User login
CASE Violent, then catatonic
Mr. T, age 52, has a long history of schizoaffective disorder, depressed type; several suicide attempts; and violent episodes. He is admitted to a mental health rehabilitation center under a forensic commitment.
Several years earlier, Mr. T had been charged with first-degree attempted murder, assault with a deadly weapon, and abuse of a dependent/geriatric adult after allegedly stabbing his mother in the upper chest and neck. At that time, Mr. T was not in psychiatric treatment and was drinking heavily. He had become obsessed with John F. Kennedy’s assassination and believed the Central Intelligence Agency (CIA), not Lee Harvey Oswald, was responsible. He feared the CIA wanted to kill him because of his knowledge, and he heard voices from his television he believed were threatening him. He acquired knives for self-protection. When his mother arrived at his apartment to take him to a psychiatric appointment, he believed she was conspiring with the CIA and attacked her. Mr. T’s mother survived her injuries. He was taken to the county jail, where psychiatric staff noted that Mr. T was psychotic.
The court found Mr. T incompetent to stand trial and sent him to a state hospital for psychiatric treatment and competency restoration. After 3 years, he was declared unable to be restored because of repeated decompensations, placed on a conservatorship, and sent back to county jail.
In the jail, Mr. T began to show signs of catatonia. He refused medications, food, and water, and became mute. He was admitted to a medical center after a 45-minute episode that appeared similar to a seizure; however, all laboratory evaluations were within normal limits, head CT was negative, and an EEG was unremarkable.
Mr. T’s catatonic state gradually resolved with increasing dosages of lorazepam, as well as clozapine. He showed improved mobility and oral intake. A month later, his train of thought was rambling and difficult to follow, circumstantial, and perseverating. However, at times he could be directed and respond to questions in a linear and logical fashion. Lorazepam was tapered, discontinued, and replaced with gabapentin because Mr. T viewed taking lorazepam as a threat to his sobriety.
Recently, Mr. T was transferred to our mental health rehabilitation center, where he expresses that he is grateful to be in a therapeutic environment. Upon admission, his medication regimen consists of clozapine, 300 mg by mouth at bedtime, duloxetine, 60 mg/d by mouth, gabapentin 600 mg by mouth 3 times a day, and docusate sodium, 250 mg/d by mouth. Our team has a discussion about the growing recognition of the pro-inflammatory state present in many patients who experience serious mental illness and the importance of augmenting standard evidence-based psychopharmacotherapy with agents that have neuroprotective properties.1,2 We offer Mr. T
[polldaddy:10375843]
The authors’ observations
Several studies have found that acute psychosis is associated with an inflammatory state, and interleukin-6 (IL-6) is a crucial biomarker. A recent meta-analysis of serum cytokines in patients with schizophrenia found that IL-6 levels were significantly increased among acutely ill patients compared with controls.3 IL-6 levels significantly decreased after treating acute episodes of schizophrenia.3 Further, levels of peripheral IL-6 mRNA levels in individuals with schizophrenia are directly correlated with severity of positive symptoms.4
Continue to: A meta-analyis reported...
A meta-analysis reported that tumor necrosis factor-alpha and IL-6 are elevated during acute psychosis3; however, IL-6 normalized with treatment, whereas tumor necrosis factor-alpha did not. This means that IL-6 is a more clinically meaningful biomarker to help gauge treatment response.
EVALUATION Elevated markers of inflammation
Laboratory testing reveals that Mr. T’s IL-6 level is 56.64 pg/mL, which is significantly elevated (reference range: 0.31 to 5.00 pg/mL). After reviewing the IL-6 results with Mr. T and explaining that there is “too much inflammation” in his brain, he agrees to take minocycline and complete follow-up IL-6 level tests to monitor his progress during treatment.
HISTORY Alcohol abuse, treatment resistance
According to Mr. T’s mother, he had met all developmental milestones and graduated from high school with plans to enter culinary school. At age 20, Mr. T began to experience psychotic symptoms, telling family members that he was being followed by FBI agents and was receiving messages from televisions. He began drinking heavily and was arrested twice for driving under the influence. In his mid-20s, he attempted suicide by overdose after his father died. Mr. T required inpatient hospitalization nearly every year thereafter. His mother, a registered nurse, was significantly involved in his care and carefully documented his treatment history.
Mr. T has had numerous medication trials, including oral and long-acting injectable risperidone, olanzapine, aripiprazole, ziprasidone, lithium, gabapentin, buspirone, quetiapine, trazodone, bupropion, and paroxetine. None of these medications were effective.
In his mid-40s, Mr. T attempted suicide by wandering into traffic and being struck by a motor vehicle. A year later, he attempted suicide by driving his car at high speed into a concrete highway median. Mr. T told first responders that he was “possessed,” and a demonic entity “forced” him to crash his car. He begged law enforcement officers at the scene to give him a gun so he could shoot himself.
Continue to: Mr. T entered an intensive outpatient treatment program...
Mr. T entered an intensive outpatient treatment program and was switched from long-acting injectable risperidone to oral aripiprazole. After taking aripiprazole for several weeks, he began to gamble compulsively at a nearby casino. Frustrated by the lack of response to psychotropic medications and his idiosyncratic response to aripiprazole, he stopped psychiatric treatment, relapsed to alcohol use, and isolated himself in his apartment shortly before stabbing his mother.
EVALUATION Pharmacogenomics testing
At the mental health rehabilitation center, Mr. T agrees to undergo pharmacogenomics testing, which suggests that he will have a normal response to selective serotonin reuptake inhibitors and is unlikely to experience adverse reactions. He does not carry the 2 alleles that place him at higher risk of serious dermatologic reactions when taking certain mood stabilizers. He is heterozygous for the C677T allele polymorphism in the MTHFR gene that is associated with reduced folic acid metabolism, moderately decreased serum folate levels, and moderately increased homocysteine levels. On the pharmacokinetic genes tested, Mr. T has the normal metabolism genotype on 5 of 6 cytochrome P450 (CYP) enzymes; he has the ultrarapid metabolizer genotype on CYP1A2. He also has normal activity and intermediate metabolizer phenotype on the 2 UGT enzymes tested, which are responsible for the glucuronidation process, a major part of phase II metabolism.
Based on these results, Mr. T’s clozapine dosage is decreased by 50% (from 300 to 150 mg/d) and he is started on fluvoxamine, 50 mg/d, because it is a strong inhibitor of CYP1A2. The reduced conversion of clozapine to norclozapine results in an average serum clozapine level of 527 ng/mL (a level of 350 ng/mL is usually therapeutic in patients with schizophrenia) and norclozapine level of 140 ng/mL (clozapine:norclozapine ratio = 3.8), which is to be expected because fluvoxamine can increase serum clozapine levels.
Due to accumulating evidence in the literature suggesting that latent infections in the CNS play a role in serious mental illnesses such as schizophrenia, Mr. T undergoes further laboratory testing.
[polldaddy:10375845]
The authors’ observations
Mr. T tested positive for TG and CMV and negative for HSV-1. We were aware of accumulating evidence that latent infections in the CNS play a role in serious mental illnesses such as schizophrenia, specifically TG5—a parasite transmitted by cats—and CMV and HSV-1,6 which are transmitted by humans. The theory that TG infection could be a factor in schizophrenia emerged in the 1990s but only in recent years received mainstream scientific attention. Toxoplasma gondii, the infectious parasite that causes toxoplasmosis, infects more than 30 million people in the United States; however, most individuals are asymptomatic because of the body’s immune response to the parasite.7
Continue to: A study of 162 individuals...
