User login
Emergency physicians (EPs) are always interested in what are the “tried-and-true” as well as the “latest-and-greatest” devices that will provide the best results for their patients. This article, while not a comprehensive list of every such device introduced over the past few years, does provide an overview of the most notable ones applicable for use in the ED.
Tonometry
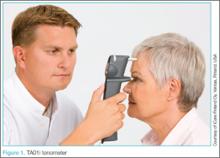
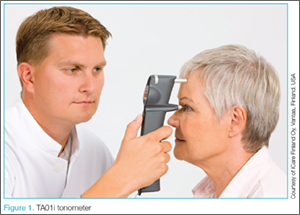
The standard iCare tonometer device (TA01i; iCare Finland Oy, Vantaa, Finland),1 began to gain acceptance in the United States in 2007 (Figure 1). Early studies2 have shown its measurement accuracy of intraocular pressure (IOP) to be equivalent to traditional tonometers such as the Tono-Pen XL Applanation Tonometer (Reichert Techonologies, Depew, New York).3
The iCare tonometer is easy to calibrate and use. Consisting of a pin inserted into a magnetic housing, the magnet quickly pushes the blunt end of the pin out to make contact with the cornea. Six quick measurements provide the clinician with an average IOP. The device can be used without anesthesia and is also applicable for at-home use.
Airway Devices
C-MAC Tip System
While direct laryngoscopy will always have a role in clinical practice, there has been a revolution in airway management over the past few years, with video laryngoscopy rapidly replacing direct laryngoscopy. The C-MAC Tip system (Karl Storz Endoscopy-America, Inc, El Segundo, California) is one of the devices currently available.4,5 While this device is not at the lower end of the cost spectrum in airway devices, it is, in this author’s opinion, among the highest quality video laryngoscopes on the market. The hub of C-MAC Tip system is a video screen that accepts input from multiple devices. The most common is the video MacIntosh blade, which is shaped like a traditional MacIntosh but with a slightly thicker handle—allowing both direct and indirect intubation.
The C-MAC Tip system is a great teaching tool, allowing learners to perform direct laryngoscopy while providing reassuring visualization to the instructor of the intubation on the screen. (No longer does the instructor need to repeatedly ask the learner what he or she is viewing!) Moreover, when required, the clinician performing the intubation can look at the screen to benefit from the superior visualization of indirect laryngoscopy.
The C-MAC can also accept input from the D-Blade, which facilitates indirect intubation of anterior airways; however, it does not allow direct intubation when secretions or blood obscure the camera, though there is a suction channel that assists in clearing secretions. The clinician can also add a nasal pharyngeal scope as well as an adult or pediatric bronchoscope. The modularity of the C-MAC system is therefore a flexible addition to any airway armamentarium.
Regarding its use in emergency medicine, in addition to cost considerations, a potential concern is the ability of the plastic adapters to hold up to frequent, repetitive use in a setting such as a busy ED.
Wireless Vital Signs Monitoring Systems
Patient vital signs monitoring systems can currently double as four-point restraints. One new device, the ViSi Mobile Monitor System (Sotera Wireless, Inc, San Diego, CA), however, may make this a thing of the past.6 This system allows for inpatient monitoring of respiratory rate (RR), pulse oximetry, continuous blood pressure (BP), and temperature, as well as a multilead electrocardiogram. The entire system attaches to a small wrist-mounted device, which connects to a hospital monitoring system through a WiFi network (Figures 2a and 2b).ViSi Mobile Monitor
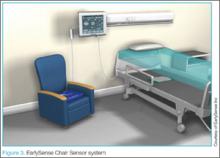
Another example of a wireless monitoring device is the EarlySense Chair Sensor system (EarlySense, Ltd, Ramat Gan, Israel) (Figure 3).7 This device assesses heart rate (HR) and RR simply by seating the patient in a chair. In the near future, this device will likely have the ability to take full vital signs. While not quite ready for prime-time in the ED, systems such as the EarlySense Chair Sensor offer a glimpse of the future in vital sign monitoring technology.
Vascular Access
Traditional intravenous (IV) line placement continues to be the standard of care for vascular access, but is not always feasible. Intraosseous needles, which have been around for decades, are seeing a new renaissance of use thanks to devices such as the Arrow EZ-IO Intraosseous Vascular Access System (Teleflex, Shavano Park, Texas).8 With these standards in mind, some new considerations are on the market, including the AV400 vein visualization system (AccuVein, Inc, Huntington, New York).
Arrow EZ-IO Intraosseous Vascular Access System
Teleflex, the maker of the EZ-IO, has recently made a push for humeral placement in order to achieve faster flow rates. Teleflex recommends using the longer needles (normally reserved for obese patients) with specific placement suggestions to facilitate retention of the needle. The Teleflex Web site8 and mobile application provide succinct, easy-to-understand instructions on placement.
AV400 Vein Visualization System
In addition to the Intraosseous Vascular Access System, the placement of an IV line may also be facilitated by laser devices such as AccuVein’s AV400 vein visualization technology. While earlier versions of both of these systems were comparable to that of a skilled technician in obtaining line placement, the latest generations of these devices have improved depth and visualization of truly difficult vascular access.
Internet-Connected Smart Glasses
In addition to the above-mentioned evolutionary changes in facilitating venous access, revolutionary technological advancements have been in development and are forthcoming. One such technology is network-connected smart glasses.
Eyes-On Glasses
Among the first in network-connected eyewear is Eyes-On Glasses (Evena Medical Inc, Los Altos, California).9 This system functions in a similar manner as other laser systems, except that the screen is a display mounted on eyeglasses, making venous placement much more intuitive (This is especially helpful for those who have difficulty translating the three-dimensional world to a two-dimensional screen).
Another variation on head-mounted technology is General Electric’s (GE) beta software for Google Glass (Google Inc, Mountain View, California) (Figure 4). This prototype links a GE ultrasound machine to Google Glass via WiFi, again simultaneously facilitating visualization of the field and the screen.10 While Google Glass is not currently available for the general public, there is still a place for it in the clinical setting.
Both the Eyes-On Glasses and Google Glass devices share a common thread: to improve patient comfort and facilitate time-consuming procedures.
Wound Care
Aquacel Ag
The EP sees a fair share of burn victims, the standard of care for which has been silver sulfadiazine and daily dressing changes. Care for burn wounds is beginning to change with the introduction of antimicrobial impregnated dressings such as Aquacel Ag (ConvaTec Inc, Greensboro, North Carolina).11 Aquacel Ag comes packaged in various forms and sizes ranging from large sheets for torsos to custom formed gloves for hands. This product is safe to use on the face and can be applied to partial thickness burns where the dermal layer is gone. The fluid from the wound moistens the bandage and helps it adhere to the skin. The dressings are then left in place until they slough-off on their own (approximately 7 to 10 days after placement). Consequently, no dressing changes are required, with cosmesis matching that of classic treatment.
While EPs may find Aquacel Ag useful in treating burns that do not require inpatient hospital admission, they also will find its use highly beneficial in treating patients with “road rash,” the abrasions that occur when one wipes-out at high speeds on asphalt (eg, motorcycle accidents). As with burn wounds, only a single application of Aquacel Ag is required on a debrided abrasion.
