User login
Venous thromboembolism (VTE) includes both deep vein thrombosis (DVT) and pulmonary embolism (PE). Although the exact incidence of VTE is unknown, an estimated 1 million people in the United States are affected each year, with about a third experiencing a recurrence within 10 years.1 VTE affects hospitalized and nonhospitalized patients, is often overlooked, and results in long-term complications including postthrombotic syndrome (PTS) for DVT, postpulmonary embolism syndrome and chronic thromboembolic pulmonary hypertension for PE, and death.2
TREATMENT
Treatment for VTE should be initiated in the following cases:
- Proximal DVT of the lower extremity
- Symptomatic distal (calf vein) DVT
- Symptomatic upper extremity DVT (axillary-subclavian veins)
- PE
- Subsegmental PE in a patient at risk for recurrence
- Surveillance for subsegmental PE in a patient with no proximal DVT and a low risk of recurrence.
In addition to anticoagulants, other more aggressive therapies for VTE may be appropriate, such as systemic thrombolysis in the case of PE or catheter-directed thrombolytic or pharmacomechnical therapies for DVT or PE, surgical intervention (acute pulmonary embolectomy), or placement of an inferior vena cava (IVC) filter.
This article reviews the management of VTE, highlighting the recent changes in treatment and prevention guidelines from the American College of Chest Physicians (ACCP).3
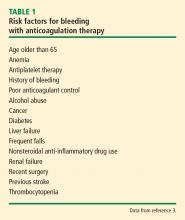
Risk of bleeding
In assessing a patient’s risk of bleeding for anticoagulation therapy (Table 1), the absence of risk factors is considered low risk for bleeding, the presence of 1 risk factor is considered intermediate risk, and 2 or more risk factors is considered high risk. Compared with low-risk patients, moderate-risk patients have a twofold increased risk of major bleeding and high-risk patients have an eightfold increased risk of major bleeding. This equates to an annualized risk of major bleeding of 0.8% for low-risk patients, 1.6% for moderate-risk patients, and greater than 6.5% for high-risk patients.3
Anticoagulants
Deciding on which anticoagulant to use depends on the indication, the patient’s underlying condition, the patient’s preference, and the patient’s risk of bleeding. Heparin, the LMWHs, fondaparinux and the DOACs (rivaroxaban and apixaban) are the only agents approved by the US Food and Drug Administration (FDA) recommended for the acute treatment phase, while the DOACs and warfarin are anticoagulation options for the long-term and extended treatment phases. The LMWHs should be used for the patient with cancer and during pregnancy.
Unfractionated heparin. UFH is administered parenterally and can be used for the prevention and treatment of VTE. Heparin remains an option for initial treatment of patients with acute VTE and is generally preferred over LMWH for patients who may require advanced therapies, such as for hemodynamically unstable PE or iliofemoral DVT. It is also recommended for patients with renal failure.3 Weight-based dosing (80 U/kg bolus followed by 18 U/kg/hour intravenous infusion) is recommended, targeting an antifactor activated clotting factor (anti-Xa) assay level of 0.3 IU/mL to 0.7 IU/mL. Heparin may also be given subcutaneously in an outpatient setting using an initial bolus of 333 U/kg followed by a subcutaneous dose of 17,500 U twice daily.5
Low-molecular-weight heparin. LMWHs are administered as weight-based subcutaneous injections and have indications for patients with acute VTE and for VTE prophylaxis. LMWHs are used for transitioning to warfarin, dabigatran, or edoxaban for long-term anticoagulation and are recommended over warfarin and DOACs for treatment of VTE in patients with cancer and in pregnant women.3
Enoxaparin (Lovenox), the most commonly used agent in the United States, is given either as a once-daily injection (1.5 mg/kg/day) or a twice-daily injection (1 mg/kg every 12 hours). It is also approved for VTE prophylaxis in patients undergoing hip or knee replacement surgery or abdominal surgery, or in patients with severely restricted mobility during acute illness. LMWH can also be given in patients with renal insufficiency (creatinine clearance [CrCL] < 30 mL/minute) after dose adjustment. No monitoring is required, although it is advised in pediatric patients, pregnant women, obese patients, and patients with renal insufficiency. If monitoring is required, an anti-Xa assay using LMWH as a reference standard should be done 4 hours after subcutaneous injection. The therapeutic range for enoxaparin is 0.5 IU/mL to 1.0 IU/mL for the 12-hour regimen and greater than 1.0 IU/mL for the once-daily dose. Other LMWHs available in the United States include dalteparin (Fragmin) and tinzaparin (Innohep). Each has its own specific indications.