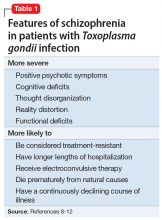
A study of 162 individuals with schizophrenia, bipolar disorder, or major depressive disorder found that this immunologic profile is associated with suicide attempts,8 which is consistent with Mr. T’s history. Research suggests that individuals with schizophrenia who have latent TG infection have a more severe form of the illness compared with patients without the infection.9-12 Many of these factors were present in Mr. T’s case (Table 18-12).
TREATMENT Improvement, then setback
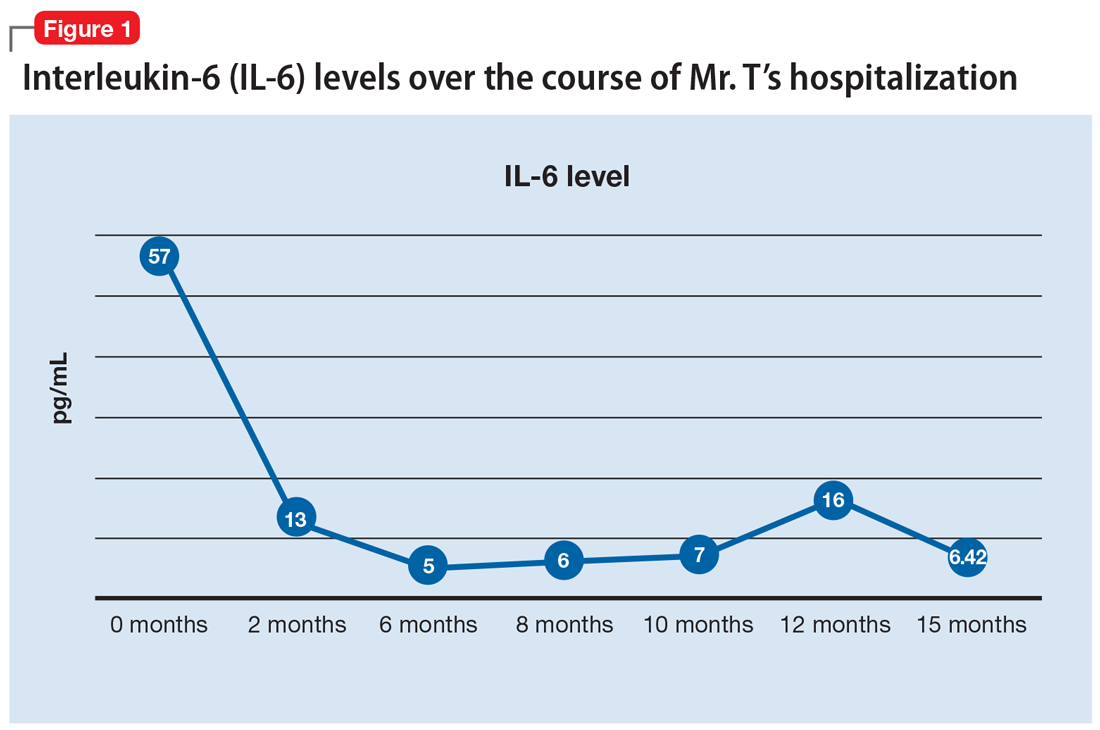
Mr. T’s medication regimen at the rehabilitation center includes clozapine, 100 mg/d; minocycline, 200 mg/d; fluvoxamine, 200 mg/d; and N-acetylcysteine, 1,200 mg/d. N-acetylcysteine is an antioxidant that could ease negative symptoms of schizophrenia by reducing oxidative stress caused by free radicals.13 Mr. T makes slow but steady improvement, and his IL-6 levels drop steadily (Figure 1).
After 6 months in the rehabilitation center, Mr. T no longer experiences catatonic symptoms and is able to participate in the therapeutic program. He is permitted to leave the facility on day passes with family members. However, approximately every 8 weeks, he continues to cycle through periods of intense anxiety, perseverates on topics, and exhibits fragmented thinking and speech. During these episodes, he has difficulty receiving and processing information.
During one of these periods, Mr. T eats 4 oleander leaves he gathered while on day pass outside of the facility. After he experiences stomach pain, nausea, and vomiting, he informs nursing staff that he ate oleander. He is brought to the emergency department, receives activated charcoal and a digoxin antidote, and is placed on continuous electrocardiogram monitoring. When asked why he made the suicide attempt, he said “I realized things will never be the same because of what happened. I felt trapped.” He later expresses regret and wants to return to the mental health rehabilitation center.
At the facility, Mr. T agrees to take 2 more agents—valproic acid and ginger root extract—that specifically target latent toxoplasmosis infection before pursuing electroconvulsive therapy. We offer valproic acid because it inhibits replication of TG in an in vitro model.14 Mr. T is started on extended-release valproic acid, 1,500 mg/d, which results in a therapeutic serum level of 74.8 µg/mL.
Continue to: Additionally, Mr. T expresses interest...
Additionally, Mr. T expresses interest in taking “natural” agents in addition to psychotropics. After reviewing the quality of available ginger root extract products, Mr. T is started on a supplement that contains 22.4 mg of gingerols and 6.7 mg of shogaols, titrated to 4 capsules twice daily.
The authors’ observations
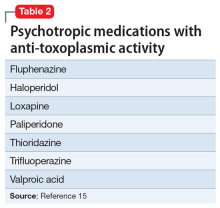
A retrospective cross-sectional analysis reported that patients with bipolar disorder who received medications with anti-toxoplasmic activity (Table 215), specifically valproic acid, had significantly fewer lifetime depressive episodes compared with patients who received medications without anti-toxoplasmic activity.15
Alternative medicine options
Research has demonstrated the beneficial effects of Chinese herbal plants for toxoplasmosis16,17 and ginger root extract has potent anti-toxoplasmic activity. A mouse model found that ginger root extract (Zingiber officinale) reduced the number of TG-infected cells by suppressing activation of apoptotic proteins the parasite induces, which prevents programmed cell death.18
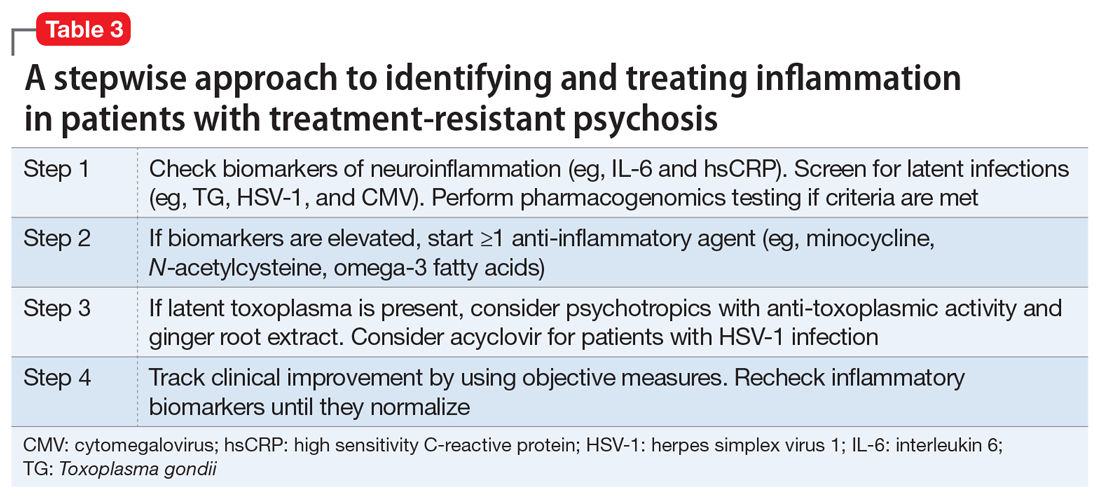
Table 3 presents a stepwise approach to identifying and treating inflammation in patients with treatment-resistant psychosis.
