User login
Traditionally, biofeedback was considered to be a stress management technique that targeted sympathetic nervous system (SNS) overdrive with an adrenal medullary system backup. Recent advances in autonomic physiology, however, have clarified that except in extreme situations, the SNS is not the key factor in day-to-day stress. Rather, the parasympathetic branch of the autonomic nervous system appears to be a more likely candidate for mediating routine stress because, unlike the SNS, which has slow-acting neurotransmitters (ie, catecholamines), the parasympathetic nervous system has the fast-acting transmitter acetylcholine.
VAGAL WITHDRAWAL: AN ALTERNATIVE TO SYMPATHETIC ACTIVATION
Porges1 first proposed the concept of vagal withdrawal as an indicator of stress and stress vulnerability; this contrasts with the idea that the stress response is a consequence of sympathetic activation and the hypothalamic-pituitary-adrenal axis response. In the vagal withdrawal model, the response to stress is stabilization of the sympathetic system followed by termination of parasympathetic activity, manifested as cardiac acceleration.
Respiratory sinus arrhythmia (RSA), or the variability in heart rate as it synchronizes with breathing, is considered an index of parasympathetic tone. In the laboratory, slow atropine infusion produces a transient paradoxical vagomimetic effect characterized by an initial increase in RSA, followed by a flattening and then a rise in the heart rate.2 This phenomenon has been measured in people during times of routine stress, such as when worrying about being late for an appointment. In such individuals, biofeedback training can result in recovery of normal RSA shortly after an episode of anxiety.
Historically, the focus of biofeedback was to cultivate low arousal, presumably reducing SNS activity, through the use of finger temperature, skin conductance training, and profound muscle relaxation. More sophisticated ways to look at both branches of the autonomic nervous system have since emerged that allow for sampling of the beat-by-beat changes in heart rate.
HEART RATE VARIABILITY BIOFEEDBACK
The concept of modifying the respiration rate (paced breathing) originated some 2,500 years ago as a component of meditation. It is being revisited today in the form of heart rate variability (HRV) biofeedback training, which is being used as a stress-management tool and a method to correct disorders in which autonomic regulation is thought to be important. HRV biofeedback involves training to increase the amplitude of HRV rhythms and thus improve autonomic homeostasis.
Normal HRV has a pattern of overlapping oscillatory frequency components, including:
- a high-frequency rhythm, 0.15 to 0.4 Hz, which is the RSA;
- a low-frequency rhythm, 0.05 to 0.15 Hz, associated with blood pressure oscillations; and
- a very-low-frequency rhythm, 0.005 to 0.05 Hz, which may regulate vascular tone and body temperature.
The goal of HRV biofeedback is to achieve respiratory rates at which resonance occurs between cardiac rhythms associated with respiration (RSA, or high-frequency oscillations) and those caused by baroreflex activity (low-frequency oscillations).
Spectral analysis has demonstrated that nearly all of the activity with HRV biofeedback occurs at a low-frequency band. The reason is that activity in the low-frequency band is related more to baroreflex activity than to HRV compared with other ranges of frequency. Breathing rates that correspond to baroreflex effects, called resonance frequency breathing, represent resonance in the cardiovascular system. Several devices are available whose mechanisms are based on the concept of achieving resonance frequency breathing. One such device is a slow-breathing monitor (Resp-e-rate) that has been approved by the US Food and Drug Administration for the adjunctive treatment of hypertension.
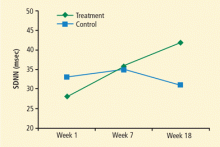
Improved HRV may suggest an improved risk status: Kleiger et al4 found that the relative risk of mortality was 5.3 times greater for people with SDNN of less than 50 msec compared with those whose SDNN was greater than 100 msec. In Del Pozo’s study, eight of 30 patients in the intervention group achieved an SDNN of greater than 50 msec (vs 0 at pretreatment) compared with three of 31 controls (vs two at pretreatment).3 As an additional benefit of HRV biofeedback, patients in the intervention group who entered the study with hypertension all became normotensive.
