Entrectinib, an investigational drug that targets several abnormal fusion proteins, showed antitumor activity and was safe in patients with several different types of advanced solid tumors. The patients had never before been exposed to drugs targeting these same genetic alterations.
“Responses can be very rapid and durable … which include colorectal, primary brain tumor, astrocytoma, fibrosarcoma, lung, and mammary analog secretory carcinoma,” Dr. Alexander Drilon of Memorial Sloan Kettering Cancer Center in New York said in a news conference at the annual meeting of the American Association for Cancer Research. “Dramatic intracranial activity … has been demonstrated both in primary brain tumor and also in metastatic.”
NTRK1/2/3, ROS1, and ALK gene–rearranged cancers produce fusion proteins that are ligand independent for their activity and thus constitutively active, driving tumor growth. Entrectinib is a pan-TRK, ROS1, ALK tyrosine kinase inhibitor that targets the abnormal fusion protein products of the genes, is highly potent at low concentrations, and has been designed to cross the blood-brain barrier (BBB). The targeted proteins are present across multiple cancers and are especially prevalent (greater than 80%) among some rare adult and pediatric cancers.
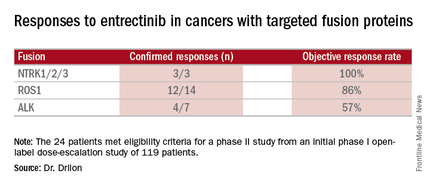
Combined data on 119 patients in two phase I trials established 600 mg orally once daily as the recommended dose to go into phase II trials. Among the 24 patients meeting eligibility criteria for a phase II trial (presence of the targeted gene fusions in their tumors, no prior treatment against these targets, and treatment at or above 600 mg daily), the confirmed response rate was 79% (19/24). Most were partial responses in terms of tumor shrinkage, but two patients had complete responses. Response rates appeared to vary according to the specific fusion protein defect.
All three cases of CNS disease with NTRK-rearranged cancers had intracranial responses, demonstrating that the drug crosses the BBB and is active. In one case, a 46-year-old man with brain metastases heavily pretreated for non–small cell lung cancer with an NTRK1 rearrangement experienced a dramatic response.
“The patient at that point was actually on hospice and was doing extremely poorly on supplemental oxygen,” Dr. Drilon said. “Within a few weeks, the patient had a dramatic clinical response to therapy … At day 26 there was almost a 50% reduction in tumor burden.” At day 317 scans showed he had a complete intracranial response to entrectinib, but he still has visceral disease on therapy past 1 year.
Responses often occurred within the first month of therapy, and many persisted for several months without disease progression, with one patient being followed for more than 2 years with clinical benefit. Nineteen of 24 patients have been on the therapy for more than 6 months, and the therapy appears to be safe and well tolerated.
Commenting on this study and others targeting specific genetic alterations leading to cancer, Dr. Louis Weiner, director of the Georgetown Lombardi Comprehensive Cancer Center in Washington, said, “You’re seeing a series of clinical trials described that aren’t necessarily targeting people with a particular cancer but rather people who have cancers characterized by particular molecular abnormalities.” Not all cancers will have identified molecular abnormalities driving them. “However, I think where you have these drivers, the proper thing to do is not to worry about whether [a drug] works in a given disease but rather whether it works for people with that particular abnormality,” he said.
For the future, the investigators plan a phase II trial called STARTRK-2. It is a multicenter, open-label, global basket study to include any solid tumors with the targeted rearrangements.
Dr. Drilon disclosed ties with Ignyta, which funded the study, and has received research funding from Foundation Medicine. Dr. Weiner disclosed ties with several pharmaceutical companies.