With respect to price, a single application of Aquacel Ag costs roughly the same as multiple dressing changes with other wound-care products.12 One concern relating to the use of advanced silver-impregnated dressings is the cost of care since silver-impregnated dressings are relatively expensive compared to traditional dressings. The higher cost, however, is partially offset by the reduced use of secondary gauze, and retention dressings, as well as improved wound healing together with the reduced costs of other care. Cost-effectiveness calculations comparing Aquacel Ag to standard of care in patients with acute and chronic wounds showed favorable results using Aquacel Ag.12-18
When using these dressings, the EP should make sure the follow-up clinic is familiar with their application so that they are not inadvertently removed at the patient’s first visit.
Medication Event Monitoring Systems
There have been a couple of recent changes in medication monitoring that are beginning to make manual pill-counting a thing of the past. Earlier generations of smart pill bottles came with a timer and an alarm that chimed and lit up to alert the patient when it was time to take his or her medication. Once the bottle was opened, the system reset itself. Unfortunately, the basic nature of these systems was not able to account for the number of pills a patient ingested at each scheduled dosing.
An example of newer and more technologically advanced pill-monitoring systems is AdhereTech’s smart wireless pill bottle (AdhereTech, New York, New York). In this system, the pill bottle can be connected to a WiFi network, allowing medication information to be shared (Figure 5).19 For example, a user could have the bottle connected to his or her provider, home healthcare worker, and family member. If a patient misses a dose of medication, the appropriate person receives notification and can make contact with the patient or family member to intervene. As with earlier generation products, these systems cannot account for or prevent a patient from either overdosing or underdosing on a medication.
The patch and sensor-enabled pill system, the Ingestible Event Marker, (Proteus Digital Health, Redwood City, California), which became available this past year, provides more advanced medication monitoring.20 This system allows tracking of individual pills through small chips imbedded in the tablet (Figure 6). The chip is then monitored through a patch worn on the patient’s body. Once connected, the physician is able to not only track when a pill bottle is opened, but also when and how many tablets the patient is ingesting. Moreover, the system has the ability to perform physiologic tracking to monitor patient response to the medication.21
Each of the above systems is a huge benefit to elderly patients and their geographically-separated families. Through these devices, children and other family members can stay apprised of a parent or other loved one’s health through these at-home monitoring systems—in a similar manner as some parents track a new teenaged driver through his or her cell phone!
Other Smart Devices
Connected devices are moving past pill bottles and smart glasses. In the same manner that many people employ fitness trackers to monitor the number of steps taken and calories burned, multiple glucometers are available that sync with a patient’s smart phone, allowing upload of the data to his or her healthcare provider. This field is also growing into commercially available HR monitors that allow easy monitoring for arrhythmias in low-risk patients.
While these devices are a boon for primary care physicians and can greatly assist in determining medication noncompliance, some potential systems issues may result in a false emergency notification akin to patients presenting to the ED for evaluation after receiving an inaccurate high BP reading on a grocery-store or home monitoring device. For instance, HR monitors with a faulty lead may cause an alert from the monitoring system noting atrial fibrillation and recommending the patient seek immediate evaluation. Similarly, a smart phone-connected glucometer may note hyperglycemia in a patient after he or she has consumed a high-sugar meal.
While there has been a reemergence in the use of traditional tourniquets, they are not effective in controlling hemorrhage at junctional sites such as the groin or axilla as there is inadequate space to accommodate the tourniquet. Two recent solutions are the Combat Ready Clamp (CRoC) and SAM Junctional Tourniquet, which are specially designed to control bleeding in an improvised explosive device or blast-type injury. As with intraosseous access devices, the use of tourniquets is also making a comeback. Both owe their new-found popularity—at least in some part—to the involvement of the United States in the recent wars in Iraq and Afghanistan. High casualty rates from improvised explosive devices countered by significant improvements in body armor have resulted in preservation of the torso at the expense of extremities. Life-threatening hemorrhage from a distal extremity can easily be controlled by a tourniquet—something this author never used as an infantryman during Desert Storm, but which is now carried on the person of every soldier in the field.
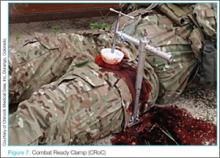
The Combat Ready Clamp (Combat Medical Systems, Harrisburg, North Carolina)22 compresses the aorta and vena cava though intra-abdominal pressure (Figure 7). While some may find this device a bit cumbersome for field use, it is definitely feasible and applicable for hospital use. A similar option, the SAM Junctional Tourniquet (SAM Medical Products, Wilsonville, Oregon),23 (Figure 8) functions in a similar manner as the CROC but uses pneumatic instead of mechanical pressure. The SAM device is definitely more “rucksack friendly,” but both products are good alternatives for controlling hemorrhages in the ED.
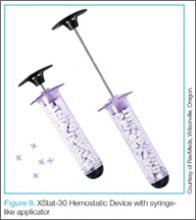
Hemostatic agents such as QuickClot (Z-Medica, Wallingford, Connecticut)24 have been in popular use for about a decade now, and the next generation of this family of treatment options has become available. The XStat-30 (RevMedx, Wilsonville, Oregon)25 (Figure 9) is one such product. Its large syringe applicator (like a large Toomey syringe) is filled with tablets of chitin. The injector is designed to be inserted into a penetrating injury and its contents injected into the wound. Upon contact with blood, the chitin tablets expand in a similar manner as children’s “hatch-and-grow” toy eggs and capsules when immersed in water. The XStat-30 provides not only hemostasis, but also some level of tamponade.
Resuscitative Endovascular Balloon Occlusion of the Aorta
The final addition to the hemostasis comeback tour is the Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA) device (Pryor Medical, San Antonio, Texas).26 Like many of the products discussed in this article, REBOA has been around for some time, but its use had fallen out of practice. A recent reemergence has shown that REBOA benefits patients with lower abdominal, pelvic, and extremity injuries.
The principal of its use is simple: An occlusive balloon is inserted into the femoral artery and advanced to roughly the level of the midabdomen. Once inflated, the balloon stops blood flow distally. While more research needs to be done on indications and outcomes, REBOA has been successfully used in England in many hospitals and even in the field.27
Summary
Some of the most notable recent evolutionary and revolutionary technological advancements to have a significant and beneficial impact on patient care have been seen in new noninvasive tonographic devices to measure IOP; video laryngoscopic devices for airway management; wireless patient vital signs monitoring systems; alternatives to traditional vascular access such as intraosseous vascular systems, laser-assisted vein visualization technology, and Internet-connected smart glasses; advances in wound-care dressings; medication monitoring systems; clamps and tourniquets to control junctional hemorrhage; and wireless, smart-phone connected glucometer devices and HR monitors. Many of these devices and systems are applicable and appropriate for use in the ED, the implementation of which will further facilitate and improve the quality of patient care.