OUTCOME Immune response, improvement
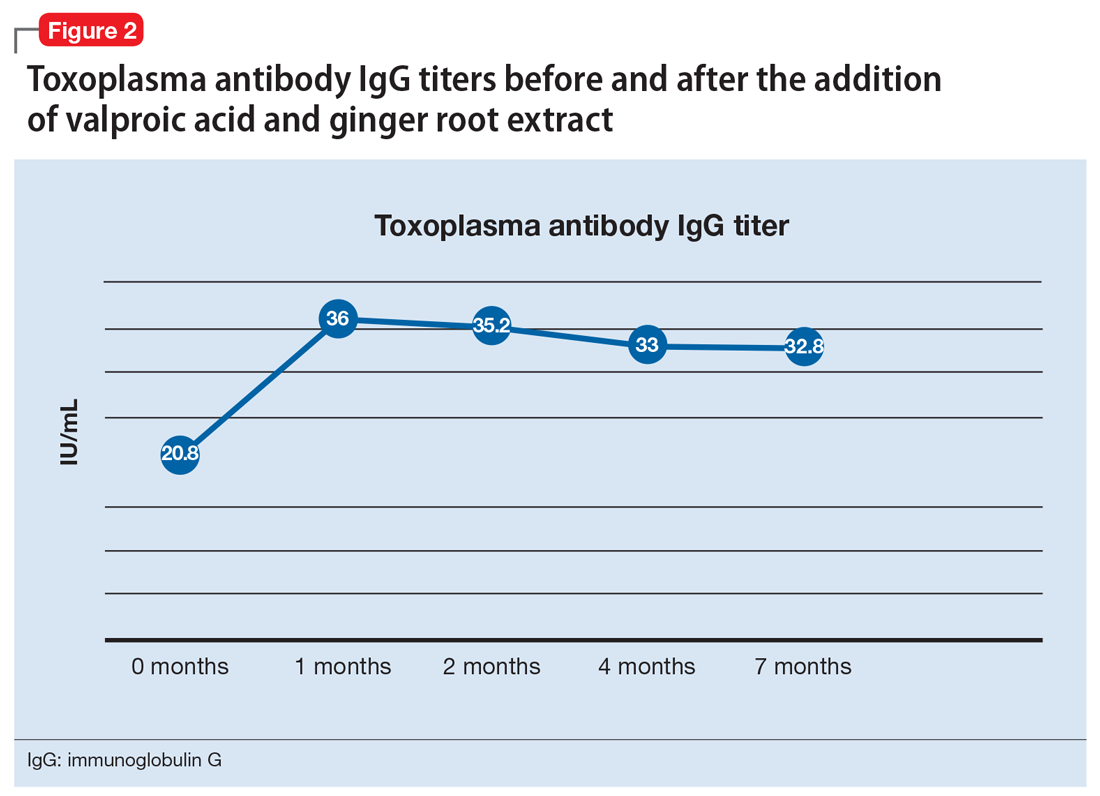
One month after the valproic acid and ginger root extract therapy is initiated, Mr. T’s toxoplasma antibody immunoglobulin G increases by 15.2 IU/mL, indicating that his immune system is mounting an enhanced response against the parasite (Figure 2). Mr. T continues to make progress while receiving the new regimen of clozapine, minocycline, valproic acid, and ginger root extract. He no longer cycles into periods of intense anxiety, perseverative thought, and fragmented thought and speech. He participates meaningfully in weekly psychotherapy and hopes to live independently and obtain gainful employment.
The District Attorney’s office dismisses his criminal charges, and Mr. T is discharged to a less restrictive level of care.
Continue to: Bottom Line
Bottom Line
Several studies have shown that neuroinflammation increases the severity of mental illness. Consider adjunct anti-inflammatory agents for patients who have elevated levels of inflammatory biomarkers and for whom standard treatment approaches do not adequately control psychiatric symptoms. Also consider testing for the presence of latent infections in the CNS, which could reveal the underlying cause of treatment resistance or the genesis of disabling psychiatric symptoms.
Related Resources
- Fond G, Macgregor A, Tamouza R, et al. Comparative analysis of anti-toxoplasmic activity of antipsychotic drugs and valproate. Eur Arch Psychiatry Clin Neurosci. 2014;264(2):179-183.
- Hamdani N, Daban-Huard C, Lajnef M, et al. Cognitive deterioration among bipolar disorder patients infected by Toxoplasma gondii is correlated to interleukin 6 levels. J Affect Disord. 2015;179:161-166.
- Monroe JM, Buckley PF, Miller BJ. Meta-analysis of antitoxoplasma gondii IgM antibodies in acute psychosis. Schizophr Bull. 2015;41(4):989-998.
Drug Brand Names
Acyclovir • Zovirax
Aripiprazole • Abilify
Bupropion • Wellbutrin
Buspirone • Buspar
Clozapine • Clozaril
Duloxetine • Cymbalta
Fluphenazine • Prolixin
Fluvoxamine • Luvox
Gabapentin • Neurontin
Haloperidol • Haldol
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Loxapine • Loxitane
Minocycline • Minocin
Olanzapine • Zyprexa
Paliperidone • Invega
Paroxetine • Paxil
Quetiapine • Seroquel
Risperidone • Risperdal, Risperdal Consta
Thioridazine • Mellaril
Trifluoperazine • Stelazine
Trazodone • Desyrel
Valproic acid • Depakote
Ziprasidone • Geodon
1. Koola MM, Raines JK, Hamilton RG, et al. Can anti-inflammatory medications improve symptoms and reduce mortality in schizophrenia? Current Psychiatry. 2016;15(5):52-57.
2. Nasrallah HA. Are you neuroprotecting your patients? 10 Adjunctive therapies to consider. Current Psychiatry. 2016;15(12):12-14.
3. Goldsmith DR, Rapaport MH, Miller BJ. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry. 2016;21(12):1696-1709.
4. Chase KA, Cone JJ, Rosen C, et al. The value of interleukin 6 as a peripheral diagnostic marker in schizophrenia. BMC Psychiatry. 2016;16:152.
5. Torrey EF, Bartko JJ, Lun ZR, et al. Antibodies to Toxoplasma gondii in patients with schizophrenia: a meta-analysis. Schizophr Bull. 2007;33(3):729-736.
6. Shirts BH, Prasad KM, Pogue-Geile MF, et al. Antibodies to cytomegalovirus and herpes simplex virus 1 associated with cognitive function in schizophrenia. Schizophr Res. 2008;106(2-3):268-274.
