User login
The author reports no financial relationships relevant to this article.
- What clinical problem is this device or drug designed to address?
- Is the problem important clinically for the well-being of my patients?
- What do I know about the basic science and physiology of the problem?
- Are there data supporting improvement in clinical outcomes, or do the studies look only at surrogate endpoints?
- Has the drug or device been studied in gynecologic patients? Is it approved for use in this population?
- Is there evidence that the new technology is more effective, safer, or less expensive than products I use now?
The process of evaluation is critical for us—as advocates for individual patients and as stewards of the diminishing resources available to treat patients in the United States.
With these questions in mind, let’s look at a surgical problem for which there are many new and potentially exciting products being promoted to gynecologic surgeons: the need to achieve hemostasis.
Bleeding is a serious clinical problem during surgery—one that can have a major impact on the well-being of the patient. We generally use sutures and electrosurgical instruments—both bipolar and monopolar—to control major vessels. Topical hemostasis agents may be useful, however, in areas where generalized oozing is present, or where the application of energy may endanger vital structures.
Many products are available for use in these situations. Some are tried and true; others, new or unapproved for use in gynecologic surgery. Your assessment and selection of the optimal product should take into account 1) the cause of bleeding and 2) the mechanism of action of the product.
Surgical bleeding has one of two causes
Although the science of coagulation is complex, bleeding occurs, from a surgical perspective, because of either of two problems:
- failure to control significant arterial and venous sources
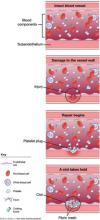
- failure of normal clotting functions, such as vasoconstriction; platelet activation and plugging ( FIGURE ); and activation of the coagulation cascade.
No topical or systemic agent adequately controls Problem #1. To address Problem #2, we have several choices to augment hemostasis, including thermal, chemical, and mechanical means. In addition, newer agents deliver additional coagulation-enhancing products such as platelets and thrombin to the operative site to supplement the patient’s natural clotting process.
FIGURE How a clot forms
Tried and true technologies
Because of long-standing experience with porcine gelatin, oxidized regenerated cellulose (ORC), and microfibrillar collagen, the Food and Drug Administration recategorized them in 2002 as Class-II devices with a clear safety profile.
Porcine gelatin
This class of products (Gelfoam, Surgifoam, Spongistan) has been around since the 1940s. The products have no intrinsic hemostatic action. They absorb 45 times their weight in blood and provide a scaff old on which platelets come into close contact, initiating the release of intrinsic and extrinsic clotting mechanisms.
Oxidized regenerated cellulose
This class (Surgicel, Oxycel) has also been around since the 1940s. The agents have acidic properties, due to their low pH level, and achieve hemostasis via denaturation of blood proteins, mechanical activation of the clotting cascade, and local vasoconstriction.
Because of its low pH, ORC is bactericidal against many common pathogens of the reproductive tract. A few studies have explored laparoscopic application of ORC to achieve hemostasis at sites of uterine perforation and for tubal bleeding secondary to sterilization.1,2 Successful hemostasis of moderate bleeding was achieved without the need for suture or conversion to laparotomy in all cases without brisk arterial bleeding.
Another ORC product (Interceed) is often used in gynecologic surgery as a barrier to adhesion formation. Its degree of oxidation, weave, and pore size differ from those of Surgicel. It requires an absolutely dry operative field to prevent adhesions.
Microfibrillar collagen
Substances derived from bovine collagen were marketed in the 1970s and 1980s. Collagen provides binding sites for platelets, which degranulate, releasing coagulation factors and initiating the clotting cascade.
Microfibrillar collagen products are available in a variety of forms (powder, sheets, loaded syringes for endoscopic placement) and are applied with pressure directly to the bleeding site. Collagen (Instat, Helistat) is supplied as a sponge, whereas microfibrillar collagen (Avitene, Superstat, Actifoam, Helitene, Hemopad, Novacol) is a tenacious powder or sheet. Microfibrillar collagen, like porcine gelatin and ORC, is absorbed by the body over time.