Fondaparinux. Fondaparinux is an indirect factor Xa inhibitor, chemically related to LMWH. It is approved for treatment of patients with acute VTE when used in combination with a VKA (warfarin) or dabigatran or edoxaban. It also has approval for VTE prophylaxis in patients undergoing hip fracture, hip or knee replacement, and abdominal surgery. Fondaparinux is administered as a once-daily subcutaneous injection of 2.5 mg for DVT prophylaxis and a body weight-based dose for the treatment of VTE (5 mg < 50 kg; 7.5 mg 50 to 100 kg; 10 mg > 100 kg).6 Fondaparinux is contraindicated in patients with severe renal impairment (CrCL les 30 mL/min) and bacterial endocarditis.6
Warfarin. Warfarin, a VKA, was the mainstay of therapy for long-term and extended treatment of VTE until the advent of the DOACs. Warfarin must be coadministered with heparin, LMWH, or fondaparinux initially and continued as overlap therapy for a minimum of 5 days until the international normalized ratio [INR] is at least 2.0 for 24 hours.4 Early initiation of a VKA on the first day of parenteral therapy is advised.
Warfarin remains the best option for patients on long-term or extended anticoagulation with liver dysfunction (elevated serum transaminases exceeding twice the upper limits of normal or active liver disease) or renal disease (CrCL < 30 mL/min), as well as patients unable to afford DOACs. Additionally, select patient populations may still be best served by warfarin as these groups were underrepresented or not included in DOAC trials, including pediatric patients, individuals with body weight less than 50 kg or greater than 150 kg, and patients with select types of thrombophilia (eg, antiphospholipid syndrome). Warfarin is also advised for patients with poor compliance, as international normalized ratio of prothrombin time (PT/INR) monitoring is required using a point-of-care testing device or during a visit to an anticoagulation clinic. DOACs do not require monitoring, and noncompliance will not be readily apparent.
Direct oral anticoagulants. The DOACs, which include the factor Xa inhibitors rivaroxaban (Xarelto), apixaban (Eliquis), and edoxaban (Savaysa) and the direct thrombin inhibitor dabigatran (Pradaxa), been studied extensively and shown to be noninferior to VKAs for treatment of VTE.7 DOACs are currently recommended by the ACCP for long-term treatment of VTE, and several have extended treatment recommendations for VTE over the VKAs.3
The advantages of DOACs include no need for PT/INR monitoring, a fixed dosage, shorter half-life, rapid onset of action (for monotherapy), and in most cases, no need for bridging for interventional or surgical procedures. Additional advantages may include a decreased burden of care for the physician and improved quality of life for the patient. DOACs are also the agents of choice for patients who prefer oral therapy (avoiding parenteral therapy), have limited access to an anticoagulation clinic (home bound or geographic inaccessibility for PT/INR monitoring), or have food or drug-drug interactions. Patients at risk of gastrointestinal bleeding or dyspepsia should avoid dabigatran, while apixaban may be preferred if there is a history of gastrointestinal bleeding.8
Rivaroxaban or apixaban can be used as monotherapy for the initial treatment of VTE, while a 5-day course of heparin, LMWH, or fondaparinux is necessary with dabigatran or edoxaban. Rivaroxaban has been approved by the FDA for use in the prevention and treatment of VTE.9,10 For VTE prophylaxis, rivaroxaban is given orally at 10 mg once daily for 35 days for patients undergoing total hip replacement surgery and for 12 days for patients undergoing knee replacement surgery. For the treatment of VTE, rivaroxaban is given orally at 15 mg twice a day for the initial 21 days of treatment, followed by once daily at 20 mg per day for long-term treatment. It is also approved for extended-duration therapy in both 10-mg and 20-mg doses. In a recently published randomized double-blind trial of rivaroxaban compared with aspirin, the risk of a recurrent event was lower with either dose of rivaroxaban compared with aspirin without an increase in bleeding.11 Rivaroxaban is contraindicated in patients with renal insufficiency (CrCL < 30 mL/min). Both the 15-mg and 20-mg tablets must be taken with food.