7. Centers for Disease Control and Prevention. Parasites - Toxoplasmosis (Toxoplasma infection). https://www.cdc.gov/parasites/toxoplasmosis/index.html. Accessed February 26, 2019.
8. Dickerson F, Wilcox HC, Adamos M, et al. Suicide attempts and markers of immune response in individuals with serious mental illness. J Psychiatr Res. 2017;87:37-43.
9. Celik T, Kartalci S, Aytas O, et al. Association between latent toxoplasmosis and clinical course of schizophrenia - continuous course of the disease is characteristic for Toxoplasma gondii-infected patients. Folia Parasitol (Praha). 2015;62. doi: 10.14411/fp.2015.015.
10. Dickerson F, Boronow J, Stallings C, et al. Toxoplasma gondii in individuals with schizophrenia: association with clinical and demographic factors and with mortality. Schizophr Bull. 2007;33(3):737-740.
11. Esshili A, Thabet S, Jemli A, et al. Toxoplasma gondii infection in schizophrenia and associated clinical features. Psychiatry Res. 2016;245:327-332.
12. Holub D, Flegr J, Dragomirecka E, et al. Differences in onset of disease and severity of psychopathology between toxoplasmosis-related and toxoplasmosis-unrelated schizophrenia. Acta Psychiatr Scand. 2013;127(3):227-238.
13. Chen AT, Chibnall JT, Nasrallah HA. Placebo-controlled augmentation trials of the antioxidant NAC in schizophrenia: a review. Ann Clin Psychiatry. 2016;28(3):190-196.
14. Jones-Brando L, Torrey EF, Yolken R. Drugs used in the treatment of schizophrenia and bipolar disorder inhibit the replication of Toxoplasma gondii. Schizophr Res. 2003;62(3):237-244.
15. Fond G, Boyer L, Gaman A, et al. Treatment with anti-toxoplasmic activity (TATA) for toxoplasma positive patients with bipolar disorders or schizophrenia: a cross-sectional study. J Psychiatr Res. 2015;63:58-64.
16. Wei HX, Wei SS, Lindsay DS, et al. A systematic review and meta-analysis of the efficacy of anti-Toxoplasma gondii medicines in humans. PLoS One. 2015;10(9):e0138204.
17. Zhuo XH, Sun HC, Huang B, et al. Evaluation of potential anti-toxoplasmosis efficiency of combined traditional herbs in a mouse model. J Zhejiang Univ Sci B. 2017;18(6):453-461.
18. Choi WH, Jiang MH, Chu JP. Antiparasitic effects of Zingiber officinale (Ginger) extract against Toxoplasma gondii. Journal of Applied Biomedicine. 2013;11:15-26.
CASE Violent, then catatonic
Mr. T, age 52, has a long history of schizoaffective disorder, depressed type; several suicide attempts; and violent episodes. He is admitted to a mental health rehabilitation center under a forensic commitment.
Several years earlier, Mr. T had been charged with first-degree attempted murder, assault with a deadly weapon, and abuse of a dependent/geriatric adult after allegedly stabbing his mother in the upper chest and neck. At that time, Mr. T was not in psychiatric treatment and was drinking heavily. He had become obsessed with John F. Kennedy’s assassination and believed the Central Intelligence Agency (CIA), not Lee Harvey Oswald, was responsible. He feared the CIA wanted to kill him because of his knowledge, and he heard voices from his television he believed were threatening him. He acquired knives for self-protection. When his mother arrived at his apartment to take him to a psychiatric appointment, he believed she was conspiring with the CIA and attacked her. Mr. T’s mother survived her injuries. He was taken to the county jail, where psychiatric staff noted that Mr. T was psychotic.
The court found Mr. T incompetent to stand trial and sent him to a state hospital for psychiatric treatment and competency restoration. After 3 years, he was declared unable to be restored because of repeated decompensations, placed on a conservatorship, and sent back to county jail.
In the jail, Mr. T began to show signs of catatonia. He refused medications, food, and water, and became mute. He was admitted to a medical center after a 45-minute episode that appeared similar to a seizure; however, all laboratory evaluations were within normal limits, head CT was negative, and an EEG was unremarkable.
Mr. T’s catatonic state gradually resolved with increasing dosages of lorazepam, as well as clozapine. He showed improved mobility and oral intake. A month later, his train of thought was rambling and difficult to follow, circumstantial, and perseverating. However, at times he could be directed and respond to questions in a linear and logical fashion. Lorazepam was tapered, discontinued, and replaced with gabapentin because Mr. T viewed taking lorazepam as a threat to his sobriety.
Recently, Mr. T was transferred to our mental health rehabilitation center, where he expresses that he is grateful to be in a therapeutic environment. Upon admission, his medication regimen consists of clozapine, 300 mg by mouth at bedtime, duloxetine, 60 mg/d by mouth, gabapentin 600 mg by mouth 3 times a day, and docusate sodium, 250 mg/d by mouth. Our team has a discussion about the growing recognition of the pro-inflammatory state present in many patients who experience serious mental illness and the importance of augmenting standard evidence-based psychopharmacotherapy with agents that have neuroprotective properties.1,2 We offer Mr. T
[polldaddy:10375843]
The authors’ observations
Several studies have found that acute psychosis is associated with an inflammatory state, and interleukin-6 (IL-6) is a crucial biomarker. A recent meta-analysis of serum cytokines in patients with schizophrenia found that IL-6 levels were significantly increased among acutely ill patients compared with controls.3 IL-6 levels significantly decreased after treating acute episodes of schizophrenia.3 Further, levels of peripheral IL-6 mRNA levels in individuals with schizophrenia are directly correlated with severity of positive symptoms.4
Continue to: A meta-analyis reported...
A meta-analysis reported that tumor necrosis factor-alpha and IL-6 are elevated during acute psychosis3; however, IL-6 normalized with treatment, whereas tumor necrosis factor-alpha did not. This means that IL-6 is a more clinically meaningful biomarker to help gauge treatment response.
EVALUATION Elevated markers of inflammation
Laboratory testing reveals that Mr. T’s IL-6 level is 56.64 pg/mL, which is significantly elevated (reference range: 0.31 to 5.00 pg/mL). After reviewing the IL-6 results with Mr. T and explaining that there is “too much inflammation” in his brain, he agrees to take minocycline and complete follow-up IL-6 level tests to monitor his progress during treatment.
HISTORY Alcohol abuse, treatment resistance
According to Mr. T’s mother, he had met all developmental milestones and graduated from high school with plans to enter culinary school. At age 20, Mr. T began to experience psychotic symptoms, telling family members that he was being followed by FBI agents and was receiving messages from televisions. He began drinking heavily and was arrested twice for driving under the influence. In his mid-20s, he attempted suicide by overdose after his father died. Mr. T required inpatient hospitalization nearly every year thereafter. His mother, a registered nurse, was significantly involved in his care and carefully documented his treatment history.
Mr. T has had numerous medication trials, including oral and long-acting injectable risperidone, olanzapine, aripiprazole, ziprasidone, lithium, gabapentin, buspirone, quetiapine, trazodone, bupropion, and paroxetine. None of these medications were effective.