No studies have evaluated microfibrillar collagen in gynecologic surgery, although case reports of successful application to sites of uterine perforation after dilation and curettage and to bleeding sites after vaginal or laparoscopic hysterectomy have been published.3,4
New products are largely unproven
Class-III devices require significantly more study before safety and efficacy can be demonstrated. The two types of products presented here—topical thrombin and tissue sealants—remain largely unproven.
Topical thrombin
This class of products has been available for more than 20 years. As a liquid (Thrombogen), topical thrombin can be supplied in a syringe and sprayed onto oozing sites. A liquid combination of collagen gelatin matrix and bovine thrombin (FloSeal) provides a structure on which clots can form; triggers topical conversion of fibrinogen to fibrin; and activates the clotting cascade. It was approved for use in 1999. CoStasis and Vitagel are similar products, approved in 2000 and 2006, respectively, that add plasma obtained from the patient at the beginning of the surgical procedure.
Thrombin and ORC don’t mix. The acidity of ORC inactivates thrombin; therefore, ORC products should not be used with any product containing bovine or human thrombin.
The theoretical advantage of products that use patients’ plasma is the addition of autologous clotting factors and platelets to the bovine collagen and thrombin mixture. Preliminary studies have shown:
- a reduction in postoperative pain in 20 orthopedic surgery patients randomized to platelet gel, compared with what was seen in 20 women in the control group5
- a reduction in the rate of sternal wound infection in cardiac surgery patients (0.3% with the gel; 1.8% without it)6
- a potentially shorter healing time when platelet gel is applied to surgical wounds.7
The facts. Labeling for topical thrombin specifically states that it is not for use in cases of infection or for postpartum hemorrhage or menorrhagia. Studies of topical thrombin products have used the time to cessation of bleeding as their primary effectiveness end-point. In practical terms, however, studies have demonstrated no reduction in the need for transfusion or chest tube drainage in re-operative cardiac surgery patients.8
Disadvantages of topical thrombin include the cost of the product (including the cost of a plasma-collection device) and the need for operating room staff to collect and combine the product for use. Topical thrombin also exposes the patient to the risk of antibody formation (see Bovine thrombin can trigger risky antibodies), catastrophic bleeding, and, even, death.
Products that contain bovine thrombin have some safety issues with regard to their antigenic reactivity. Patients may develop antibodies to the bovine product that cross-react with human thrombin and factor Va. Associated with all products that contain bovine thrombin is a black box warning that states that the product may be associated with severe bleeding, thrombosis, and, rarely, death, because of antibody formation.
In one case report, a very complicated patient who required systemic anticoagulation for a mechanical aortic valve underwent hysterectomy, with topical thrombin administered at the end of the procedure in an effort to avert postoperative hemorrhage.1 She developed antibodies to the bovine thrombin, which caused significant and severe coagulation defects.
No clinical studies have assessed these products in gynecologic surgery.
Reference
1. Sharma JB, Malhotra M, Pundir P. Laparoscopic oxidized cellulose (Surgicel) application for small uterine perforations. Int J Gynaecol Obstet. 2003;83:271-275.
This last set of products has been approved for use in cardiopulmonary bypass procedures, in patients who have splenic injury, and to close a colostomy. They are “tissue glues” that also have hemostatic properties.
Tisseel is a combination of human thrombin, human “sealer protein” (fibrinogen), and aprotinin, a synthetic inhibitor of fibrinolysis that prevents premature degradation of a clot once it has formed. In clinical studies, this product has reduced the need for splenectomy in patients who have bleeding that is difficult to control.
Disadvantages of tissue-sealing products. These products have not been studied in gynecologic patients. They have the significant disadvantage of containing products derived from pooled human plasma. Although precautions have been taken to reduce transmission of infectious disease, viral transmission may occur. Anaphylaxis is an additional risk.