Apixaban is also approved for monotherapy of VTE and was found to be noninferior to standard therapy of LMWH and warfarin with less bleeding.12 Apixaban is used for VTE prophylaxis in patients undergoing hip or knee replacement surgery, given at 2.5 mg twice daily beginning 12 to 24 hours postoperatively for 35 days (hip) or 12 days (knee). The acute-phase dosage is 10 mg twice daily for 7 days followed by 5 mg twice daily for long-term treatment of VTE. The recommended dose should be reduced to 2.5 mg twice daily in patients that meet 2 of the following criteria: age 80 or older; body weight of 60 kg or less; or with a serum creatinine 1.5 mg/dL or greater. Apixaban is also approved for extended treatment of VTE. In a randomized, double-blind study of 2 doses (2.5 mg and 5 mg, twice daily) of apixaban compared with placebo, apixaban reduced the risk of recurrent VTE without increasing the risk of bleeding.13
Both dabigatran and edoxaban require an initial 5-day overlap with a parenteral anticoagulant.14,15 Dabigatran is given at 150 mg orally twice daily if the CrCL is greater than 30 mL/min for the long-term treatment of VTE. Edoxaban is given orally at 60 mg once daily but reduced to 30 mg once daily if the CrCL is 30 mL/min to 50 mL/min, if body weight is 60 kg or less, or with use of certain P-glycoprotein inhibitors. Dabigatran has been evaluated in 2 double-blind, randomized controlled trials comparing the extended use of dabigatran with warfarin or placebo in patients with VTE.16 Dabigatran carried a lower risk of major or clinically relevant bleeding than warfarin but a higher risk than placebo. Dabigatran was noninferior to warfarin but significantly reduced the rate of recurrence in the placebo group.16
The major side effect observed with all DOACs is bleeding, but they have been proven safer particularly in the terms of major bleeding compared with the standard heparin-LMWH-VKA regimen for treatment of VTE.17–19 The risk of major bleeding, and in particular intracranial bleeding, has been shown to be less with DOACs compared with VKAs in 2 meta-analysis trials.17,18 Of the 4 new DOACs, only dabigatran currently has an anticoagulant-reversing agent (idarucizumab), although an antidote for the other 3 agents is awaiting FDA approval.20
Subsegmental pulmonary embolism
There is debate as to the need for treatment of patients with subsegmental PE. The most recent guidelines advise clinical surveillance over anticoagulation for patients with a low risk for recurrent VTE and no evidence for a proximal DVT.3 However, individuals who are hospitalized, have reduced mobility, have active cancer or are being treated with chemotherapy, or have a low cardiopulmonary reserve should be considered for anticoagulation unless they have a high bleeding risk.
Thrombolytic therapy
Thrombolytic therapy may be beneficial in select patients with VTE and can be delivered systemically or locally per catheter-directed therapy (CDT). Both routes carry an increased risk of hemorrhage compared with standard anticoagulation. The Catheter-Directed-Venous Thrombolysis (CaVenT) trial and Thrombus Obliteration by Rapid Percutaneous Endovenous Intervention in Deep Venous Occlusion (TORPEDO) trial compared CDT with standard therapy.21,22 In CaVEnT, CDT resulted in increased clinical benefit during the 5-year follow-up but did not result in improved quality of life.21 In the TORPEDO trial, patients with proximal DVT receiving percutaneous endovenous intervention and anticoagulation compared with anticoagulation alone demonstrated superiority in the reduction of PTS at greater than 2 years.22 Early results of the Acute Venous Thrombosis: Thrombus Removal With Adjunctive Catheter-directed Thrombolysis (ATTRACT) trial show that most patients with DVT did not have a long-term benefit from CDT, buy they did have reduced leg pain and swelling and some had reduced risk of moderate-to-severe PTS.23
The 2012 and 2016 ACCP guidelines advise anticoagulant therapy over CDT for patients with acute DVT of the leg but suggest patients who may benefit are those with iliofemoral DVT with symptoms for less than 14 days, good functional status, a life expectancy greater than 1 year, and a low risk of bleeding.3,4 This is in contrast to the 2008 CHEST guidelines that recommended patients who have extensive proximal DVT, who have a high risk of limb gangrene, who are at low risk of bleeding, and who otherwise have good functional status be given CDT if the expertise and resources are available.24 It has been suggested that CDT promotes early recanalization and minimizes the incidence of PTS.