In his mid-40s, Mr. T attempted suicide by wandering into traffic and being struck by a motor vehicle. A year later, he attempted suicide by driving his car at high speed into a concrete highway median. Mr. T told first responders that he was “possessed,” and a demonic entity “forced” him to crash his car. He begged law enforcement officers at the scene to give him a gun so he could shoot himself.
Continue to: Mr. T entered an intensive outpatient treatment program...
Mr. T entered an intensive outpatient treatment program and was switched from long-acting injectable risperidone to oral aripiprazole. After taking aripiprazole for several weeks, he began to gamble compulsively at a nearby casino. Frustrated by the lack of response to psychotropic medications and his idiosyncratic response to aripiprazole, he stopped psychiatric treatment, relapsed to alcohol use, and isolated himself in his apartment shortly before stabbing his mother.
EVALUATION Pharmacogenomics testing
At the mental health rehabilitation center, Mr. T agrees to undergo pharmacogenomics testing, which suggests that he will have a normal response to selective serotonin reuptake inhibitors and is unlikely to experience adverse reactions. He does not carry the 2 alleles that place him at higher risk of serious dermatologic reactions when taking certain mood stabilizers. He is heterozygous for the C677T allele polymorphism in the MTHFR gene that is associated with reduced folic acid metabolism, moderately decreased serum folate levels, and moderately increased homocysteine levels. On the pharmacokinetic genes tested, Mr. T has the normal metabolism genotype on 5 of 6 cytochrome P450 (CYP) enzymes; he has the ultrarapid metabolizer genotype on CYP1A2. He also has normal activity and intermediate metabolizer phenotype on the 2 UGT enzymes tested, which are responsible for the glucuronidation process, a major part of phase II metabolism.
Based on these results, Mr. T’s clozapine dosage is decreased by 50% (from 300 to 150 mg/d) and he is started on fluvoxamine, 50 mg/d, because it is a strong inhibitor of CYP1A2. The reduced conversion of clozapine to norclozapine results in an average serum clozapine level of 527 ng/mL (a level of 350 ng/mL is usually therapeutic in patients with schizophrenia) and norclozapine level of 140 ng/mL (clozapine:norclozapine ratio = 3.8), which is to be expected because fluvoxamine can increase serum clozapine levels.
Due to accumulating evidence in the literature suggesting that latent infections in the CNS play a role in serious mental illnesses such as schizophrenia, Mr. T undergoes further laboratory testing.
[polldaddy:10375845]
The authors’ observations
Mr. T tested positive for TG and CMV and negative for HSV-1. We were aware of accumulating evidence that latent infections in the CNS play a role in serious mental illnesses such as schizophrenia, specifically TG5—a parasite transmitted by cats—and CMV and HSV-1,6 which are transmitted by humans. The theory that TG infection could be a factor in schizophrenia emerged in the 1990s but only in recent years received mainstream scientific attention. Toxoplasma gondii, the infectious parasite that causes toxoplasmosis, infects more than 30 million people in the United States; however, most individuals are asymptomatic because of the body’s immune response to the parasite.7
Continue to: A study of 162 individuals...
A study of 162 individuals with schizophrenia, bipolar disorder, or major depressive disorder found that this immunologic profile is associated with suicide attempts,8 which is consistent with Mr. T’s history. Research suggests that individuals with schizophrenia who have latent TG infection have a more severe form of the illness compared with patients without the infection.9-12 Many of these factors were present in Mr. T’s case (Table 18-12).
TREATMENT Improvement, then setback
Mr. T’s medication regimen at the rehabilitation center includes clozapine, 100 mg/d; minocycline, 200 mg/d; fluvoxamine, 200 mg/d; and N-acetylcysteine, 1,200 mg/d. N-acetylcysteine is an antioxidant that could ease negative symptoms of schizophrenia by reducing oxidative stress caused by free radicals.13 Mr. T makes slow but steady improvement, and his IL-6 levels drop steadily (Figure 1).
After 6 months in the rehabilitation center, Mr. T no longer experiences catatonic symptoms and is able to participate in the therapeutic program. He is permitted to leave the facility on day passes with family members. However, approximately every 8 weeks, he continues to cycle through periods of intense anxiety, perseverates on topics, and exhibits fragmented thinking and speech. During these episodes, he has difficulty receiving and processing information.
During one of these periods, Mr. T eats 4 oleander leaves he gathered while on day pass outside of the facility. After he experiences stomach pain, nausea, and vomiting, he informs nursing staff that he ate oleander. He is brought to the emergency department, receives activated charcoal and a digoxin antidote, and is placed on continuous electrocardiogram monitoring. When asked why he made the suicide attempt, he said “I realized things will never be the same because of what happened. I felt trapped.” He later expresses regret and wants to return to the mental health rehabilitation center.
At the facility, Mr. T agrees to take 2 more agents—valproic acid and ginger root extract—that specifically target latent toxoplasmosis infection before pursuing electroconvulsive therapy. We offer valproic acid because it inhibits replication of TG in an in vitro model.14 Mr. T is started on extended-release valproic acid, 1,500 mg/d, which results in a therapeutic serum level of 74.8 µg/mL.
Continue to: Additionally, Mr. T expresses interest...
Additionally, Mr. T expresses interest in taking “natural” agents in addition to psychotropics. After reviewing the quality of available ginger root extract products, Mr. T is started on a supplement that contains 22.4 mg of gingerols and 6.7 mg of shogaols, titrated to 4 capsules twice daily.
The authors’ observations
A retrospective cross-sectional analysis reported that patients with bipolar disorder who received medications with anti-toxoplasmic activity (Table 215), specifically valproic acid, had significantly fewer lifetime depressive episodes compared with patients who received medications without anti-toxoplasmic activity.15
Alternative medicine options
Research has demonstrated the beneficial effects of Chinese herbal plants for toxoplasmosis16,17 and ginger root extract has potent anti-toxoplasmic activity. A mouse model found that ginger root extract (Zingiber officinale) reduced the number of TG-infected cells by suppressing activation of apoptotic proteins the parasite induces, which prevents programmed cell death.18
Table 3 presents a stepwise approach to identifying and treating inflammation in patients with treatment-resistant psychosis.
OUTCOME Immune response, improvement
One month after the valproic acid and ginger root extract therapy is initiated, Mr. T’s toxoplasma antibody immunoglobulin G increases by 15.2 IU/mL, indicating that his immune system is mounting an enhanced response against the parasite (Figure 2). Mr. T continues to make progress while receiving the new regimen of clozapine, minocycline, valproic acid, and ginger root extract. He no longer cycles into periods of intense anxiety, perseverative thought, and fragmented thought and speech. He participates meaningfully in weekly psychotherapy and hopes to live independently and obtain gainful employment.
The District Attorney’s office dismisses his criminal charges, and Mr. T is discharged to a less restrictive level of care.
Continue to: Bottom Line
Bottom Line
Several studies have shown that neuroinflammation increases the severity of mental illness. Consider adjunct anti-inflammatory agents for patients who have elevated levels of inflammatory biomarkers and for whom standard treatment approaches do not adequately control psychiatric symptoms. Also consider testing for the presence of latent infections in the CNS, which could reveal the underlying cause of treatment resistance or the genesis of disabling psychiatric symptoms.
Related Resources
- Fond G, Macgregor A, Tamouza R, et al. Comparative analysis of anti-toxoplasmic activity of antipsychotic drugs and valproate. Eur Arch Psychiatry Clin Neurosci. 2014;264(2):179-183.