In a meta-analysis, van Dixhoorn and White5 found fewer cardiac events, fewer episodes of angina, and less occurrence of arrhythmia and exercise-induced ischemia from intensive supervised relaxation therapy in patients with ischemic heart disease. Improvements in scales of depression and anxiety were also observed with relaxation therapy.
Other studies have shown biofeedback to have beneficial effects based on the Posttraumatic Stress Disorder Checklist, the Hamilton Depression Rating Scale, and, in patients with mild to moderate heartfailure, the 6-minute walk test.6–8
The proposed mechanism for the beneficial effects of biofeedback found in clinical trials is improvement in baroreflex function, producing greater reflex efficiency and improved modulation of autonomic activity.
CONCLUSION
A shift in emphasis to vagal withdrawal has led to new forms of biofeedback that probably potentiate many of the same mechanisms thought to be present in Eastern practices such as yoga and tai chi. Results from small-scale trials have been promising for HRV biofeedback as a means of modifying responses to stress and promoting homeostatic processes that reduce the intensity of symptoms and improve surrogate markers associated with a number of disorders.
- Porges SW. Cardiac vagal tone: a physiological index of stress. Neurosci Biobehav Rev 1995; 19:225–233.
- Médigue C, Girard A, Laude D, Monti A, Wargon M, ElghoziJ-L. Relationship between pulse interval and respiratory sinusarrhythmia: a time- and frequency-domain analysis of the effects ofatropine. Eur J Physiol 2001; 441:650–655.
- Del Pozo JM, Gevirtz RN, Scher B, Guarneri E. Biofeedbacktreatment increases heart rate variability in patients withknown coronary artery disease. Am Heart J 2004; 147:e11. http://download.journals.elsevierhealth.com/pdfs/journals/0002-8703/PIIS0002870303007191.pdf. Accessed May 2, 2011.
- Kleiger RE, Miller JP, Bigger JT Jr, Moss AJ. Decreased heart ratevariability and its association with increased mortality after acutemyocardial infarciton. Am J Cardiol 1987; 59:256–262.
- van Dixhoorn JV, White A. Relaxation therapy for rehabilitationand prevention in ischaemic heart disease: a systematic review andmeta-analysis. Eur J Cardiovasc Prev Rehabil 2005; 12:193–202.
- Karavidas MK, Lehrer PM, Vaschillo E, et al. Preliminary resultsof an open label study of heart rate variability biofeedback for thetreatment of major depression. Appl Psychophysiol Biofeedback2007; 32:19–30.
- Zucker TL, Samuelson KW, Muench F, Greenberg MA, GevirtzRN. The effects of respiratory sinus arrhythmia biofeedback onheart rate variability and posttraumatic stress disorder symptoms: apilot study. Appl Psychophysiol Biofeedback 2009; 34:135–143.
- Swanson KS, Gevirtz RN, Brown M, Spira J, Guarneri E, StoletniyL. The effect of biofeedback on function in patients with heartfailure. Appl Psychophysiol Biofeedback 2009; 34:71–91.
Traditionally, biofeedback was considered to be a stress management technique that targeted sympathetic nervous system (SNS) overdrive with an adrenal medullary system backup. Recent advances in autonomic physiology, however, have clarified that except in extreme situations, the SNS is not the key factor in day-to-day stress. Rather, the parasympathetic branch of the autonomic nervous system appears to be a more likely candidate for mediating routine stress because, unlike the SNS, which has slow-acting neurotransmitters (ie, catecholamines), the parasympathetic nervous system has the fast-acting transmitter acetylcholine.
VAGAL WITHDRAWAL: AN ALTERNATIVE TO SYMPATHETIC ACTIVATION
Porges1 first proposed the concept of vagal withdrawal as an indicator of stress and stress vulnerability; this contrasts with the idea that the stress response is a consequence of sympathetic activation and the hypothalamic-pituitary-adrenal axis response. In the vagal withdrawal model, the response to stress is stabilization of the sympathetic system followed by termination of parasympathetic activity, manifested as cardiac acceleration.
Respiratory sinus arrhythmia (RSA), or the variability in heart rate as it synchronizes with breathing, is considered an index of parasympathetic tone. In the laboratory, slow atropine infusion produces a transient paradoxical vagomimetic effect characterized by an initial increase in RSA, followed by a flattening and then a rise in the heart rate.2 This phenomenon has been measured in people during times of routine stress, such as when worrying about being late for an appointment. In such individuals, biofeedback training can result in recovery of normal RSA shortly after an episode of anxiety.