Many products are available to help the ObGyn surgeon achieve hemostasis in tough situations. Most of the time, we face generalized oozing after treatment of extensive endometriosis or adhesiolysis; in these cases, older topical agents should serve us well. Patients who experience massive bleeding are not likely to benefit from the use of any of the products described in this article.
Extensive bleeding from uterine incisions—at cesarean section or after myomectomy—might respond to topical thrombin, platelet gel products, or tissue sealants, but these products have not been studied in our patients. They also are expensive and carry some risk for our patients.
Don’t overlook two strategies for extremely high-risk situations:
- Cell-saver technology can help avert transfusion in patients expected to lose a substantial amount of blood
- Intravenous recombinant activated factor VII (NovoSeven) can be life-saving for women who experience postpartum hemorrhage, placenta percreta, or retroperitoneal sarcoma and for whom our standard strategies have failed.—BARBARA S. LEVY, MD
1. Sharma JB, Malhotra M, Pundir P. Laparoscopic oxidized cellulose (Surgicel) application for small uterine perforations. Int J Gynaecol Obstet. 2003;83:271-275.
2. Sharma JB, Malhotra M. Topical oxidized cellulose for tubal hemorrhage hemostasis during laparoscopic sterilization. Int J Gynaecol Obstet. 2003;82:221-222.
3. Borten M, Friedman EA. Translaparoscopic hemostasis with microfibrillar collagen in lieu of laparotomy. A report of two cases. J Reprod Med. 1983;28:804-806.
4. Holub Z, Jabor A. Laparoscopic management of bleeding after laparoscopic or vaginal hysterectomy. JSLS. 2004;8:235-238.
5. Zavadil DP, Satterlee CC, Costigan JM, Holt DW, Shostrom VK. Autologous platelet gel and platelet-poor plasma reduce pain with total shoulder arthroplasty. J Extra Corpor Technol. 2007;39:177-182.
6. Trowbridge CC, Stammers AH, Woods E, Yen BR, Klayman M. Use of platelet gel and its effects on infection in cardiac surgery. J Extra Corpor Technol. 2005;37:381-386.
7. Hom DB, Linzie MB, Huang TC. The healing effects of autologous platelet gel on acute human skin wounds. Arch Facial Plast Surg. 2007;9:174-183.
8. Wajon P, Gibson J, Calcroft R, Hughes C, Thrift B. Intraoperative plateletpheresis and autologous platelet gel do not reduce chest tube drainage or allogeneic blood transfusion after reoperative coronary artery bypass graft. Anesth Analg. 2001;93:536-542.
The author reports no financial relationships relevant to this article.
- What clinical problem is this device or drug designed to address?
- Is the problem important clinically for the well-being of my patients?
- What do I know about the basic science and physiology of the problem?
- Are there data supporting improvement in clinical outcomes, or do the studies look only at surrogate endpoints?
- Has the drug or device been studied in gynecologic patients? Is it approved for use in this population?
- Is there evidence that the new technology is more effective, safer, or less expensive than products I use now?
The process of evaluation is critical for us—as advocates for individual patients and as stewards of the diminishing resources available to treat patients in the United States.
With these questions in mind, let’s look at a surgical problem for which there are many new and potentially exciting products being promoted to gynecologic surgeons: the need to achieve hemostasis.
Bleeding is a serious clinical problem during surgery—one that can have a major impact on the well-being of the patient. We generally use sutures and electrosurgical instruments—both bipolar and monopolar—to control major vessels. Topical hemostasis agents may be useful, however, in areas where generalized oozing is present, or where the application of energy may endanger vital structures.
Many products are available for use in these situations. Some are tried and true; others, new or unapproved for use in gynecologic surgery. Your assessment and selection of the optimal product should take into account 1) the cause of bleeding and 2) the mechanism of action of the product.