- Hamdani N, Daban-Huard C, Lajnef M, et al. Cognitive deterioration among bipolar disorder patients infected by Toxoplasma gondii is correlated to interleukin 6 levels. J Affect Disord. 2015;179:161-166.
- Monroe JM, Buckley PF, Miller BJ. Meta-analysis of antitoxoplasma gondii IgM antibodies in acute psychosis. Schizophr Bull. 2015;41(4):989-998.
Drug Brand Names
Acyclovir • Zovirax
Aripiprazole • Abilify
Bupropion • Wellbutrin
Buspirone • Buspar
Clozapine • Clozaril
Duloxetine • Cymbalta
Fluphenazine • Prolixin
Fluvoxamine • Luvox
Gabapentin • Neurontin
Haloperidol • Haldol
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Loxapine • Loxitane
Minocycline • Minocin
Olanzapine • Zyprexa
Paliperidone • Invega
Paroxetine • Paxil
Quetiapine • Seroquel
Risperidone • Risperdal, Risperdal Consta
Thioridazine • Mellaril
Trifluoperazine • Stelazine
Trazodone • Desyrel
Valproic acid • Depakote
Ziprasidone • Geodon
CASE Violent, then catatonic
Mr. T, age 52, has a long history of schizoaffective disorder, depressed type; several suicide attempts; and violent episodes. He is admitted to a mental health rehabilitation center under a forensic commitment.
Several years earlier, Mr. T had been charged with first-degree attempted murder, assault with a deadly weapon, and abuse of a dependent/geriatric adult after allegedly stabbing his mother in the upper chest and neck. At that time, Mr. T was not in psychiatric treatment and was drinking heavily. He had become obsessed with John F. Kennedy’s assassination and believed the Central Intelligence Agency (CIA), not Lee Harvey Oswald, was responsible. He feared the CIA wanted to kill him because of his knowledge, and he heard voices from his television he believed were threatening him. He acquired knives for self-protection. When his mother arrived at his apartment to take him to a psychiatric appointment, he believed she was conspiring with the CIA and attacked her. Mr. T’s mother survived her injuries. He was taken to the county jail, where psychiatric staff noted that Mr. T was psychotic.
The court found Mr. T incompetent to stand trial and sent him to a state hospital for psychiatric treatment and competency restoration. After 3 years, he was declared unable to be restored because of repeated decompensations, placed on a conservatorship, and sent back to county jail.
In the jail, Mr. T began to show signs of catatonia. He refused medications, food, and water, and became mute. He was admitted to a medical center after a 45-minute episode that appeared similar to a seizure; however, all laboratory evaluations were within normal limits, head CT was negative, and an EEG was unremarkable.
Mr. T’s catatonic state gradually resolved with increasing dosages of lorazepam, as well as clozapine. He showed improved mobility and oral intake. A month later, his train of thought was rambling and difficult to follow, circumstantial, and perseverating. However, at times he could be directed and respond to questions in a linear and logical fashion. Lorazepam was tapered, discontinued, and replaced with gabapentin because Mr. T viewed taking lorazepam as a threat to his sobriety.
Recently, Mr. T was transferred to our mental health rehabilitation center, where he expresses that he is grateful to be in a therapeutic environment. Upon admission, his medication regimen consists of clozapine, 300 mg by mouth at bedtime, duloxetine, 60 mg/d by mouth, gabapentin 600 mg by mouth 3 times a day, and docusate sodium, 250 mg/d by mouth. Our team has a discussion about the growing recognition of the pro-inflammatory state present in many patients who experience serious mental illness and the importance of augmenting standard evidence-based psychopharmacotherapy with agents that have neuroprotective properties.1,2 We offer Mr. T
[polldaddy:10375843]
The authors’ observations
Several studies have found that acute psychosis is associated with an inflammatory state, and interleukin-6 (IL-6) is a crucial biomarker. A recent meta-analysis of serum cytokines in patients with schizophrenia found that IL-6 levels were significantly increased among acutely ill patients compared with controls.3 IL-6 levels significantly decreased after treating acute episodes of schizophrenia.3 Further, levels of peripheral IL-6 mRNA levels in individuals with schizophrenia are directly correlated with severity of positive symptoms.4
Continue to: A meta-analyis reported...
A meta-analysis reported that tumor necrosis factor-alpha and IL-6 are elevated during acute psychosis3; however, IL-6 normalized with treatment, whereas tumor necrosis factor-alpha did not. This means that IL-6 is a more clinically meaningful biomarker to help gauge treatment response.
EVALUATION Elevated markers of inflammation
Laboratory testing reveals that Mr. T’s IL-6 level is 56.64 pg/mL, which is significantly elevated (reference range: 0.31 to 5.00 pg/mL). After reviewing the IL-6 results with Mr. T and explaining that there is “too much inflammation” in his brain, he agrees to take minocycline and complete follow-up IL-6 level tests to monitor his progress during treatment.
HISTORY Alcohol abuse, treatment resistance
According to Mr. T’s mother, he had met all developmental milestones and graduated from high school with plans to enter culinary school. At age 20, Mr. T began to experience psychotic symptoms, telling family members that he was being followed by FBI agents and was receiving messages from televisions. He began drinking heavily and was arrested twice for driving under the influence. In his mid-20s, he attempted suicide by overdose after his father died. Mr. T required inpatient hospitalization nearly every year thereafter. His mother, a registered nurse, was significantly involved in his care and carefully documented his treatment history.
Mr. T has had numerous medication trials, including oral and long-acting injectable risperidone, olanzapine, aripiprazole, ziprasidone, lithium, gabapentin, buspirone, quetiapine, trazodone, bupropion, and paroxetine. None of these medications were effective.
In his mid-40s, Mr. T attempted suicide by wandering into traffic and being struck by a motor vehicle. A year later, he attempted suicide by driving his car at high speed into a concrete highway median. Mr. T told first responders that he was “possessed,” and a demonic entity “forced” him to crash his car. He begged law enforcement officers at the scene to give him a gun so he could shoot himself.
Continue to: Mr. T entered an intensive outpatient treatment program...
Mr. T entered an intensive outpatient treatment program and was switched from long-acting injectable risperidone to oral aripiprazole. After taking aripiprazole for several weeks, he began to gamble compulsively at a nearby casino. Frustrated by the lack of response to psychotropic medications and his idiosyncratic response to aripiprazole, he stopped psychiatric treatment, relapsed to alcohol use, and isolated himself in his apartment shortly before stabbing his mother.
EVALUATION Pharmacogenomics testing
At the mental health rehabilitation center, Mr. T agrees to undergo pharmacogenomics testing, which suggests that he will have a normal response to selective serotonin reuptake inhibitors and is unlikely to experience adverse reactions. He does not carry the 2 alleles that place him at higher risk of serious dermatologic reactions when taking certain mood stabilizers. He is heterozygous for the C677T allele polymorphism in the MTHFR gene that is associated with reduced folic acid metabolism, moderately decreased serum folate levels, and moderately increased homocysteine levels. On the pharmacokinetic genes tested, Mr. T has the normal metabolism genotype on 5 of 6 cytochrome P450 (CYP) enzymes; he has the ultrarapid metabolizer genotype on CYP1A2. He also has normal activity and intermediate metabolizer phenotype on the 2 UGT enzymes tested, which are responsible for the glucuronidation process, a major part of phase II metabolism.