Dr Wagner is an assistant professor of emergency medicine; program director for the emergency medicine residency program; and director of augmented learning at Barnes-Jewish Hospital, Saint Louis, Missouri.
The author invites readers to contact him via Twitter @TheTechDoc with suggestions for future devices.
The views expressed in this article are those of the author and do not represent the views or opinions of the editorial staff, the editorial board or the publisher.
- iCare Tonometer. iCare Finland. http://www.icaretonometer.com/products/icare-ta01/. Accessed June 2, 2015.
- García-Resúa C, González-Meijome JM, Gilino J, Yebra-Pimentel E. Accuracy of the new ICare rebound tonometer vs. other portable tonometers in healthy eyes. Optom Vis Sci. 2006;83(2):102-107.
- Reichert Technologies. Tono-Pen & Ocu-Film +. http://www.reichert.com/products.cfm?pcId=474. Accessed June 2, 2015.
- Karl Storz-Endoskope. From Laryngoscopy to Video Laryngoscopy. The history of endotracheal intubation. https://www.karlstorz.com/cps/rde/xbcr/karlstorz_assets/ASSETS/2133990.pdf. Accessed May 5, 2015.
- Lipe DN, Lindstrom R, Tauferner D, Mitchell C, Moffett P. Evaluation of Karl Storz C-MAC Tip Device Versus Traditional Airway Suction in a Cadaver Model. West J Emerg Med. 2014;15(4):548-553.
- ViSi Mobile. Sotera Wireless. http://www.visimobile.com/. Accessed May 6, 2015.
- EarlySense Chair Sensor Receives FDA Clearance [press release]. Waltham, MA:Early Sense; July 2, 2014. http://www.earlysense.com/news-and-events/news/jul-2-2014/. Accessed May 6, 2015.
- Arrow EZ-10. Teleflex. http://www.arrowezio.com/. Accessed May 6, 2015.
- Evena Medical Eyes-On Glass 1.0. http://evenamed.com/~even5672/~even5672/products/glasses. Accessed May 6, 2015.
- Wu TS, Dameff CJ, Tully JL. Ultrasound-guided central venous access using google glass. J Emerg Med. 2014;47(6):668-675.
- Aquacel Ag Dressing. ConvaTec. http://www.convatec.com/wound-skin/aquacel-ag-dressing. Accessed May 6, 2015.
- A review of the applications of the hydrofiber dressing with silver (Aquacel Ag) in wound care. Ther Clin Risk Manag. 2010;6:21-27.
- Caruso DM, Foster KN, Blome-Eberwein SA, et al. Randomized clinical study of Hydrofiber dressing with silver or silver sulfadiazine in the management of partial-thickness burns. J Burn Care Res. 2006;27(3):298–309.
- Kaźmierski M, Mańkowski P, Jankowski A, Harasymczuk J. Comparison of the results of operative and conservative treatment of deep dermal partial-thickness scalds in children. Eur J Pediatr Surg. 2007;17(5):354–361.
- Lohana P, Potokar TS. Aquacel Ag in paediatric burns: a prospective audit. Ann Burns Fire Disasters. 2006;19(3):144-147.
- Paddock HN, Fabia R, Giles S, et al. A silver-impregnated antimicrobial dressing reduces hospital costs for pediatric burn patients. J Pediatr Surg. 2007;42(1):211–213.
- Saba SC, Tsai R, Glat P. Clinical evaluation comparing the efficacy of aquacel ag hydrofiber dressing versus petrolatum gauze with antibiotic ointment in partial-thickness burns in a pediatric burn center. J Burn Care Res. 2009;30(3):380–385.
- Scanlon E, Karlsmark T, Leaper DJ, et al. Cost-effective faster wound healing with a sustained silver-releasing foam dressing in delayed healing leg ulcers-a health-economic analysis. Int Wound J. 2005;2(2):150-160.
- Smart Wireless Pill Bottles. AdhereTech. http://adheretech.com/. Accessed May 6, 2015.
- Proteus Digital Health. http://www.proteus.com/. Accessed May 6, 2015.
- Kim E. ‘Digital pill’ with chip inside gets FDA green light. CNN Money. http://money.cnn.com/2012/08/03/technology/startups/ingestible-sensor-proteus/. Accessed May 6, 2015.
- CROC Combat Ready Clamp (CRoC). Combat Medical. http://combatmedicalsystems.com/products/prod_massivehem_croc/ Accessed May 6, 2015.
- SAM Junctional Tourniquet. SAM Medical Products. http://www.sammedical.com/products/the-sam-junctional-tourniquet/. Accessed May 6, 2015.
- QuikClot hemostatic devices help patients survive traumatic blood loss. QuikClot. http://www.quikclot.com/. Accessed May 6, 2015.
- Revmedx. Revolutionary Medical Technologies. http://www.revmedx.com/#!xstat-dressing/c2500. Accessed May 6, 2015.
- Stannard A, Eliason JL, Rasmussen TE. Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA) as an adjunct for hemorrhagic shock J Trauma. 2011;71(6):1869-1872.
- London’s Air Ambulance Performs World’s First Prehospital REBOA. EMSWORLD – Patient Care. http://www.emsworld.com/news/11545597/londons-air-ambulance-performs-worlds-first-prehospital-reboa. Published July 2, 2014. Accessed May 6, 2015.
Emergency physicians (EPs) are always interested in what are the “tried-and-true” as well as the “latest-and-greatest” devices that will provide the best results for their patients. This article, while not a comprehensive list of every such device introduced over the past few years, does provide an overview of the most notable ones applicable for use in the ED.
Tonometry
The standard iCare tonometer device (TA01i; iCare Finland Oy, Vantaa, Finland),1 began to gain acceptance in the United States in 2007 (Figure 1). Early studies2 have shown its measurement accuracy of intraocular pressure (IOP) to be equivalent to traditional tonometers such as the Tono-Pen XL Applanation Tonometer (Reichert Techonologies, Depew, New York).3
The iCare tonometer is easy to calibrate and use. Consisting of a pin inserted into a magnetic housing, the magnet quickly pushes the blunt end of the pin out to make contact with the cornea. Six quick measurements provide the clinician with an average IOP. The device can be used without anesthesia and is also applicable for at-home use.
Airway Devices
C-MAC Tip System
While direct laryngoscopy will always have a role in clinical practice, there has been a revolution in airway management over the past few years, with video laryngoscopy rapidly replacing direct laryngoscopy. The C-MAC Tip system (Karl Storz Endoscopy-America, Inc, El Segundo, California) is one of the devices currently available.4,5 While this device is not at the lower end of the cost spectrum in airway devices, it is, in this author’s opinion, among the highest quality video laryngoscopes on the market. The hub of C-MAC Tip system is a video screen that accepts input from multiple devices. The most common is the video MacIntosh blade, which is shaped like a traditional MacIntosh but with a slightly thicker handle—allowing both direct and indirect intubation.
The C-MAC Tip system is a great teaching tool, allowing learners to perform direct laryngoscopy while providing reassuring visualization to the instructor of the intubation on the screen. (No longer does the instructor need to repeatedly ask the learner what he or she is viewing!) Moreover, when required, the clinician performing the intubation can look at the screen to benefit from the superior visualization of indirect laryngoscopy.