Thrombolytic therapy for acute PE remains controversial because there is no clearly established short-term mortality benefit. In the Pulmonary Embolism Thrombolysis (PEITHO) trial, thrombolysis prevented hemodynamic decompensation but increased the risk of major hemorrhage and stroke.25 A lower dose (50 mg) of thrombolytic therapy was studied in the Moderate Pulmonary Embolism Treated With Thrombolysis (MOPPET) trial and was found to be safe and effective in the treatment of moderate PE.26
CDT has also been shown to be effective in the treatment of PE. The Ultrasound Acceleration Thrombolysis of Pulmonary Embolism (ULTIMA) trial demonstrated that catheter-directed thrombolysis with ultrasonographic guidance in patients with acute intermediate-risk PE was superior in reversing right ventricular dilatation without an increase in bleeding complications compared with UFH.27 The Ultrasound-Facilitated, Catheter-Directed, Low-Dose Fibrinolysis for Acute Massive and Submassive Pulmonary Embolism (SEATTLE II) study found that this approach decreased right ventricular dilation, decreased pulmonary hypertension, decreased anatomic burden, and minimized the risk of intracranial hemorrhage in patients with massive and submassive PE.28
Alteplase (Activase) is a recombinant tissue-type plasminogen activator approved by the FDA for treatment of acute PE. Alteplase is administered as a 100-mg infusion over 2 hours. Because of favorable outcomes with prompt recognition and anticoagulation for PE, the ACCP guidelines recommend systemic thrombolysis for hemodynamically unstable patients (systolic blood pressure < 90 mm Hg) with acute PE and a low risk of bleeding using a peripheral vein.3 These guidelines also recommend thrombolysis for the patient whose condition deteriorates after starting anticoagulant therapy but who have yet to develop hypotension.
If the appropriate expertise is available, CDT is suggested for patients with acute PE if they have hypotension and a high bleeding risk, have failed systemic thrombolysis, or are in shock that is likely to cause death before systemic thrombolysis can take effect.3 An area of ongoing debate is whether there is a benefit for thrombolytic therapy in patients with submassive PE who are hemodynamically stable but have evidence of right ventricular dysfunction on echocardiography or computed tomographic angiography. Bleeding remains the most serious complication of thrombolytic therapy.4
Surgical interventions: Pulmonary embolectomy and IVC filters
Pulmonary embolectomy. According to ACCP guidelines, surgical pulmonary embolectomy for the initial treatment of PE is reserved for patients with massive PE (documented angiographically, if possible), shock despite heparin and resuscitation efforts, and failure of thrombolytic therapy or a contraindication to its use.4 To date, there have been no randomized trials evaluating this procedure. Pooled data published by Stein et al29 reported a 20% operative mortality rate in patients undergoing pulmonary embolectomy between 1985 and 2005 compared with 32% in patients undergoing the procedure before 1985. A more recent retrospective review of 214 patients undergoing surgical embolectomy for massive and submassive PE reported an in-hospital mortality rate of 11.7%, with the highest death rate (32.1%) in patients who had a preoperative cardiac arrest.30 The use of surgical embolectomy has also been reported in patients with intermediate-risk to high-risk conditions (defined as elevated biomarkers and evidence of right heart strain on computed tomographic angiography or echocardiography).19
IVC filters. Current guidelines recommend against routine use of IVC filters for patients with DVT or PE who are able to be treated with anticoagulants.3 Absolute indications for the placement of IVC filters include a contraindication to anticoagulation, complications of anticoagulation, and recurrent thromboembolism despite adequate anticoagulant therapy.4 Relative indications for IVC filters are massive PE, iliocaval DVT, free-floating proximal DVT, cardiac or pulmonary insufficiency, high risk of complications from anticoagulation (frequent falls, ataxia), and poor compliance.
Retrievable filters may be considered for situations in which anticoagulation is temporarily contraindicated or there is a short duration of PE risk.31 The current consensus guidelines advise that indications for placing a retrievable IVC filter are the same as for placing a permanent device.31 An IVC filter alone is not effective therapy for VTE, and resumption of anticoagulation is recommended as soon as possible after placement.