Based on these results, Mr. T’s clozapine dosage is decreased by 50% (from 300 to 150 mg/d) and he is started on fluvoxamine, 50 mg/d, because it is a strong inhibitor of CYP1A2. The reduced conversion of clozapine to norclozapine results in an average serum clozapine level of 527 ng/mL (a level of 350 ng/mL is usually therapeutic in patients with schizophrenia) and norclozapine level of 140 ng/mL (clozapine:norclozapine ratio = 3.8), which is to be expected because fluvoxamine can increase serum clozapine levels.
Due to accumulating evidence in the literature suggesting that latent infections in the CNS play a role in serious mental illnesses such as schizophrenia, Mr. T undergoes further laboratory testing.
[polldaddy:10375845]
The authors’ observations
Mr. T tested positive for TG and CMV and negative for HSV-1. We were aware of accumulating evidence that latent infections in the CNS play a role in serious mental illnesses such as schizophrenia, specifically TG5—a parasite transmitted by cats—and CMV and HSV-1,6 which are transmitted by humans. The theory that TG infection could be a factor in schizophrenia emerged in the 1990s but only in recent years received mainstream scientific attention. Toxoplasma gondii, the infectious parasite that causes toxoplasmosis, infects more than 30 million people in the United States; however, most individuals are asymptomatic because of the body’s immune response to the parasite.7
Continue to: A study of 162 individuals...
A study of 162 individuals with schizophrenia, bipolar disorder, or major depressive disorder found that this immunologic profile is associated with suicide attempts,8 which is consistent with Mr. T’s history. Research suggests that individuals with schizophrenia who have latent TG infection have a more severe form of the illness compared with patients without the infection.9-12 Many of these factors were present in Mr. T’s case (Table 18-12).
TREATMENT Improvement, then setback
Mr. T’s medication regimen at the rehabilitation center includes clozapine, 100 mg/d; minocycline, 200 mg/d; fluvoxamine, 200 mg/d; and N-acetylcysteine, 1,200 mg/d. N-acetylcysteine is an antioxidant that could ease negative symptoms of schizophrenia by reducing oxidative stress caused by free radicals.13 Mr. T makes slow but steady improvement, and his IL-6 levels drop steadily (Figure 1).
After 6 months in the rehabilitation center, Mr. T no longer experiences catatonic symptoms and is able to participate in the therapeutic program. He is permitted to leave the facility on day passes with family members. However, approximately every 8 weeks, he continues to cycle through periods of intense anxiety, perseverates on topics, and exhibits fragmented thinking and speech. During these episodes, he has difficulty receiving and processing information.
During one of these periods, Mr. T eats 4 oleander leaves he gathered while on day pass outside of the facility. After he experiences stomach pain, nausea, and vomiting, he informs nursing staff that he ate oleander. He is brought to the emergency department, receives activated charcoal and a digoxin antidote, and is placed on continuous electrocardiogram monitoring. When asked why he made the suicide attempt, he said “I realized things will never be the same because of what happened. I felt trapped.” He later expresses regret and wants to return to the mental health rehabilitation center.
At the facility, Mr. T agrees to take 2 more agents—valproic acid and ginger root extract—that specifically target latent toxoplasmosis infection before pursuing electroconvulsive therapy. We offer valproic acid because it inhibits replication of TG in an in vitro model.14 Mr. T is started on extended-release valproic acid, 1,500 mg/d, which results in a therapeutic serum level of 74.8 µg/mL.
Continue to: Additionally, Mr. T expresses interest...
Additionally, Mr. T expresses interest in taking “natural” agents in addition to psychotropics. After reviewing the quality of available ginger root extract products, Mr. T is started on a supplement that contains 22.4 mg of gingerols and 6.7 mg of shogaols, titrated to 4 capsules twice daily.
The authors’ observations
A retrospective cross-sectional analysis reported that patients with bipolar disorder who received medications with anti-toxoplasmic activity (Table 215), specifically valproic acid, had significantly fewer lifetime depressive episodes compared with patients who received medications without anti-toxoplasmic activity.15
Alternative medicine options
Research has demonstrated the beneficial effects of Chinese herbal plants for toxoplasmosis16,17 and ginger root extract has potent anti-toxoplasmic activity. A mouse model found that ginger root extract (Zingiber officinale) reduced the number of TG-infected cells by suppressing activation of apoptotic proteins the parasite induces, which prevents programmed cell death.18
Table 3 presents a stepwise approach to identifying and treating inflammation in patients with treatment-resistant psychosis.
OUTCOME Immune response, improvement
One month after the valproic acid and ginger root extract therapy is initiated, Mr. T’s toxoplasma antibody immunoglobulin G increases by 15.2 IU/mL, indicating that his immune system is mounting an enhanced response against the parasite (Figure 2). Mr. T continues to make progress while receiving the new regimen of clozapine, minocycline, valproic acid, and ginger root extract. He no longer cycles into periods of intense anxiety, perseverative thought, and fragmented thought and speech. He participates meaningfully in weekly psychotherapy and hopes to live independently and obtain gainful employment.
The District Attorney’s office dismisses his criminal charges, and Mr. T is discharged to a less restrictive level of care.
Continue to: Bottom Line
Bottom Line
Several studies have shown that neuroinflammation increases the severity of mental illness. Consider adjunct anti-inflammatory agents for patients who have elevated levels of inflammatory biomarkers and for whom standard treatment approaches do not adequately control psychiatric symptoms. Also consider testing for the presence of latent infections in the CNS, which could reveal the underlying cause of treatment resistance or the genesis of disabling psychiatric symptoms.
Related Resources
- Fond G, Macgregor A, Tamouza R, et al. Comparative analysis of anti-toxoplasmic activity of antipsychotic drugs and valproate. Eur Arch Psychiatry Clin Neurosci. 2014;264(2):179-183.
- Hamdani N, Daban-Huard C, Lajnef M, et al. Cognitive deterioration among bipolar disorder patients infected by Toxoplasma gondii is correlated to interleukin 6 levels. J Affect Disord. 2015;179:161-166.
- Monroe JM, Buckley PF, Miller BJ. Meta-analysis of antitoxoplasma gondii IgM antibodies in acute psychosis. Schizophr Bull. 2015;41(4):989-998.
Drug Brand Names
Acyclovir • Zovirax
Aripiprazole • Abilify
Bupropion • Wellbutrin
Buspirone • Buspar
Clozapine • Clozaril
Duloxetine • Cymbalta
Fluphenazine • Prolixin
Fluvoxamine • Luvox
Gabapentin • Neurontin
Haloperidol • Haldol
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Loxapine • Loxitane
Minocycline • Minocin
Olanzapine • Zyprexa
Paliperidone • Invega
Paroxetine • Paxil
Quetiapine • Seroquel
Risperidone • Risperdal, Risperdal Consta
Thioridazine • Mellaril
Trifluoperazine • Stelazine
Trazodone • Desyrel
Valproic acid • Depakote
Ziprasidone • Geodon
1. Koola MM, Raines JK, Hamilton RG, et al. Can anti-inflammatory medications improve symptoms and reduce mortality in schizophrenia? Current Psychiatry. 2016;15(5):52-57.