Surgical bleeding has one of two causes
Although the science of coagulation is complex, bleeding occurs, from a surgical perspective, because of either of two problems:
- failure to control significant arterial and venous sources
- failure of normal clotting functions, such as vasoconstriction; platelet activation and plugging ( FIGURE ); and activation of the coagulation cascade.
No topical or systemic agent adequately controls Problem #1. To address Problem #2, we have several choices to augment hemostasis, including thermal, chemical, and mechanical means. In addition, newer agents deliver additional coagulation-enhancing products such as platelets and thrombin to the operative site to supplement the patient’s natural clotting process.
FIGURE How a clot forms
Tried and true technologies
Because of long-standing experience with porcine gelatin, oxidized regenerated cellulose (ORC), and microfibrillar collagen, the Food and Drug Administration recategorized them in 2002 as Class-II devices with a clear safety profile.
Porcine gelatin
This class of products (Gelfoam, Surgifoam, Spongistan) has been around since the 1940s. The products have no intrinsic hemostatic action. They absorb 45 times their weight in blood and provide a scaff old on which platelets come into close contact, initiating the release of intrinsic and extrinsic clotting mechanisms.
Oxidized regenerated cellulose
This class (Surgicel, Oxycel) has also been around since the 1940s. The agents have acidic properties, due to their low pH level, and achieve hemostasis via denaturation of blood proteins, mechanical activation of the clotting cascade, and local vasoconstriction.
Because of its low pH, ORC is bactericidal against many common pathogens of the reproductive tract. A few studies have explored laparoscopic application of ORC to achieve hemostasis at sites of uterine perforation and for tubal bleeding secondary to sterilization.1,2 Successful hemostasis of moderate bleeding was achieved without the need for suture or conversion to laparotomy in all cases without brisk arterial bleeding.
Another ORC product (Interceed) is often used in gynecologic surgery as a barrier to adhesion formation. Its degree of oxidation, weave, and pore size differ from those of Surgicel. It requires an absolutely dry operative field to prevent adhesions.
Microfibrillar collagen
Substances derived from bovine collagen were marketed in the 1970s and 1980s. Collagen provides binding sites for platelets, which degranulate, releasing coagulation factors and initiating the clotting cascade.
Microfibrillar collagen products are available in a variety of forms (powder, sheets, loaded syringes for endoscopic placement) and are applied with pressure directly to the bleeding site. Collagen (Instat, Helistat) is supplied as a sponge, whereas microfibrillar collagen (Avitene, Superstat, Actifoam, Helitene, Hemopad, Novacol) is a tenacious powder or sheet. Microfibrillar collagen, like porcine gelatin and ORC, is absorbed by the body over time.
No studies have evaluated microfibrillar collagen in gynecologic surgery, although case reports of successful application to sites of uterine perforation after dilation and curettage and to bleeding sites after vaginal or laparoscopic hysterectomy have been published.3,4
New products are largely unproven
Class-III devices require significantly more study before safety and efficacy can be demonstrated. The two types of products presented here—topical thrombin and tissue sealants—remain largely unproven.
Topical thrombin
This class of products has been available for more than 20 years. As a liquid (Thrombogen), topical thrombin can be supplied in a syringe and sprayed onto oozing sites. A liquid combination of collagen gelatin matrix and bovine thrombin (FloSeal) provides a structure on which clots can form; triggers topical conversion of fibrinogen to fibrin; and activates the clotting cascade. It was approved for use in 1999. CoStasis and Vitagel are similar products, approved in 2000 and 2006, respectively, that add plasma obtained from the patient at the beginning of the surgical procedure.
Thrombin and ORC don’t mix. The acidity of ORC inactivates thrombin; therefore, ORC products should not be used with any product containing bovine or human thrombin.
