone-density assessment, and colonoscopy. Osteopenia (femoral neck T-score, –1.8) was noted on dual-energy x-ray absorptiometry (DXA), and alendronate 70 mg per week was started.
Today, she reports 6 months of worsening vaginal dryness. In addition, she mentions concerns about taking alendronate based on recent news reports describing serious potential adverse effects.
This patient is taking osteoporosis medication unnecessarily
BMD screening was not indicated in this patient at age 52. Schnatz and colleagues found that 41% of 612 women who had been referred for and underwent DXA screening did not meet the criteria for such screening, according to the 2006 Osteoporosis Position Statement of The North American Menopause Society.1 In addition, nearly 18% of those women who underwent DXA screening without the proper indications for it were being treated for osteopenia or osteoporosis with a bisphosphonate, raloxifene, or calcitonin therapy.
These groups should be screened for BMD2–4:
- women aged 65 and older
- women younger than age 65 who are at high risk for fracture
- adults with fracture after age 50
- adults with a medical condition or who are taking a medication that is associated with low bone mass.
Bone-health treatment was not indicated in this patient at age 52. The World Health Organization’s fracture risk assessment tool (FRAX) indicated that the patient’s risk of having a major osteoporotic fracture in the next 10 years was 5.4%, and her calculated risk of hip fracture in the next 10 years was 0.5% (FIGURE). Relying on both the patient’s T-score and FRAX results, alendronate therapy should not have been initiated.
These groups meet the criteria for treatment with a prescription medication according to FRAX results4:
- >20% risk of major osteoporotic fracture in next 10 years
- >3% risk of hip fracture in the next 10 years.

FRAX calculation tool
What now?
This patient is at low risk for osteoporosis. With an estimated rate of bone loss of 0.5% per year, over the next 8 years (from age 57 to age 65), she is unlikely to have osteoporosis at age 65. Therefore, her next BMD screening should be when she reaches age 65, according to current screening guidelines.4
Treatment holiday. Although bisphosphonate side effects, including esophageal irritation and osteonecrosis of the jaw and atypical femur fracture, are rare, it makes no sense for this patient to continue her bisphosphonate since its use was not indicated in the first place. Since bisphosphonates have been shown to accumulate in bone, their treatment effect can last up to 2 years after discontinuing therapy. Ceasing treatment for 1 to 2 years, after 3 to 5 years of continuous treatment, is a good option to minimize drug exposure and the risk of complications while preserving bone density and fracture risk reduction in patients at low risk for fracture. In patients at high risk for fracture, the 1- to 2-year drug holiday should occur after 10 years of bisphosphonate treatment.5,6
Your patient’s lifestyle can help her avoid fracture. Tell her.
Counsel your patients to exercise (30 to 45 minutes per day of weight-bearing exercise), limit their alcohol intake, and ensure adequate calcium and vitamin D intake. Food sources, rather than supplement sources, of calcium are better for bone health. The total daily calcium and vitamin D intake for women should be7-10:
- Calcium: Age older than 50 years = 1200 mg/d
- Vitamin D: Until age 70 = 600 IU/d; after age 70 = 800 IU/d.
We want to hear from you! Tell us what you think.
1. Schnatz PF, Marakovitz KA, Dubois M, O’Sullivan DM. Osteoporosis screening and treatment guidelines: are they being followed? Menopause. 2011;18(10):1072-1078.
2. North American Menopause Society. Management of osteoporosis in postmenopausal women: 2010 position statement. Menopause. 2010;17(1):25-54.
3. US Preventive Services Task Force. Screening for osteoporosis: recommendation statement. http://www.uspreventiveservicestaskforce.org/uspstf10/osteoporosis/osteors.htm. Published January 18 2011. Accessed December 6, 2012.
4. National Osteoporosis Foundation. Clinician’s Guide to Prevention and Treatment of Osteoporosis. http://www.nof.org/files/nof/public/content/file/344/upload/159.pdf. Revised January 2010. Accessed December 6 2012.
5. Watts NB, Diab D. Long-term use of bisphosphonates in osteoporosis. J Clin Endocrinol Metab. 2010;95(4):1555-1565.
6. Black DM, Bauer DC, Schwartz AV, Cummings ST, Rosen CJ. Continuing bisphosphonate treatment for osteoporosis—for whom and for how long? N Engl J Med. 2012;366(22):2051-2053.
7. Manson JE, Bassuk SS. NAMS Practice Pearl: Calcium supplements: Do they help or harm? North American Menopause Society. http://www.menopause.org/publications/clinical-practice-materials/practice-pearls. Published September 6 2012. Accessed December 6, 2012.
8. Jackson RD, LaCrois AZ, Gass M, et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354(7):669-683.
9. Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96(1):53-58.
10. Ross AC, Taylor CL, Yaktine AL, Del Valle HB. eds; Committee to Review Dietary Reference Intakes for Vitamin D and Calcium, Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D. Washington DC: The National Academies Press; 2011.
1. Schnatz PF, Marakovitz KA, Dubois M, O’Sullivan DM. Osteoporosis screening and treatment guidelines: are they being followed? Menopause. 2011;18(10):1072-1078.
2. North American Menopause Society. Management of osteoporosis in postmenopausal women: 2010 position statement. Menopause. 2010;17(1):25-54.
3. US Preventive Services Task Force. Screening for osteoporosis: recommendation statement. http://www.uspreventiveservicestaskforce.org/uspstf10/osteoporosis/osteors.htm. Published January 18 2011. Accessed December 6, 2012.
4. National Osteoporosis Foundation. Clinician’s Guide to Prevention and Treatment of Osteoporosis. http://www.nof.org/files/nof/public/content/file/344/upload/159.pdf. Revised January 2010. Accessed December 6 2012.
5. Watts NB, Diab D. Long-term use of bisphosphonates in osteoporosis. J Clin Endocrinol Metab. 2010;95(4):1555-1565.
6. Black DM, Bauer DC, Schwartz AV, Cummings ST, Rosen CJ. Continuing bisphosphonate treatment for osteoporosis—for whom and for how long? N Engl J Med. 2012;366(22):2051-2053.
7. Manson JE, Bassuk SS. NAMS Practice Pearl: Calcium supplements: Do they help or harm? North American Menopause Society. http://www.menopause.org/publications/clinical-practice-materials/practice-pearls. Published September 6 2012. Accessed December 6, 2012.
8. Jackson RD, LaCrois AZ, Gass M, et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354(7):669-683.
9. Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96(1):53-58.
10. Ross AC, Taylor CL, Yaktine AL, Del Valle HB. eds; Committee to Review Dietary Reference Intakes for Vitamin D and Calcium, Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D. Washington DC: The National Academies Press; 2011.