The C-MAC can also accept input from the D-Blade, which facilitates indirect intubation of anterior airways; however, it does not allow direct intubation when secretions or blood obscure the camera, though there is a suction channel that assists in clearing secretions. The clinician can also add a nasal pharyngeal scope as well as an adult or pediatric bronchoscope. The modularity of the C-MAC system is therefore a flexible addition to any airway armamentarium.
Regarding its use in emergency medicine, in addition to cost considerations, a potential concern is the ability of the plastic adapters to hold up to frequent, repetitive use in a setting such as a busy ED.
Wireless Vital Signs Monitoring Systems
Patient vital signs monitoring systems can currently double as four-point restraints. One new device, the ViSi Mobile Monitor System (Sotera Wireless, Inc, San Diego, CA), however, may make this a thing of the past.6 This system allows for inpatient monitoring of respiratory rate (RR), pulse oximetry, continuous blood pressure (BP), and temperature, as well as a multilead electrocardiogram. The entire system attaches to a small wrist-mounted device, which connects to a hospital monitoring system through a WiFi network (Figures 2a and 2b).ViSi Mobile Monitor
Another example of a wireless monitoring device is the EarlySense Chair Sensor system (EarlySense, Ltd, Ramat Gan, Israel) (Figure 3).7 This device assesses heart rate (HR) and RR simply by seating the patient in a chair. In the near future, this device will likely have the ability to take full vital signs. While not quite ready for prime-time in the ED, systems such as the EarlySense Chair Sensor offer a glimpse of the future in vital sign monitoring technology.
Vascular Access
Traditional intravenous (IV) line placement continues to be the standard of care for vascular access, but is not always feasible. Intraosseous needles, which have been around for decades, are seeing a new renaissance of use thanks to devices such as the Arrow EZ-IO Intraosseous Vascular Access System (Teleflex, Shavano Park, Texas).8 With these standards in mind, some new considerations are on the market, including the AV400 vein visualization system (AccuVein, Inc, Huntington, New York).
Arrow EZ-IO Intraosseous Vascular Access System
Teleflex, the maker of the EZ-IO, has recently made a push for humeral placement in order to achieve faster flow rates. Teleflex recommends using the longer needles (normally reserved for obese patients) with specific placement suggestions to facilitate retention of the needle. The Teleflex Web site8 and mobile application provide succinct, easy-to-understand instructions on placement.
AV400 Vein Visualization System
In addition to the Intraosseous Vascular Access System, the placement of an IV line may also be facilitated by laser devices such as AccuVein’s AV400 vein visualization technology. While earlier versions of both of these systems were comparable to that of a skilled technician in obtaining line placement, the latest generations of these devices have improved depth and visualization of truly difficult vascular access.
Internet-Connected Smart Glasses
In addition to the above-mentioned evolutionary changes in facilitating venous access, revolutionary technological advancements have been in development and are forthcoming. One such technology is network-connected smart glasses.
Eyes-On Glasses
Among the first in network-connected eyewear is Eyes-On Glasses (Evena Medical Inc, Los Altos, California).9 This system functions in a similar manner as other laser systems, except that the screen is a display mounted on eyeglasses, making venous placement much more intuitive (This is especially helpful for those who have difficulty translating the three-dimensional world to a two-dimensional screen).
Another variation on head-mounted technology is General Electric’s (GE) beta software for Google Glass (Google Inc, Mountain View, California) (Figure 4). This prototype links a GE ultrasound machine to Google Glass via WiFi, again simultaneously facilitating visualization of the field and the screen.10 While Google Glass is not currently available for the general public, there is still a place for it in the clinical setting.
Both the Eyes-On Glasses and Google Glass devices share a common thread: to improve patient comfort and facilitate time-consuming procedures.
Wound Care
Aquacel Ag
The EP sees a fair share of burn victims, the standard of care for which has been silver sulfadiazine and daily dressing changes. Care for burn wounds is beginning to change with the introduction of antimicrobial impregnated dressings such as Aquacel Ag (ConvaTec Inc, Greensboro, North Carolina).11 Aquacel Ag comes packaged in various forms and sizes ranging from large sheets for torsos to custom formed gloves for hands. This product is safe to use on the face and can be applied to partial thickness burns where the dermal layer is gone. The fluid from the wound moistens the bandage and helps it adhere to the skin. The dressings are then left in place until they slough-off on their own (approximately 7 to 10 days after placement). Consequently, no dressing changes are required, with cosmesis matching that of classic treatment.
While EPs may find Aquacel Ag useful in treating burns that do not require inpatient hospital admission, they also will find its use highly beneficial in treating patients with “road rash,” the abrasions that occur when one wipes-out at high speeds on asphalt (eg, motorcycle accidents). As with burn wounds, only a single application of Aquacel Ag is required on a debrided abrasion.
With respect to price, a single application of Aquacel Ag costs roughly the same as multiple dressing changes with other wound-care products.12 One concern relating to the use of advanced silver-impregnated dressings is the cost of care since silver-impregnated dressings are relatively expensive compared to traditional dressings. The higher cost, however, is partially offset by the reduced use of secondary gauze, and retention dressings, as well as improved wound healing together with the reduced costs of other care. Cost-effectiveness calculations comparing Aquacel Ag to standard of care in patients with acute and chronic wounds showed favorable results using Aquacel Ag.12-18
When using these dressings, the EP should make sure the follow-up clinic is familiar with their application so that they are not inadvertently removed at the patient’s first visit.
Medication Event Monitoring Systems
There have been a couple of recent changes in medication monitoring that are beginning to make manual pill-counting a thing of the past. Earlier generations of smart pill bottles came with a timer and an alarm that chimed and lit up to alert the patient when it was time to take his or her medication. Once the bottle was opened, the system reset itself. Unfortunately, the basic nature of these systems was not able to account for the number of pills a patient ingested at each scheduled dosing.
An example of newer and more technologically advanced pill-monitoring systems is AdhereTech’s smart wireless pill bottle (AdhereTech, New York, New York). In this system, the pill bottle can be connected to a WiFi network, allowing medication information to be shared (Figure 5).19 For example, a user could have the bottle connected to his or her provider, home healthcare worker, and family member. If a patient misses a dose of medication, the appropriate person receives notification and can make contact with the patient or family member to intervene. As with earlier generation products, these systems cannot account for or prevent a patient from either overdosing or underdosing on a medication.
The patch and sensor-enabled pill system, the Ingestible Event Marker, (Proteus Digital Health, Redwood City, California), which became available this past year, provides more advanced medication monitoring.20 This system allows tracking of individual pills through small chips imbedded in the tablet (Figure 6). The chip is then monitored through a patch worn on the patient’s body. Once connected, the physician is able to not only track when a pill bottle is opened, but also when and how many tablets the patient is ingesting. Moreover, the system has the ability to perform physiologic tracking to monitor patient response to the medication.21
Each of the above systems is a huge benefit to elderly patients and their geographically-separated families. Through these devices, children and other family members can stay apprised of a parent or other loved one’s health through these at-home monitoring systems—in a similar manner as some parents track a new teenaged driver through his or her cell phone!