The theoretical advantage of products that use patients’ plasma is the addition of autologous clotting factors and platelets to the bovine collagen and thrombin mixture. Preliminary studies have shown:
- a reduction in postoperative pain in 20 orthopedic surgery patients randomized to platelet gel, compared with what was seen in 20 women in the control group5
- a reduction in the rate of sternal wound infection in cardiac surgery patients (0.3% with the gel; 1.8% without it)6
- a potentially shorter healing time when platelet gel is applied to surgical wounds.7
The facts. Labeling for topical thrombin specifically states that it is not for use in cases of infection or for postpartum hemorrhage or menorrhagia. Studies of topical thrombin products have used the time to cessation of bleeding as their primary effectiveness end-point. In practical terms, however, studies have demonstrated no reduction in the need for transfusion or chest tube drainage in re-operative cardiac surgery patients.8
Disadvantages of topical thrombin include the cost of the product (including the cost of a plasma-collection device) and the need for operating room staff to collect and combine the product for use. Topical thrombin also exposes the patient to the risk of antibody formation (see Bovine thrombin can trigger risky antibodies), catastrophic bleeding, and, even, death.
Products that contain bovine thrombin have some safety issues with regard to their antigenic reactivity. Patients may develop antibodies to the bovine product that cross-react with human thrombin and factor Va. Associated with all products that contain bovine thrombin is a black box warning that states that the product may be associated with severe bleeding, thrombosis, and, rarely, death, because of antibody formation.
In one case report, a very complicated patient who required systemic anticoagulation for a mechanical aortic valve underwent hysterectomy, with topical thrombin administered at the end of the procedure in an effort to avert postoperative hemorrhage.1 She developed antibodies to the bovine thrombin, which caused significant and severe coagulation defects.
No clinical studies have assessed these products in gynecologic surgery.
Reference
1. Sharma JB, Malhotra M, Pundir P. Laparoscopic oxidized cellulose (Surgicel) application for small uterine perforations. Int J Gynaecol Obstet. 2003;83:271-275.
This last set of products has been approved for use in cardiopulmonary bypass procedures, in patients who have splenic injury, and to close a colostomy. They are “tissue glues” that also have hemostatic properties.
Tisseel is a combination of human thrombin, human “sealer protein” (fibrinogen), and aprotinin, a synthetic inhibitor of fibrinolysis that prevents premature degradation of a clot once it has formed. In clinical studies, this product has reduced the need for splenectomy in patients who have bleeding that is difficult to control.
Disadvantages of tissue-sealing products. These products have not been studied in gynecologic patients. They have the significant disadvantage of containing products derived from pooled human plasma. Although precautions have been taken to reduce transmission of infectious disease, viral transmission may occur. Anaphylaxis is an additional risk.
Many products are available to help the ObGyn surgeon achieve hemostasis in tough situations. Most of the time, we face generalized oozing after treatment of extensive endometriosis or adhesiolysis; in these cases, older topical agents should serve us well. Patients who experience massive bleeding are not likely to benefit from the use of any of the products described in this article.
Extensive bleeding from uterine incisions—at cesarean section or after myomectomy—might respond to topical thrombin, platelet gel products, or tissue sealants, but these products have not been studied in our patients. They also are expensive and carry some risk for our patients.
Don’t overlook two strategies for extremely high-risk situations:
- Cell-saver technology can help avert transfusion in patients expected to lose a substantial amount of blood
- Intravenous recombinant activated factor VII (NovoSeven) can be life-saving for women who experience postpartum hemorrhage, placenta percreta, or retroperitoneal sarcoma and for whom our standard strategies have failed.—BARBARA S. LEVY, MD
The author reports no financial relationships relevant to this article.
- What clinical problem is this device or drug designed to address?
- Is the problem important clinically for the well-being of my patients?
- What do I know about the basic science and physiology of the problem?
- Are there data supporting improvement in clinical outcomes, or do the studies look only at surrogate endpoints?
- Has the drug or device been studied in gynecologic patients? Is it approved for use in this population?