2. Nasrallah HA. Are you neuroprotecting your patients? 10 Adjunctive therapies to consider. Current Psychiatry. 2016;15(12):12-14.
3. Goldsmith DR, Rapaport MH, Miller BJ. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry. 2016;21(12):1696-1709.
4. Chase KA, Cone JJ, Rosen C, et al. The value of interleukin 6 as a peripheral diagnostic marker in schizophrenia. BMC Psychiatry. 2016;16:152.
5. Torrey EF, Bartko JJ, Lun ZR, et al. Antibodies to Toxoplasma gondii in patients with schizophrenia: a meta-analysis. Schizophr Bull. 2007;33(3):729-736.
6. Shirts BH, Prasad KM, Pogue-Geile MF, et al. Antibodies to cytomegalovirus and herpes simplex virus 1 associated with cognitive function in schizophrenia. Schizophr Res. 2008;106(2-3):268-274.
7. Centers for Disease Control and Prevention. Parasites - Toxoplasmosis (Toxoplasma infection). https://www.cdc.gov/parasites/toxoplasmosis/index.html. Accessed February 26, 2019.
8. Dickerson F, Wilcox HC, Adamos M, et al. Suicide attempts and markers of immune response in individuals with serious mental illness. J Psychiatr Res. 2017;87:37-43.
9. Celik T, Kartalci S, Aytas O, et al. Association between latent toxoplasmosis and clinical course of schizophrenia - continuous course of the disease is characteristic for Toxoplasma gondii-infected patients. Folia Parasitol (Praha). 2015;62. doi: 10.14411/fp.2015.015.
10. Dickerson F, Boronow J, Stallings C, et al. Toxoplasma gondii in individuals with schizophrenia: association with clinical and demographic factors and with mortality. Schizophr Bull. 2007;33(3):737-740.
11. Esshili A, Thabet S, Jemli A, et al. Toxoplasma gondii infection in schizophrenia and associated clinical features. Psychiatry Res. 2016;245:327-332.
12. Holub D, Flegr J, Dragomirecka E, et al. Differences in onset of disease and severity of psychopathology between toxoplasmosis-related and toxoplasmosis-unrelated schizophrenia. Acta Psychiatr Scand. 2013;127(3):227-238.
13. Chen AT, Chibnall JT, Nasrallah HA. Placebo-controlled augmentation trials of the antioxidant NAC in schizophrenia: a review. Ann Clin Psychiatry. 2016;28(3):190-196.
14. Jones-Brando L, Torrey EF, Yolken R. Drugs used in the treatment of schizophrenia and bipolar disorder inhibit the replication of Toxoplasma gondii. Schizophr Res. 2003;62(3):237-244.
15. Fond G, Boyer L, Gaman A, et al. Treatment with anti-toxoplasmic activity (TATA) for toxoplasma positive patients with bipolar disorders or schizophrenia: a cross-sectional study. J Psychiatr Res. 2015;63:58-64.
16. Wei HX, Wei SS, Lindsay DS, et al. A systematic review and meta-analysis of the efficacy of anti-Toxoplasma gondii medicines in humans. PLoS One. 2015;10(9):e0138204.
17. Zhuo XH, Sun HC, Huang B, et al. Evaluation of potential anti-toxoplasmosis efficiency of combined traditional herbs in a mouse model. J Zhejiang Univ Sci B. 2017;18(6):453-461.
18. Choi WH, Jiang MH, Chu JP. Antiparasitic effects of Zingiber officinale (Ginger) extract against Toxoplasma gondii. Journal of Applied Biomedicine. 2013;11:15-26.
1. Koola MM, Raines JK, Hamilton RG, et al. Can anti-inflammatory medications improve symptoms and reduce mortality in schizophrenia? Current Psychiatry. 2016;15(5):52-57.
2. Nasrallah HA. Are you neuroprotecting your patients? 10 Adjunctive therapies to consider. Current Psychiatry. 2016;15(12):12-14.
3. Goldsmith DR, Rapaport MH, Miller BJ. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry. 2016;21(12):1696-1709.
4. Chase KA, Cone JJ, Rosen C, et al. The value of interleukin 6 as a peripheral diagnostic marker in schizophrenia. BMC Psychiatry. 2016;16:152.
5. Torrey EF, Bartko JJ, Lun ZR, et al. Antibodies to Toxoplasma gondii in patients with schizophrenia: a meta-analysis. Schizophr Bull. 2007;33(3):729-736.
6. Shirts BH, Prasad KM, Pogue-Geile MF, et al. Antibodies to cytomegalovirus and herpes simplex virus 1 associated with cognitive function in schizophrenia. Schizophr Res. 2008;106(2-3):268-274.
7. Centers for Disease Control and Prevention. Parasites - Toxoplasmosis (Toxoplasma infection). https://www.cdc.gov/parasites/toxoplasmosis/index.html. Accessed February 26, 2019.
8. Dickerson F, Wilcox HC, Adamos M, et al. Suicide attempts and markers of immune response in individuals with serious mental illness. J Psychiatr Res. 2017;87:37-43.
9. Celik T, Kartalci S, Aytas O, et al. Association between latent toxoplasmosis and clinical course of schizophrenia - continuous course of the disease is characteristic for Toxoplasma gondii-infected patients. Folia Parasitol (Praha). 2015;62. doi: 10.14411/fp.2015.015.
10. Dickerson F, Boronow J, Stallings C, et al. Toxoplasma gondii in individuals with schizophrenia: association with clinical and demographic factors and with mortality. Schizophr Bull. 2007;33(3):737-740.
11. Esshili A, Thabet S, Jemli A, et al. Toxoplasma gondii infection in schizophrenia and associated clinical features. Psychiatry Res. 2016;245:327-332.
12. Holub D, Flegr J, Dragomirecka E, et al. Differences in onset of disease and severity of psychopathology between toxoplasmosis-related and toxoplasmosis-unrelated schizophrenia. Acta Psychiatr Scand. 2013;127(3):227-238.
13. Chen AT, Chibnall JT, Nasrallah HA. Placebo-controlled augmentation trials of the antioxidant NAC in schizophrenia: a review. Ann Clin Psychiatry. 2016;28(3):190-196.
14. Jones-Brando L, Torrey EF, Yolken R. Drugs used in the treatment of schizophrenia and bipolar disorder inhibit the replication of Toxoplasma gondii. Schizophr Res. 2003;62(3):237-244.
15. Fond G, Boyer L, Gaman A, et al. Treatment with anti-toxoplasmic activity (TATA) for toxoplasma positive patients with bipolar disorders or schizophrenia: a cross-sectional study. J Psychiatr Res. 2015;63:58-64.
16. Wei HX, Wei SS, Lindsay DS, et al. A systematic review and meta-analysis of the efficacy of anti-Toxoplasma gondii medicines in humans. PLoS One. 2015;10(9):e0138204.
17. Zhuo XH, Sun HC, Huang B, et al. Evaluation of potential anti-toxoplasmosis efficiency of combined traditional herbs in a mouse model. J Zhejiang Univ Sci B. 2017;18(6):453-461.
18. Choi WH, Jiang MH, Chu JP. Antiparasitic effects of Zingiber officinale (Ginger) extract against Toxoplasma gondii. Journal of Applied Biomedicine. 2013;11:15-26.