Historically, the focus of biofeedback was to cultivate low arousal, presumably reducing SNS activity, through the use of finger temperature, skin conductance training, and profound muscle relaxation. More sophisticated ways to look at both branches of the autonomic nervous system have since emerged that allow for sampling of the beat-by-beat changes in heart rate.
HEART RATE VARIABILITY BIOFEEDBACK
The concept of modifying the respiration rate (paced breathing) originated some 2,500 years ago as a component of meditation. It is being revisited today in the form of heart rate variability (HRV) biofeedback training, which is being used as a stress-management tool and a method to correct disorders in which autonomic regulation is thought to be important. HRV biofeedback involves training to increase the amplitude of HRV rhythms and thus improve autonomic homeostasis.
Normal HRV has a pattern of overlapping oscillatory frequency components, including:
- a high-frequency rhythm, 0.15 to 0.4 Hz, which is the RSA;
- a low-frequency rhythm, 0.05 to 0.15 Hz, associated with blood pressure oscillations; and
- a very-low-frequency rhythm, 0.005 to 0.05 Hz, which may regulate vascular tone and body temperature.
The goal of HRV biofeedback is to achieve respiratory rates at which resonance occurs between cardiac rhythms associated with respiration (RSA, or high-frequency oscillations) and those caused by baroreflex activity (low-frequency oscillations).
Spectral analysis has demonstrated that nearly all of the activity with HRV biofeedback occurs at a low-frequency band. The reason is that activity in the low-frequency band is related more to baroreflex activity than to HRV compared with other ranges of frequency. Breathing rates that correspond to baroreflex effects, called resonance frequency breathing, represent resonance in the cardiovascular system. Several devices are available whose mechanisms are based on the concept of achieving resonance frequency breathing. One such device is a slow-breathing monitor (Resp-e-rate) that has been approved by the US Food and Drug Administration for the adjunctive treatment of hypertension.
Improved HRV may suggest an improved risk status: Kleiger et al4 found that the relative risk of mortality was 5.3 times greater for people with SDNN of less than 50 msec compared with those whose SDNN was greater than 100 msec. In Del Pozo’s study, eight of 30 patients in the intervention group achieved an SDNN of greater than 50 msec (vs 0 at pretreatment) compared with three of 31 controls (vs two at pretreatment).3 As an additional benefit of HRV biofeedback, patients in the intervention group who entered the study with hypertension all became normotensive.
In a meta-analysis, van Dixhoorn and White5 found fewer cardiac events, fewer episodes of angina, and less occurrence of arrhythmia and exercise-induced ischemia from intensive supervised relaxation therapy in patients with ischemic heart disease. Improvements in scales of depression and anxiety were also observed with relaxation therapy.
Other studies have shown biofeedback to have beneficial effects based on the Posttraumatic Stress Disorder Checklist, the Hamilton Depression Rating Scale, and, in patients with mild to moderate heartfailure, the 6-minute walk test.6–8
The proposed mechanism for the beneficial effects of biofeedback found in clinical trials is improvement in baroreflex function, producing greater reflex efficiency and improved modulation of autonomic activity.
CONCLUSION
A shift in emphasis to vagal withdrawal has led to new forms of biofeedback that probably potentiate many of the same mechanisms thought to be present in Eastern practices such as yoga and tai chi. Results from small-scale trials have been promising for HRV biofeedback as a means of modifying responses to stress and promoting homeostatic processes that reduce the intensity of symptoms and improve surrogate markers associated with a number of disorders.
Traditionally, biofeedback was considered to be a stress management technique that targeted sympathetic nervous system (SNS) overdrive with an adrenal medullary system backup. Recent advances in autonomic physiology, however, have clarified that except in extreme situations, the SNS is not the key factor in day-to-day stress. Rather, the parasympathetic branch of the autonomic nervous system appears to be a more likely candidate for mediating routine stress because, unlike the SNS, which has slow-acting neurotransmitters (ie, catecholamines), the parasympathetic nervous system has the fast-acting transmitter acetylcholine.