Other Smart Devices
Connected devices are moving past pill bottles and smart glasses. In the same manner that many people employ fitness trackers to monitor the number of steps taken and calories burned, multiple glucometers are available that sync with a patient’s smart phone, allowing upload of the data to his or her healthcare provider. This field is also growing into commercially available HR monitors that allow easy monitoring for arrhythmias in low-risk patients.
While these devices are a boon for primary care physicians and can greatly assist in determining medication noncompliance, some potential systems issues may result in a false emergency notification akin to patients presenting to the ED for evaluation after receiving an inaccurate high BP reading on a grocery-store or home monitoring device. For instance, HR monitors with a faulty lead may cause an alert from the monitoring system noting atrial fibrillation and recommending the patient seek immediate evaluation. Similarly, a smart phone-connected glucometer may note hyperglycemia in a patient after he or she has consumed a high-sugar meal.
While there has been a reemergence in the use of traditional tourniquets, they are not effective in controlling hemorrhage at junctional sites such as the groin or axilla as there is inadequate space to accommodate the tourniquet. Two recent solutions are the Combat Ready Clamp (CRoC) and SAM Junctional Tourniquet, which are specially designed to control bleeding in an improvised explosive device or blast-type injury. As with intraosseous access devices, the use of tourniquets is also making a comeback. Both owe their new-found popularity—at least in some part—to the involvement of the United States in the recent wars in Iraq and Afghanistan. High casualty rates from improvised explosive devices countered by significant improvements in body armor have resulted in preservation of the torso at the expense of extremities. Life-threatening hemorrhage from a distal extremity can easily be controlled by a tourniquet—something this author never used as an infantryman during Desert Storm, but which is now carried on the person of every soldier in the field.
The Combat Ready Clamp (Combat Medical Systems, Harrisburg, North Carolina)22 compresses the aorta and vena cava though intra-abdominal pressure (Figure 7). While some may find this device a bit cumbersome for field use, it is definitely feasible and applicable for hospital use. A similar option, the SAM Junctional Tourniquet (SAM Medical Products, Wilsonville, Oregon),23 (Figure 8) functions in a similar manner as the CROC but uses pneumatic instead of mechanical pressure. The SAM device is definitely more “rucksack friendly,” but both products are good alternatives for controlling hemorrhages in the ED.
Hemostatic agents such as QuickClot (Z-Medica, Wallingford, Connecticut)24 have been in popular use for about a decade now, and the next generation of this family of treatment options has become available. The XStat-30 (RevMedx, Wilsonville, Oregon)25 (Figure 9) is one such product. Its large syringe applicator (like a large Toomey syringe) is filled with tablets of chitin. The injector is designed to be inserted into a penetrating injury and its contents injected into the wound. Upon contact with blood, the chitin tablets expand in a similar manner as children’s “hatch-and-grow” toy eggs and capsules when immersed in water. The XStat-30 provides not only hemostasis, but also some level of tamponade.
Resuscitative Endovascular Balloon Occlusion of the Aorta
The final addition to the hemostasis comeback tour is the Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA) device (Pryor Medical, San Antonio, Texas).26 Like many of the products discussed in this article, REBOA has been around for some time, but its use had fallen out of practice. A recent reemergence has shown that REBOA benefits patients with lower abdominal, pelvic, and extremity injuries.
The principal of its use is simple: An occlusive balloon is inserted into the femoral artery and advanced to roughly the level of the midabdomen. Once inflated, the balloon stops blood flow distally. While more research needs to be done on indications and outcomes, REBOA has been successfully used in England in many hospitals and even in the field.27
Summary
Some of the most notable recent evolutionary and revolutionary technological advancements to have a significant and beneficial impact on patient care have been seen in new noninvasive tonographic devices to measure IOP; video laryngoscopic devices for airway management; wireless patient vital signs monitoring systems; alternatives to traditional vascular access such as intraosseous vascular systems, laser-assisted vein visualization technology, and Internet-connected smart glasses; advances in wound-care dressings; medication monitoring systems; clamps and tourniquets to control junctional hemorrhage; and wireless, smart-phone connected glucometer devices and HR monitors. Many of these devices and systems are applicable and appropriate for use in the ED, the implementation of which will further facilitate and improve the quality of patient care.
Dr Wagner is an assistant professor of emergency medicine; program director for the emergency medicine residency program; and director of augmented learning at Barnes-Jewish Hospital, Saint Louis, Missouri.
The author invites readers to contact him via Twitter @TheTechDoc with suggestions for future devices.
The views expressed in this article are those of the author and do not represent the views or opinions of the editorial staff, the editorial board or the publisher.
Emergency physicians (EPs) are always interested in what are the “tried-and-true” as well as the “latest-and-greatest” devices that will provide the best results for their patients. This article, while not a comprehensive list of every such device introduced over the past few years, does provide an overview of the most notable ones applicable for use in the ED.
Tonometry
The standard iCare tonometer device (TA01i; iCare Finland Oy, Vantaa, Finland),1 began to gain acceptance in the United States in 2007 (Figure 1). Early studies2 have shown its measurement accuracy of intraocular pressure (IOP) to be equivalent to traditional tonometers such as the Tono-Pen XL Applanation Tonometer (Reichert Techonologies, Depew, New York).3
The iCare tonometer is easy to calibrate and use. Consisting of a pin inserted into a magnetic housing, the magnet quickly pushes the blunt end of the pin out to make contact with the cornea. Six quick measurements provide the clinician with an average IOP. The device can be used without anesthesia and is also applicable for at-home use.
Airway Devices
C-MAC Tip System
While direct laryngoscopy will always have a role in clinical practice, there has been a revolution in airway management over the past few years, with video laryngoscopy rapidly replacing direct laryngoscopy. The C-MAC Tip system (Karl Storz Endoscopy-America, Inc, El Segundo, California) is one of the devices currently available.4,5 While this device is not at the lower end of the cost spectrum in airway devices, it is, in this author’s opinion, among the highest quality video laryngoscopes on the market. The hub of C-MAC Tip system is a video screen that accepts input from multiple devices. The most common is the video MacIntosh blade, which is shaped like a traditional MacIntosh but with a slightly thicker handle—allowing both direct and indirect intubation.
The C-MAC Tip system is a great teaching tool, allowing learners to perform direct laryngoscopy while providing reassuring visualization to the instructor of the intubation on the screen. (No longer does the instructor need to repeatedly ask the learner what he or she is viewing!) Moreover, when required, the clinician performing the intubation can look at the screen to benefit from the superior visualization of indirect laryngoscopy.
The C-MAC can also accept input from the D-Blade, which facilitates indirect intubation of anterior airways; however, it does not allow direct intubation when secretions or blood obscure the camera, though there is a suction channel that assists in clearing secretions. The clinician can also add a nasal pharyngeal scope as well as an adult or pediatric bronchoscope. The modularity of the C-MAC system is therefore a flexible addition to any airway armamentarium.