- Is there evidence that the new technology is more effective, safer, or less expensive than products I use now?
The process of evaluation is critical for us—as advocates for individual patients and as stewards of the diminishing resources available to treat patients in the United States.
With these questions in mind, let’s look at a surgical problem for which there are many new and potentially exciting products being promoted to gynecologic surgeons: the need to achieve hemostasis.
Bleeding is a serious clinical problem during surgery—one that can have a major impact on the well-being of the patient. We generally use sutures and electrosurgical instruments—both bipolar and monopolar—to control major vessels. Topical hemostasis agents may be useful, however, in areas where generalized oozing is present, or where the application of energy may endanger vital structures.
Many products are available for use in these situations. Some are tried and true; others, new or unapproved for use in gynecologic surgery. Your assessment and selection of the optimal product should take into account 1) the cause of bleeding and 2) the mechanism of action of the product.
Surgical bleeding has one of two causes
Although the science of coagulation is complex, bleeding occurs, from a surgical perspective, because of either of two problems:
- failure to control significant arterial and venous sources
- failure of normal clotting functions, such as vasoconstriction; platelet activation and plugging ( FIGURE ); and activation of the coagulation cascade.
No topical or systemic agent adequately controls Problem #1. To address Problem #2, we have several choices to augment hemostasis, including thermal, chemical, and mechanical means. In addition, newer agents deliver additional coagulation-enhancing products such as platelets and thrombin to the operative site to supplement the patient’s natural clotting process.
FIGURE How a clot forms
Tried and true technologies
Because of long-standing experience with porcine gelatin, oxidized regenerated cellulose (ORC), and microfibrillar collagen, the Food and Drug Administration recategorized them in 2002 as Class-II devices with a clear safety profile.
Porcine gelatin
This class of products (Gelfoam, Surgifoam, Spongistan) has been around since the 1940s. The products have no intrinsic hemostatic action. They absorb 45 times their weight in blood and provide a scaff old on which platelets come into close contact, initiating the release of intrinsic and extrinsic clotting mechanisms.
Oxidized regenerated cellulose
This class (Surgicel, Oxycel) has also been around since the 1940s. The agents have acidic properties, due to their low pH level, and achieve hemostasis via denaturation of blood proteins, mechanical activation of the clotting cascade, and local vasoconstriction.
Because of its low pH, ORC is bactericidal against many common pathogens of the reproductive tract. A few studies have explored laparoscopic application of ORC to achieve hemostasis at sites of uterine perforation and for tubal bleeding secondary to sterilization.1,2 Successful hemostasis of moderate bleeding was achieved without the need for suture or conversion to laparotomy in all cases without brisk arterial bleeding.
Another ORC product (Interceed) is often used in gynecologic surgery as a barrier to adhesion formation. Its degree of oxidation, weave, and pore size differ from those of Surgicel. It requires an absolutely dry operative field to prevent adhesions.
Microfibrillar collagen
Substances derived from bovine collagen were marketed in the 1970s and 1980s. Collagen provides binding sites for platelets, which degranulate, releasing coagulation factors and initiating the clotting cascade.
Microfibrillar collagen products are available in a variety of forms (powder, sheets, loaded syringes for endoscopic placement) and are applied with pressure directly to the bleeding site. Collagen (Instat, Helistat) is supplied as a sponge, whereas microfibrillar collagen (Avitene, Superstat, Actifoam, Helitene, Hemopad, Novacol) is a tenacious powder or sheet. Microfibrillar collagen, like porcine gelatin and ORC, is absorbed by the body over time.
No studies have evaluated microfibrillar collagen in gynecologic surgery, although case reports of successful application to sites of uterine perforation after dilation and curettage and to bleeding sites after vaginal or laparoscopic hysterectomy have been published.3,4
New products are largely unproven
Class-III devices require significantly more study before safety and efficacy can be demonstrated. The two types of products presented here—topical thrombin and tissue sealants—remain largely unproven.