VAGAL WITHDRAWAL: AN ALTERNATIVE TO SYMPATHETIC ACTIVATION
Porges1 first proposed the concept of vagal withdrawal as an indicator of stress and stress vulnerability; this contrasts with the idea that the stress response is a consequence of sympathetic activation and the hypothalamic-pituitary-adrenal axis response. In the vagal withdrawal model, the response to stress is stabilization of the sympathetic system followed by termination of parasympathetic activity, manifested as cardiac acceleration.
Respiratory sinus arrhythmia (RSA), or the variability in heart rate as it synchronizes with breathing, is considered an index of parasympathetic tone. In the laboratory, slow atropine infusion produces a transient paradoxical vagomimetic effect characterized by an initial increase in RSA, followed by a flattening and then a rise in the heart rate.2 This phenomenon has been measured in people during times of routine stress, such as when worrying about being late for an appointment. In such individuals, biofeedback training can result in recovery of normal RSA shortly after an episode of anxiety.
Historically, the focus of biofeedback was to cultivate low arousal, presumably reducing SNS activity, through the use of finger temperature, skin conductance training, and profound muscle relaxation. More sophisticated ways to look at both branches of the autonomic nervous system have since emerged that allow for sampling of the beat-by-beat changes in heart rate.
HEART RATE VARIABILITY BIOFEEDBACK
The concept of modifying the respiration rate (paced breathing) originated some 2,500 years ago as a component of meditation. It is being revisited today in the form of heart rate variability (HRV) biofeedback training, which is being used as a stress-management tool and a method to correct disorders in which autonomic regulation is thought to be important. HRV biofeedback involves training to increase the amplitude of HRV rhythms and thus improve autonomic homeostasis.
Normal HRV has a pattern of overlapping oscillatory frequency components, including:
- a high-frequency rhythm, 0.15 to 0.4 Hz, which is the RSA;
- a low-frequency rhythm, 0.05 to 0.15 Hz, associated with blood pressure oscillations; and
- a very-low-frequency rhythm, 0.005 to 0.05 Hz, which may regulate vascular tone and body temperature.
The goal of HRV biofeedback is to achieve respiratory rates at which resonance occurs between cardiac rhythms associated with respiration (RSA, or high-frequency oscillations) and those caused by baroreflex activity (low-frequency oscillations).
Spectral analysis has demonstrated that nearly all of the activity with HRV biofeedback occurs at a low-frequency band. The reason is that activity in the low-frequency band is related more to baroreflex activity than to HRV compared with other ranges of frequency. Breathing rates that correspond to baroreflex effects, called resonance frequency breathing, represent resonance in the cardiovascular system. Several devices are available whose mechanisms are based on the concept of achieving resonance frequency breathing. One such device is a slow-breathing monitor (Resp-e-rate) that has been approved by the US Food and Drug Administration for the adjunctive treatment of hypertension.
Improved HRV may suggest an improved risk status: Kleiger et al4 found that the relative risk of mortality was 5.3 times greater for people with SDNN of less than 50 msec compared with those whose SDNN was greater than 100 msec. In Del Pozo’s study, eight of 30 patients in the intervention group achieved an SDNN of greater than 50 msec (vs 0 at pretreatment) compared with three of 31 controls (vs two at pretreatment).3 As an additional benefit of HRV biofeedback, patients in the intervention group who entered the study with hypertension all became normotensive.
In a meta-analysis, van Dixhoorn and White5 found fewer cardiac events, fewer episodes of angina, and less occurrence of arrhythmia and exercise-induced ischemia from intensive supervised relaxation therapy in patients with ischemic heart disease. Improvements in scales of depression and anxiety were also observed with relaxation therapy.
Other studies have shown biofeedback to have beneficial effects based on the Posttraumatic Stress Disorder Checklist, the Hamilton Depression Rating Scale, and, in patients with mild to moderate heartfailure, the 6-minute walk test.6–8
The proposed mechanism for the beneficial effects of biofeedback found in clinical trials is improvement in baroreflex function, producing greater reflex efficiency and improved modulation of autonomic activity.