Regarding its use in emergency medicine, in addition to cost considerations, a potential concern is the ability of the plastic adapters to hold up to frequent, repetitive use in a setting such as a busy ED.
Wireless Vital Signs Monitoring Systems
Patient vital signs monitoring systems can currently double as four-point restraints. One new device, the ViSi Mobile Monitor System (Sotera Wireless, Inc, San Diego, CA), however, may make this a thing of the past.6 This system allows for inpatient monitoring of respiratory rate (RR), pulse oximetry, continuous blood pressure (BP), and temperature, as well as a multilead electrocardiogram. The entire system attaches to a small wrist-mounted device, which connects to a hospital monitoring system through a WiFi network (Figures 2a and 2b).ViSi Mobile Monitor
Another example of a wireless monitoring device is the EarlySense Chair Sensor system (EarlySense, Ltd, Ramat Gan, Israel) (Figure 3).7 This device assesses heart rate (HR) and RR simply by seating the patient in a chair. In the near future, this device will likely have the ability to take full vital signs. While not quite ready for prime-time in the ED, systems such as the EarlySense Chair Sensor offer a glimpse of the future in vital sign monitoring technology.
Vascular Access
Traditional intravenous (IV) line placement continues to be the standard of care for vascular access, but is not always feasible. Intraosseous needles, which have been around for decades, are seeing a new renaissance of use thanks to devices such as the Arrow EZ-IO Intraosseous Vascular Access System (Teleflex, Shavano Park, Texas).8 With these standards in mind, some new considerations are on the market, including the AV400 vein visualization system (AccuVein, Inc, Huntington, New York).
Arrow EZ-IO Intraosseous Vascular Access System
Teleflex, the maker of the EZ-IO, has recently made a push for humeral placement in order to achieve faster flow rates. Teleflex recommends using the longer needles (normally reserved for obese patients) with specific placement suggestions to facilitate retention of the needle. The Teleflex Web site8 and mobile application provide succinct, easy-to-understand instructions on placement.
AV400 Vein Visualization System
In addition to the Intraosseous Vascular Access System, the placement of an IV line may also be facilitated by laser devices such as AccuVein’s AV400 vein visualization technology. While earlier versions of both of these systems were comparable to that of a skilled technician in obtaining line placement, the latest generations of these devices have improved depth and visualization of truly difficult vascular access.
Internet-Connected Smart Glasses
In addition to the above-mentioned evolutionary changes in facilitating venous access, revolutionary technological advancements have been in development and are forthcoming. One such technology is network-connected smart glasses.
Eyes-On Glasses
Among the first in network-connected eyewear is Eyes-On Glasses (Evena Medical Inc, Los Altos, California).9 This system functions in a similar manner as other laser systems, except that the screen is a display mounted on eyeglasses, making venous placement much more intuitive (This is especially helpful for those who have difficulty translating the three-dimensional world to a two-dimensional screen).
Another variation on head-mounted technology is General Electric’s (GE) beta software for Google Glass (Google Inc, Mountain View, California) (Figure 4). This prototype links a GE ultrasound machine to Google Glass via WiFi, again simultaneously facilitating visualization of the field and the screen.10 While Google Glass is not currently available for the general public, there is still a place for it in the clinical setting.
Both the Eyes-On Glasses and Google Glass devices share a common thread: to improve patient comfort and facilitate time-consuming procedures.
Wound Care
Aquacel Ag
The EP sees a fair share of burn victims, the standard of care for which has been silver sulfadiazine and daily dressing changes. Care for burn wounds is beginning to change with the introduction of antimicrobial impregnated dressings such as Aquacel Ag (ConvaTec Inc, Greensboro, North Carolina).11 Aquacel Ag comes packaged in various forms and sizes ranging from large sheets for torsos to custom formed gloves for hands. This product is safe to use on the face and can be applied to partial thickness burns where the dermal layer is gone. The fluid from the wound moistens the bandage and helps it adhere to the skin. The dressings are then left in place until they slough-off on their own (approximately 7 to 10 days after placement). Consequently, no dressing changes are required, with cosmesis matching that of classic treatment.
While EPs may find Aquacel Ag useful in treating burns that do not require inpatient hospital admission, they also will find its use highly beneficial in treating patients with “road rash,” the abrasions that occur when one wipes-out at high speeds on asphalt (eg, motorcycle accidents). As with burn wounds, only a single application of Aquacel Ag is required on a debrided abrasion.
With respect to price, a single application of Aquacel Ag costs roughly the same as multiple dressing changes with other wound-care products.12 One concern relating to the use of advanced silver-impregnated dressings is the cost of care since silver-impregnated dressings are relatively expensive compared to traditional dressings. The higher cost, however, is partially offset by the reduced use of secondary gauze, and retention dressings, as well as improved wound healing together with the reduced costs of other care. Cost-effectiveness calculations comparing Aquacel Ag to standard of care in patients with acute and chronic wounds showed favorable results using Aquacel Ag.12-18
When using these dressings, the EP should make sure the follow-up clinic is familiar with their application so that they are not inadvertently removed at the patient’s first visit.
Medication Event Monitoring Systems
There have been a couple of recent changes in medication monitoring that are beginning to make manual pill-counting a thing of the past. Earlier generations of smart pill bottles came with a timer and an alarm that chimed and lit up to alert the patient when it was time to take his or her medication. Once the bottle was opened, the system reset itself. Unfortunately, the basic nature of these systems was not able to account for the number of pills a patient ingested at each scheduled dosing.
An example of newer and more technologically advanced pill-monitoring systems is AdhereTech’s smart wireless pill bottle (AdhereTech, New York, New York). In this system, the pill bottle can be connected to a WiFi network, allowing medication information to be shared (Figure 5).19 For example, a user could have the bottle connected to his or her provider, home healthcare worker, and family member. If a patient misses a dose of medication, the appropriate person receives notification and can make contact with the patient or family member to intervene. As with earlier generation products, these systems cannot account for or prevent a patient from either overdosing or underdosing on a medication.
The patch and sensor-enabled pill system, the Ingestible Event Marker, (Proteus Digital Health, Redwood City, California), which became available this past year, provides more advanced medication monitoring.20 This system allows tracking of individual pills through small chips imbedded in the tablet (Figure 6). The chip is then monitored through a patch worn on the patient’s body. Once connected, the physician is able to not only track when a pill bottle is opened, but also when and how many tablets the patient is ingesting. Moreover, the system has the ability to perform physiologic tracking to monitor patient response to the medication.21
Each of the above systems is a huge benefit to elderly patients and their geographically-separated families. Through these devices, children and other family members can stay apprised of a parent or other loved one’s health through these at-home monitoring systems—in a similar manner as some parents track a new teenaged driver through his or her cell phone!
Other Smart Devices
Connected devices are moving past pill bottles and smart glasses. In the same manner that many people employ fitness trackers to monitor the number of steps taken and calories burned, multiple glucometers are available that sync with a patient’s smart phone, allowing upload of the data to his or her healthcare provider. This field is also growing into commercially available HR monitors that allow easy monitoring for arrhythmias in low-risk patients.
