User login
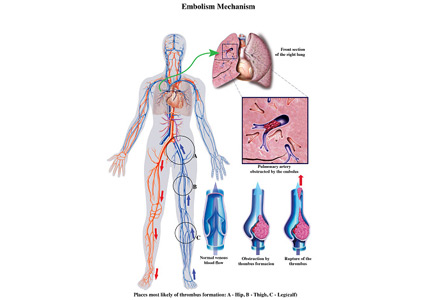
To the Editor: I read with interest the review on submassive pulmonary embolism by Ataya et al1 in the December 2016 issue. I had 3 questions or observations for the authors
First, systemic thrombolytic therapy for massive or hemodynamically unstable pulmonary embolism is given a grade 2C recommendation, similar to the level for select patients with submassive pulmonary embolism with low bleeding risk but at high risk of developing hypotension. The reference for this is the 2012 American College of Chest Physicians guidelines.2 I would like to point out that these guidelines were updated and published in February 2016,3 and systemic thrombolytic therapy for massive pulmonary embolism now carries a grade 2B recommendation. Thrombolytic therapy still has a grade 2C recommendation for select patients with submassive pulmonary embolism.
Second, the Moderate Pulmonary Embolism Treated With Thrombolysis (MOPETT) trial is described as a randomized trial in patients with moderate pulmonary hypertension and right ventricular dysfunction. I would like to point out that right ventricular dysfunction was not a criterion for enrollment in the trial.4
Finally, catheter-directed thrombolytic therapy is mentioned as an option for select patients with submassive and massive pulmonary embolism. The advantage is believed to be due to local action of the drug with fewer systemic effects. Since the protocol involves alteplase for 12 or 24 hours with a maximum dose of 24 mg, and since in most cases pulmonary embolism originates in the lower extremity, are we not exposing these patients to further clot propagation for 12 or 24 hours without the benefit of concomitant systemic anticoagulation or an inferior vena cava filter?
- Ataya A, Cope J, Shahmohammadi A, Alnuaimat H. Do patients with submassive pulmonary embolism benefit from thrombolytic therapy? Cleve Clin J Med 2016; 83:923–932.
- Kearon C, Akl EA, Comerota AJ, et al; American College of Chest Physicians. Antithrombotic therapy for VTE disease: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e419S–e494S.
- Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest 2016; 149:315–352.
- Sharifi M, Bay C, Skrocki L, Rahimi F, Mehdipour M; “MOPETT” Investigators. Moderate pulmonary embolism treated with thrombolysis (from the “MOPETT” Trial). Am J Cardiol 2013; 111:273–277.
To the Editor: I read with interest the review on submassive pulmonary embolism by Ataya et al1 in the December 2016 issue. I had 3 questions or observations for the authors
First, systemic thrombolytic therapy for massive or hemodynamically unstable pulmonary embolism is given a grade 2C recommendation, similar to the level for select patients with submassive pulmonary embolism with low bleeding risk but at high risk of developing hypotension. The reference for this is the 2012 American College of Chest Physicians guidelines.2 I would like to point out that these guidelines were updated and published in February 2016,3 and systemic thrombolytic therapy for massive pulmonary embolism now carries a grade 2B recommendation. Thrombolytic therapy still has a grade 2C recommendation for select patients with submassive pulmonary embolism.
Second, the Moderate Pulmonary Embolism Treated With Thrombolysis (MOPETT) trial is described as a randomized trial in patients with moderate pulmonary hypertension and right ventricular dysfunction. I would like to point out that right ventricular dysfunction was not a criterion for enrollment in the trial.4
Finally, catheter-directed thrombolytic therapy is mentioned as an option for select patients with submassive and massive pulmonary embolism. The advantage is believed to be due to local action of the drug with fewer systemic effects. Since the protocol involves alteplase for 12 or 24 hours with a maximum dose of 24 mg, and since in most cases pulmonary embolism originates in the lower extremity, are we not exposing these patients to further clot propagation for 12 or 24 hours without the benefit of concomitant systemic anticoagulation or an inferior vena cava filter?
To the Editor: I read with interest the review on submassive pulmonary embolism by Ataya et al1 in the December 2016 issue. I had 3 questions or observations for the authors
First, systemic thrombolytic therapy for massive or hemodynamically unstable pulmonary embolism is given a grade 2C recommendation, similar to the level for select patients with submassive pulmonary embolism with low bleeding risk but at high risk of developing hypotension. The reference for this is the 2012 American College of Chest Physicians guidelines.2 I would like to point out that these guidelines were updated and published in February 2016,3 and systemic thrombolytic therapy for massive pulmonary embolism now carries a grade 2B recommendation. Thrombolytic therapy still has a grade 2C recommendation for select patients with submassive pulmonary embolism.
Second, the Moderate Pulmonary Embolism Treated With Thrombolysis (MOPETT) trial is described as a randomized trial in patients with moderate pulmonary hypertension and right ventricular dysfunction. I would like to point out that right ventricular dysfunction was not a criterion for enrollment in the trial.4
Finally, catheter-directed thrombolytic therapy is mentioned as an option for select patients with submassive and massive pulmonary embolism. The advantage is believed to be due to local action of the drug with fewer systemic effects. Since the protocol involves alteplase for 12 or 24 hours with a maximum dose of 24 mg, and since in most cases pulmonary embolism originates in the lower extremity, are we not exposing these patients to further clot propagation for 12 or 24 hours without the benefit of concomitant systemic anticoagulation or an inferior vena cava filter?
- Ataya A, Cope J, Shahmohammadi A, Alnuaimat H. Do patients with submassive pulmonary embolism benefit from thrombolytic therapy? Cleve Clin J Med 2016; 83:923–932.
- Kearon C, Akl EA, Comerota AJ, et al; American College of Chest Physicians. Antithrombotic therapy for VTE disease: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e419S–e494S.
- Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest 2016; 149:315–352.
- Sharifi M, Bay C, Skrocki L, Rahimi F, Mehdipour M; “MOPETT” Investigators. Moderate pulmonary embolism treated with thrombolysis (from the “MOPETT” Trial). Am J Cardiol 2013; 111:273–277.
- Ataya A, Cope J, Shahmohammadi A, Alnuaimat H. Do patients with submassive pulmonary embolism benefit from thrombolytic therapy? Cleve Clin J Med 2016; 83:923–932.
- Kearon C, Akl EA, Comerota AJ, et al; American College of Chest Physicians. Antithrombotic therapy for VTE disease: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e419S–e494S.
- Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest 2016; 149:315–352.
- Sharifi M, Bay C, Skrocki L, Rahimi F, Mehdipour M; “MOPETT” Investigators. Moderate pulmonary embolism treated with thrombolysis (from the “MOPETT” Trial). Am J Cardiol 2013; 111:273–277.