CONCLUSION
A shift in emphasis to vagal withdrawal has led to new forms of biofeedback that probably potentiate many of the same mechanisms thought to be present in Eastern practices such as yoga and tai chi. Results from small-scale trials have been promising for HRV biofeedback as a means of modifying responses to stress and promoting homeostatic processes that reduce the intensity of symptoms and improve surrogate markers associated with a number of disorders.
- Porges SW. Cardiac vagal tone: a physiological index of stress. Neurosci Biobehav Rev 1995; 19:225–233.
- Médigue C, Girard A, Laude D, Monti A, Wargon M, ElghoziJ-L. Relationship between pulse interval and respiratory sinusarrhythmia: a time- and frequency-domain analysis of the effects ofatropine. Eur J Physiol 2001; 441:650–655.
- Del Pozo JM, Gevirtz RN, Scher B, Guarneri E. Biofeedbacktreatment increases heart rate variability in patients withknown coronary artery disease. Am Heart J 2004; 147:e11. http://download.journals.elsevierhealth.com/pdfs/journals/0002-8703/PIIS0002870303007191.pdf. Accessed May 2, 2011.
- Kleiger RE, Miller JP, Bigger JT Jr, Moss AJ. Decreased heart ratevariability and its association with increased mortality after acutemyocardial infarciton. Am J Cardiol 1987; 59:256–262.
- van Dixhoorn JV, White A. Relaxation therapy for rehabilitationand prevention in ischaemic heart disease: a systematic review andmeta-analysis. Eur J Cardiovasc Prev Rehabil 2005; 12:193–202.
- Karavidas MK, Lehrer PM, Vaschillo E, et al. Preliminary resultsof an open label study of heart rate variability biofeedback for thetreatment of major depression. Appl Psychophysiol Biofeedback2007; 32:19–30.
- Zucker TL, Samuelson KW, Muench F, Greenberg MA, GevirtzRN. The effects of respiratory sinus arrhythmia biofeedback onheart rate variability and posttraumatic stress disorder symptoms: apilot study. Appl Psychophysiol Biofeedback 2009; 34:135–143.
- Swanson KS, Gevirtz RN, Brown M, Spira J, Guarneri E, StoletniyL. The effect of biofeedback on function in patients with heartfailure. Appl Psychophysiol Biofeedback 2009; 34:71–91.
- Porges SW. Cardiac vagal tone: a physiological index of stress. Neurosci Biobehav Rev 1995; 19:225–233.
- Médigue C, Girard A, Laude D, Monti A, Wargon M, ElghoziJ-L. Relationship between pulse interval and respiratory sinusarrhythmia: a time- and frequency-domain analysis of the effects ofatropine. Eur J Physiol 2001; 441:650–655.
- Del Pozo JM, Gevirtz RN, Scher B, Guarneri E. Biofeedbacktreatment increases heart rate variability in patients withknown coronary artery disease. Am Heart J 2004; 147:e11. http://download.journals.elsevierhealth.com/pdfs/journals/0002-8703/PIIS0002870303007191.pdf. Accessed May 2, 2011.
- Kleiger RE, Miller JP, Bigger JT Jr, Moss AJ. Decreased heart ratevariability and its association with increased mortality after acutemyocardial infarciton. Am J Cardiol 1987; 59:256–262.
- van Dixhoorn JV, White A. Relaxation therapy for rehabilitationand prevention in ischaemic heart disease: a systematic review andmeta-analysis. Eur J Cardiovasc Prev Rehabil 2005; 12:193–202.
- Karavidas MK, Lehrer PM, Vaschillo E, et al. Preliminary resultsof an open label study of heart rate variability biofeedback for thetreatment of major depression. Appl Psychophysiol Biofeedback2007; 32:19–30.
- Zucker TL, Samuelson KW, Muench F, Greenberg MA, GevirtzRN. The effects of respiratory sinus arrhythmia biofeedback onheart rate variability and posttraumatic stress disorder symptoms: apilot study. Appl Psychophysiol Biofeedback 2009; 34:135–143.
- Swanson KS, Gevirtz RN, Brown M, Spira J, Guarneri E, StoletniyL. The effect of biofeedback on function in patients with heartfailure. Appl Psychophysiol Biofeedback 2009; 34:71–91.