While these devices are a boon for primary care physicians and can greatly assist in determining medication noncompliance, some potential systems issues may result in a false emergency notification akin to patients presenting to the ED for evaluation after receiving an inaccurate high BP reading on a grocery-store or home monitoring device. For instance, HR monitors with a faulty lead may cause an alert from the monitoring system noting atrial fibrillation and recommending the patient seek immediate evaluation. Similarly, a smart phone-connected glucometer may note hyperglycemia in a patient after he or she has consumed a high-sugar meal.
While there has been a reemergence in the use of traditional tourniquets, they are not effective in controlling hemorrhage at junctional sites such as the groin or axilla as there is inadequate space to accommodate the tourniquet. Two recent solutions are the Combat Ready Clamp (CRoC) and SAM Junctional Tourniquet, which are specially designed to control bleeding in an improvised explosive device or blast-type injury. As with intraosseous access devices, the use of tourniquets is also making a comeback. Both owe their new-found popularity—at least in some part—to the involvement of the United States in the recent wars in Iraq and Afghanistan. High casualty rates from improvised explosive devices countered by significant improvements in body armor have resulted in preservation of the torso at the expense of extremities. Life-threatening hemorrhage from a distal extremity can easily be controlled by a tourniquet—something this author never used as an infantryman during Desert Storm, but which is now carried on the person of every soldier in the field.
The Combat Ready Clamp (Combat Medical Systems, Harrisburg, North Carolina)22 compresses the aorta and vena cava though intra-abdominal pressure (Figure 7). While some may find this device a bit cumbersome for field use, it is definitely feasible and applicable for hospital use. A similar option, the SAM Junctional Tourniquet (SAM Medical Products, Wilsonville, Oregon),23 (Figure 8) functions in a similar manner as the CROC but uses pneumatic instead of mechanical pressure. The SAM device is definitely more “rucksack friendly,” but both products are good alternatives for controlling hemorrhages in the ED.
Hemostatic agents such as QuickClot (Z-Medica, Wallingford, Connecticut)24 have been in popular use for about a decade now, and the next generation of this family of treatment options has become available. The XStat-30 (RevMedx, Wilsonville, Oregon)25 (Figure 9) is one such product. Its large syringe applicator (like a large Toomey syringe) is filled with tablets of chitin. The injector is designed to be inserted into a penetrating injury and its contents injected into the wound. Upon contact with blood, the chitin tablets expand in a similar manner as children’s “hatch-and-grow” toy eggs and capsules when immersed in water. The XStat-30 provides not only hemostasis, but also some level of tamponade.
Resuscitative Endovascular Balloon Occlusion of the Aorta
The final addition to the hemostasis comeback tour is the Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA) device (Pryor Medical, San Antonio, Texas).26 Like many of the products discussed in this article, REBOA has been around for some time, but its use had fallen out of practice. A recent reemergence has shown that REBOA benefits patients with lower abdominal, pelvic, and extremity injuries.
The principal of its use is simple: An occlusive balloon is inserted into the femoral artery and advanced to roughly the level of the midabdomen. Once inflated, the balloon stops blood flow distally. While more research needs to be done on indications and outcomes, REBOA has been successfully used in England in many hospitals and even in the field.27
Summary
Some of the most notable recent evolutionary and revolutionary technological advancements to have a significant and beneficial impact on patient care have been seen in new noninvasive tonographic devices to measure IOP; video laryngoscopic devices for airway management; wireless patient vital signs monitoring systems; alternatives to traditional vascular access such as intraosseous vascular systems, laser-assisted vein visualization technology, and Internet-connected smart glasses; advances in wound-care dressings; medication monitoring systems; clamps and tourniquets to control junctional hemorrhage; and wireless, smart-phone connected glucometer devices and HR monitors. Many of these devices and systems are applicable and appropriate for use in the ED, the implementation of which will further facilitate and improve the quality of patient care.
Dr Wagner is an assistant professor of emergency medicine; program director for the emergency medicine residency program; and director of augmented learning at Barnes-Jewish Hospital, Saint Louis, Missouri.
The author invites readers to contact him via Twitter @TheTechDoc with suggestions for future devices.
The views expressed in this article are those of the author and do not represent the views or opinions of the editorial staff, the editorial board or the publisher.
- iCare Tonometer. iCare Finland. http://www.icaretonometer.com/products/icare-ta01/. Accessed June 2, 2015.
- García-Resúa C, González-Meijome JM, Gilino J, Yebra-Pimentel E. Accuracy of the new ICare rebound tonometer vs. other portable tonometers in healthy eyes. Optom Vis Sci. 2006;83(2):102-107.
- Reichert Technologies. Tono-Pen & Ocu-Film +. http://www.reichert.com/products.cfm?pcId=474. Accessed June 2, 2015.
- Karl Storz-Endoskope. From Laryngoscopy to Video Laryngoscopy. The history of endotracheal intubation. https://www.karlstorz.com/cps/rde/xbcr/karlstorz_assets/ASSETS/2133990.pdf. Accessed May 5, 2015.
- Lipe DN, Lindstrom R, Tauferner D, Mitchell C, Moffett P. Evaluation of Karl Storz C-MAC Tip Device Versus Traditional Airway Suction in a Cadaver Model. West J Emerg Med. 2014;15(4):548-553.
- ViSi Mobile. Sotera Wireless. http://www.visimobile.com/. Accessed May 6, 2015.
- EarlySense Chair Sensor Receives FDA Clearance [press release]. Waltham, MA:Early Sense; July 2, 2014. http://www.earlysense.com/news-and-events/news/jul-2-2014/. Accessed May 6, 2015.
- Arrow EZ-10. Teleflex. http://www.arrowezio.com/. Accessed May 6, 2015.
- Evena Medical Eyes-On Glass 1.0. http://evenamed.com/~even5672/~even5672/products/glasses. Accessed May 6, 2015.
- Wu TS, Dameff CJ, Tully JL. Ultrasound-guided central venous access using google glass. J Emerg Med. 2014;47(6):668-675.
- Aquacel Ag Dressing. ConvaTec. http://www.convatec.com/wound-skin/aquacel-ag-dressing. Accessed May 6, 2015.
- A review of the applications of the hydrofiber dressing with silver (Aquacel Ag) in wound care. Ther Clin Risk Manag. 2010;6:21-27.
- Caruso DM, Foster KN, Blome-Eberwein SA, et al. Randomized clinical study of Hydrofiber dressing with silver or silver sulfadiazine in the management of partial-thickness burns. J Burn Care Res. 2006;27(3):298–309.
- Kaźmierski M, Mańkowski P, Jankowski A, Harasymczuk J. Comparison of the results of operative and conservative treatment of deep dermal partial-thickness scalds in children. Eur J Pediatr Surg. 2007;17(5):354–361.
- Lohana P, Potokar TS. Aquacel Ag in paediatric burns: a prospective audit. Ann Burns Fire Disasters. 2006;19(3):144-147.