Topical thrombin
This class of products has been available for more than 20 years. As a liquid (Thrombogen), topical thrombin can be supplied in a syringe and sprayed onto oozing sites. A liquid combination of collagen gelatin matrix and bovine thrombin (FloSeal) provides a structure on which clots can form; triggers topical conversion of fibrinogen to fibrin; and activates the clotting cascade. It was approved for use in 1999. CoStasis and Vitagel are similar products, approved in 2000 and 2006, respectively, that add plasma obtained from the patient at the beginning of the surgical procedure.
Thrombin and ORC don’t mix. The acidity of ORC inactivates thrombin; therefore, ORC products should not be used with any product containing bovine or human thrombin.
The theoretical advantage of products that use patients’ plasma is the addition of autologous clotting factors and platelets to the bovine collagen and thrombin mixture. Preliminary studies have shown:
- a reduction in postoperative pain in 20 orthopedic surgery patients randomized to platelet gel, compared with what was seen in 20 women in the control group5
- a reduction in the rate of sternal wound infection in cardiac surgery patients (0.3% with the gel; 1.8% without it)6
- a potentially shorter healing time when platelet gel is applied to surgical wounds.7
The facts. Labeling for topical thrombin specifically states that it is not for use in cases of infection or for postpartum hemorrhage or menorrhagia. Studies of topical thrombin products have used the time to cessation of bleeding as their primary effectiveness end-point. In practical terms, however, studies have demonstrated no reduction in the need for transfusion or chest tube drainage in re-operative cardiac surgery patients.8
Disadvantages of topical thrombin include the cost of the product (including the cost of a plasma-collection device) and the need for operating room staff to collect and combine the product for use. Topical thrombin also exposes the patient to the risk of antibody formation (see Bovine thrombin can trigger risky antibodies), catastrophic bleeding, and, even, death.
Products that contain bovine thrombin have some safety issues with regard to their antigenic reactivity. Patients may develop antibodies to the bovine product that cross-react with human thrombin and factor Va. Associated with all products that contain bovine thrombin is a black box warning that states that the product may be associated with severe bleeding, thrombosis, and, rarely, death, because of antibody formation.
In one case report, a very complicated patient who required systemic anticoagulation for a mechanical aortic valve underwent hysterectomy, with topical thrombin administered at the end of the procedure in an effort to avert postoperative hemorrhage.1 She developed antibodies to the bovine thrombin, which caused significant and severe coagulation defects.
No clinical studies have assessed these products in gynecologic surgery.
Reference
1. Sharma JB, Malhotra M, Pundir P. Laparoscopic oxidized cellulose (Surgicel) application for small uterine perforations. Int J Gynaecol Obstet. 2003;83:271-275.
This last set of products has been approved for use in cardiopulmonary bypass procedures, in patients who have splenic injury, and to close a colostomy. They are “tissue glues” that also have hemostatic properties.
Tisseel is a combination of human thrombin, human “sealer protein” (fibrinogen), and aprotinin, a synthetic inhibitor of fibrinolysis that prevents premature degradation of a clot once it has formed. In clinical studies, this product has reduced the need for splenectomy in patients who have bleeding that is difficult to control.
Disadvantages of tissue-sealing products. These products have not been studied in gynecologic patients. They have the significant disadvantage of containing products derived from pooled human plasma. Although precautions have been taken to reduce transmission of infectious disease, viral transmission may occur. Anaphylaxis is an additional risk.
Many products are available to help the ObGyn surgeon achieve hemostasis in tough situations. Most of the time, we face generalized oozing after treatment of extensive endometriosis or adhesiolysis; in these cases, older topical agents should serve us well. Patients who experience massive bleeding are not likely to benefit from the use of any of the products described in this article.