- Paddock HN, Fabia R, Giles S, et al. A silver-impregnated antimicrobial dressing reduces hospital costs for pediatric burn patients. J Pediatr Surg. 2007;42(1):211–213.
- Saba SC, Tsai R, Glat P. Clinical evaluation comparing the efficacy of aquacel ag hydrofiber dressing versus petrolatum gauze with antibiotic ointment in partial-thickness burns in a pediatric burn center. J Burn Care Res. 2009;30(3):380–385.
- Scanlon E, Karlsmark T, Leaper DJ, et al. Cost-effective faster wound healing with a sustained silver-releasing foam dressing in delayed healing leg ulcers-a health-economic analysis. Int Wound J. 2005;2(2):150-160.
- Smart Wireless Pill Bottles. AdhereTech. http://adheretech.com/. Accessed May 6, 2015.
- Proteus Digital Health. http://www.proteus.com/. Accessed May 6, 2015.
- Kim E. ‘Digital pill’ with chip inside gets FDA green light. CNN Money. http://money.cnn.com/2012/08/03/technology/startups/ingestible-sensor-proteus/. Accessed May 6, 2015.
- CROC Combat Ready Clamp (CRoC). Combat Medical. http://combatmedicalsystems.com/products/prod_massivehem_croc/ Accessed May 6, 2015.
- SAM Junctional Tourniquet. SAM Medical Products. http://www.sammedical.com/products/the-sam-junctional-tourniquet/. Accessed May 6, 2015.
- QuikClot hemostatic devices help patients survive traumatic blood loss. QuikClot. http://www.quikclot.com/. Accessed May 6, 2015.
- Revmedx. Revolutionary Medical Technologies. http://www.revmedx.com/#!xstat-dressing/c2500. Accessed May 6, 2015.
- Stannard A, Eliason JL, Rasmussen TE. Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA) as an adjunct for hemorrhagic shock J Trauma. 2011;71(6):1869-1872.
- London’s Air Ambulance Performs World’s First Prehospital REBOA. EMSWORLD – Patient Care. http://www.emsworld.com/news/11545597/londons-air-ambulance-performs-worlds-first-prehospital-reboa. Published July 2, 2014. Accessed May 6, 2015.
- iCare Tonometer. iCare Finland. http://www.icaretonometer.com/products/icare-ta01/. Accessed June 2, 2015.
- García-Resúa C, González-Meijome JM, Gilino J, Yebra-Pimentel E. Accuracy of the new ICare rebound tonometer vs. other portable tonometers in healthy eyes. Optom Vis Sci. 2006;83(2):102-107.
- Reichert Technologies. Tono-Pen & Ocu-Film +. http://www.reichert.com/products.cfm?pcId=474. Accessed June 2, 2015.
- Karl Storz-Endoskope. From Laryngoscopy to Video Laryngoscopy. The history of endotracheal intubation. https://www.karlstorz.com/cps/rde/xbcr/karlstorz_assets/ASSETS/2133990.pdf. Accessed May 5, 2015.
- Lipe DN, Lindstrom R, Tauferner D, Mitchell C, Moffett P. Evaluation of Karl Storz C-MAC Tip Device Versus Traditional Airway Suction in a Cadaver Model. West J Emerg Med. 2014;15(4):548-553.
- ViSi Mobile. Sotera Wireless. http://www.visimobile.com/. Accessed May 6, 2015.
- EarlySense Chair Sensor Receives FDA Clearance [press release]. Waltham, MA:Early Sense; July 2, 2014. http://www.earlysense.com/news-and-events/news/jul-2-2014/. Accessed May 6, 2015.
- Arrow EZ-10. Teleflex. http://www.arrowezio.com/. Accessed May 6, 2015.
- Evena Medical Eyes-On Glass 1.0. http://evenamed.com/~even5672/~even5672/products/glasses. Accessed May 6, 2015.
- Wu TS, Dameff CJ, Tully JL. Ultrasound-guided central venous access using google glass. J Emerg Med. 2014;47(6):668-675.
- Aquacel Ag Dressing. ConvaTec. http://www.convatec.com/wound-skin/aquacel-ag-dressing. Accessed May 6, 2015.
- A review of the applications of the hydrofiber dressing with silver (Aquacel Ag) in wound care. Ther Clin Risk Manag. 2010;6:21-27.
- Caruso DM, Foster KN, Blome-Eberwein SA, et al. Randomized clinical study of Hydrofiber dressing with silver or silver sulfadiazine in the management of partial-thickness burns. J Burn Care Res. 2006;27(3):298–309.
- Kaźmierski M, Mańkowski P, Jankowski A, Harasymczuk J. Comparison of the results of operative and conservative treatment of deep dermal partial-thickness scalds in children. Eur J Pediatr Surg. 2007;17(5):354–361.
- Lohana P, Potokar TS. Aquacel Ag in paediatric burns: a prospective audit. Ann Burns Fire Disasters. 2006;19(3):144-147.
- Paddock HN, Fabia R, Giles S, et al. A silver-impregnated antimicrobial dressing reduces hospital costs for pediatric burn patients. J Pediatr Surg. 2007;42(1):211–213.
- Saba SC, Tsai R, Glat P. Clinical evaluation comparing the efficacy of aquacel ag hydrofiber dressing versus petrolatum gauze with antibiotic ointment in partial-thickness burns in a pediatric burn center. J Burn Care Res. 2009;30(3):380–385.
- Scanlon E, Karlsmark T, Leaper DJ, et al. Cost-effective faster wound healing with a sustained silver-releasing foam dressing in delayed healing leg ulcers-a health-economic analysis. Int Wound J. 2005;2(2):150-160.
- Smart Wireless Pill Bottles. AdhereTech. http://adheretech.com/. Accessed May 6, 2015.
- Proteus Digital Health. http://www.proteus.com/. Accessed May 6, 2015.
- Kim E. ‘Digital pill’ with chip inside gets FDA green light. CNN Money. http://money.cnn.com/2012/08/03/technology/startups/ingestible-sensor-proteus/. Accessed May 6, 2015.
- CROC Combat Ready Clamp (CRoC). Combat Medical. http://combatmedicalsystems.com/products/prod_massivehem_croc/ Accessed May 6, 2015.
- SAM Junctional Tourniquet. SAM Medical Products. http://www.sammedical.com/products/the-sam-junctional-tourniquet/. Accessed May 6, 2015.
- QuikClot hemostatic devices help patients survive traumatic blood loss. QuikClot. http://www.quikclot.com/. Accessed May 6, 2015.
- Revmedx. Revolutionary Medical Technologies. http://www.revmedx.com/#!xstat-dressing/c2500. Accessed May 6, 2015.
- Stannard A, Eliason JL, Rasmussen TE. Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA) as an adjunct for hemorrhagic shock J Trauma. 2011;71(6):1869-1872.
- London’s Air Ambulance Performs World’s First Prehospital REBOA. EMSWORLD – Patient Care. http://www.emsworld.com/news/11545597/londons-air-ambulance-performs-worlds-first-prehospital-reboa. Published July 2, 2014. Accessed May 6, 2015.