Extensive bleeding from uterine incisions—at cesarean section or after myomectomy—might respond to topical thrombin, platelet gel products, or tissue sealants, but these products have not been studied in our patients. They also are expensive and carry some risk for our patients.
Don’t overlook two strategies for extremely high-risk situations:
- Cell-saver technology can help avert transfusion in patients expected to lose a substantial amount of blood
- Intravenous recombinant activated factor VII (NovoSeven) can be life-saving for women who experience postpartum hemorrhage, placenta percreta, or retroperitoneal sarcoma and for whom our standard strategies have failed.—BARBARA S. LEVY, MD
1. Sharma JB, Malhotra M, Pundir P. Laparoscopic oxidized cellulose (Surgicel) application for small uterine perforations. Int J Gynaecol Obstet. 2003;83:271-275.
2. Sharma JB, Malhotra M. Topical oxidized cellulose for tubal hemorrhage hemostasis during laparoscopic sterilization. Int J Gynaecol Obstet. 2003;82:221-222.
3. Borten M, Friedman EA. Translaparoscopic hemostasis with microfibrillar collagen in lieu of laparotomy. A report of two cases. J Reprod Med. 1983;28:804-806.
4. Holub Z, Jabor A. Laparoscopic management of bleeding after laparoscopic or vaginal hysterectomy. JSLS. 2004;8:235-238.
5. Zavadil DP, Satterlee CC, Costigan JM, Holt DW, Shostrom VK. Autologous platelet gel and platelet-poor plasma reduce pain with total shoulder arthroplasty. J Extra Corpor Technol. 2007;39:177-182.
6. Trowbridge CC, Stammers AH, Woods E, Yen BR, Klayman M. Use of platelet gel and its effects on infection in cardiac surgery. J Extra Corpor Technol. 2005;37:381-386.
7. Hom DB, Linzie MB, Huang TC. The healing effects of autologous platelet gel on acute human skin wounds. Arch Facial Plast Surg. 2007;9:174-183.
8. Wajon P, Gibson J, Calcroft R, Hughes C, Thrift B. Intraoperative plateletpheresis and autologous platelet gel do not reduce chest tube drainage or allogeneic blood transfusion after reoperative coronary artery bypass graft. Anesth Analg. 2001;93:536-542.
1. Sharma JB, Malhotra M, Pundir P. Laparoscopic oxidized cellulose (Surgicel) application for small uterine perforations. Int J Gynaecol Obstet. 2003;83:271-275.
2. Sharma JB, Malhotra M. Topical oxidized cellulose for tubal hemorrhage hemostasis during laparoscopic sterilization. Int J Gynaecol Obstet. 2003;82:221-222.
3. Borten M, Friedman EA. Translaparoscopic hemostasis with microfibrillar collagen in lieu of laparotomy. A report of two cases. J Reprod Med. 1983;28:804-806.
4. Holub Z, Jabor A. Laparoscopic management of bleeding after laparoscopic or vaginal hysterectomy. JSLS. 2004;8:235-238.
5. Zavadil DP, Satterlee CC, Costigan JM, Holt DW, Shostrom VK. Autologous platelet gel and platelet-poor plasma reduce pain with total shoulder arthroplasty. J Extra Corpor Technol. 2007;39:177-182.
6. Trowbridge CC, Stammers AH, Woods E, Yen BR, Klayman M. Use of platelet gel and its effects on infection in cardiac surgery. J Extra Corpor Technol. 2005;37:381-386.
7. Hom DB, Linzie MB, Huang TC. The healing effects of autologous platelet gel on acute human skin wounds. Arch Facial Plast Surg. 2007;9:174-183.
8. Wajon P, Gibson J, Calcroft R, Hughes C, Thrift B. Intraoperative plateletpheresis and autologous platelet gel do not reduce chest tube drainage or allogeneic blood transfusion after reoperative coronary artery bypass graft. Anesth Analg. 2001;93:536-542.