DURATION OF TREATMENT
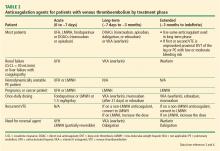
Current guidelines recommend 3 months of anticoagulation (long-term) for patients with an episode of acute proximal or isolated distal DVT of the leg or PE resulting from surgery or a nonsurgical transient cause.3 Patients who have the antiphospholipid syndrome, who are homozygous for factor V Leiden, or who are doubly heterozygous for factor V Leiden and prothrombin gene mutation should be considered for longer (extended) anticoagulation. Extended anticoagulation is also recommended in patients with active cancer and in patients who have unexplained recurrent VTE (Table 2).3
The duration of treatment for unprovoked VTE remains controversial. In the most recent ACCP guidelines, indefinite or extended anticoagulation is indicated for patients with a low or moderate risk of bleeding for a first (and second) unprovoked VTE.4 Patients with a high risk of bleeding with a first (or second) unprovoked VTE that is a proximal DVT of the leg or PE be treated for 3 months.3,4 Three DOACs (rivaroxaban, apixaban, and dabigatran) have extended-duration indications. The 2016 ACCP guidelines suggest aspirin over no treatment for the patient who has decided to stop anticoagulation therapy, although the guidelines do not consider aspirin a reasonable alternative to anticoagulation.34,35 Use of markers such as residual venous obstruction and D-dimer level in conjunction with the DASH score have been studied in an effort to predict the risk of recurrence and thus the duration of anticoagulation.36,37 Residual venous obstruction appears to be less useful than the D-dimer level as an indicator for recurrence. The D-dimer used in conjunction with the DASH prediction score may help to calculate recurrence risk based on the following predictors: abnormal D-dimer 3 weeks after stopping anticoagulation, age under 50, male sex, and hormone use at the time of the VTE.38 DASH score assessment may help physicians decide whether to continue anticoagulation therapy but it has not been shown to be helpful in men.4 A more recent study confirmed the validity of the DASH score with better prediction in patients under age 65. The recurrence rate was higher in the older population, suggesting that this population should be considered for prolonged treatment if the bleeding risk is acceptable.39 Other prediction tools include the Vienna prediction model and the clinical decision rule “Men continue and HER DOO2”—ie, HER = hyperpigmentation, edema, redness; DOO = D-dimer ≥ 250 μg/L, obesity body mass index ≥ 30 kg/m2, old age (≥ 65); 2 = high risk if more than 2 of these factors.40,41
SCREENING AND PREVENTION
Nearly 60% of all VTE events occur in hospitals and nursing homes.42 Yet anticoagulant prophylaxis is used in only 16% to 33% of at-risk hospitalized medical patients compared with 90% of at-risk hospitalized surgical patients.43 Adequate prophylaxis can reduce the incidence of VTE as demonstrated in a meta-analysis involving 19,958 patients, which revealed a 64% reduction in relative risk (RR) of a fatal PE, 58% reduction in RR of a symptomic PE, and a 53% reduction in RR of a symptomatic DVT.43
The consequences of VTE include symptomatic DVT and PE, fatal PE, the cost of investigating symptomatic patients, the risk and cost of treatment (bleeding), PTS, and chronic thromboembolic pulmonary hypertension. Heparin, enoxaparin, and fondaparinux are approved agents for prophylactic but each agent has specific indications. Factor Xa inhibitors, rivaroxaban, and apixaban are approved for use in patients undergoing total knee or hip replacement. More recently, the factor Xa inhibitor, betrixaban, has been approved for VTE prophylaxis for up to 42 days in adult patients hospitalized for acute medical illness.44 For patients with increased bleeding risk who are unable to receive pharmacologic prophylaxis, intermittent pneumatic compression devices or graduated compression stockings should be used.
Compression stockings
Current ACCP guidelines advise against routine use of compression stockings to prevent PTS in patients who have had a DVT.3 While current evidence suggests compression stockings do not prevent PTS, they reduce symptoms of acute or chronic DVT for some patients.
- Centers for Disease Control and Prevention. Venous thromboembolism (blood clots). https://www.cdc.gov/ncbddd/dvt/data.html. Updated June 22, 2015. Reviewed April 6, 2017. Accessed October 24, 2017.
- Klok FA, van der Hulle T, den Exter PL, Lankeit M, Huisman MV, Konstantinides S. The post-PE syndrome: A new concept for chronic complications of pulmonary embolism. Blood Rev 2014; 28:221–226.
- Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest 2016; 149:315–352.
- Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2012; 141(suppl 2):e419S–494S.
- Kearon C, Ginsberg JS, Julian JA, et al; Fixed-Dose Heparin (FIDO) Investigators. Comparison of fixed-dose weight-adjusted unfractionated heparin and low-molecular-weight heparin for acute treatment of venous thromboembolism. JAMA 2006; 296:935–942.
- Arixtra [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2010. https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/021345s023lbl.pdf. Accessed October 24, 2017.
- Adam SS, McDuffie JR, Ortel TL, Williams Jr JW. Comparative effectiveness of warfarin and new oral anticoagulants for the management of atrial fibrillation and venous thromboembolism: a systematic review. Ann Intern Med 2012; 157:796–807.
- Yeh CH, Gross PL, Weitz JI. Evolving use of new oral anticoagulants for treatment of venous thromboembolism. Blood 2014; 124:1020–1028.
- EINSTEIN–PE Investigators; Büller HR, Prins MH, Lensin AW, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med 2012; 366:1287–1297.
- EINSTEIN Investigators; Bauersachs R, Berkowitz SD, Brenner B, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010; 363:2499–2510.
- Weitz JI, Lensing AWA, Prins MH, et al; EINSTEIN CHOICE Investigators. Rivaroxaban or aspirin for extended treatment of venous thromboembolism. N Engl J Med 2017; 376:1211–1222.
- Agnelli G, Buller HR, Cohen A, et al; AMPLIFY Investigators. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med 2013; 369:799–808.
- Agnelli G, Buller HR, Cohen A, et al; AMPLIFY-EXT Investigators. Apixaban for extended treatment of venous thromboembolism. N Engl J Med 2013; 368:699–708.
- Schulman S, Kearon C, Kakkar AK, et al; RE-COVER Study Group. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009; 361:2342–2352.
- The Hokusai-VTE Investigators; Büller HR, Décousus H, Grosso MA, et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med 2013; 369:1406–1415.
- Schulman S, Kearon C, Kakkar AK, et al; RE-MEDY Trial Investigators; RE-SONATE Trial Investigators. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med 2013; 368:709–718.
- van Es N, Coppens M, Schulman S, Middeldorp S, Büller HR. Direct oral anticoagulants compared with vitamin K antagonists for acute venous thromboembolism: evidence from phase 3 trials. Blood 2014; 124:1968–1975.
- Chai-Adisaksopha C, Crowther M, Isayama T, Lim W. The impact of bleeding complications in patients receiving target-specific oral anticoagulants: a systematic review and meta-analysis. Blood 2014; 124:2450–2458.
- Konstantinides SV, Torbicki A, Agnelli G, et al; Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 2014; 35:3033–3069, 3069a–3069k.
- Pollack CV Jr, Reilly PA, Eikelboom J, et al. Idarucizumab for dabigatran reversal. N Engl J Med 2015; 373:511–520.
- Haig Y, Enden T, Grøtta O, et al; CaVenT Study Group. Post-thrombotic syndrome after catheter-directed thrombolysis for deep vein thrombosis (CaVenT): 5-year follow-up results of an open-label, randomised controlled trial. Lancet Haematol 2016; 3:e64–e71.
- Sharifi M, Bay C, Mehdipour M, Sharifi J; TORPEDO Investigators. Thrombus obliteration by rapid percutaneous endovenous intervention in deep venous occlusion (TORPEDO) trial: midterm results. J Endovasc Ther 2012; 19:273–280.
- Society of Interventional Radiology. Pivotal study of minimally invasive therapy improves the care of patients with deep vein thrombosis [news release]. https://www.sirweb.org/advocacy-and-outreach/media/news-release-archive/news-release-ATTRACT-Trial. Published March 6, 2017. Accessed November 28, 2017.
- Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians evidence-based clinical practice guidelines (8th ed). Chest 2008; 133(suppl 6):454S–545S.
- Meyer G, Vicaut E, Danays T, et al; PEITHO Investigators. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med 2014; 370:1402–1411.
- Sharifi M, Bay C, Skrocki L, Rahimi F, Mehdipour M; “MOPETT” Investigators. Moderate pulmonary embolism treated with thrombolysis (from the “MOPETT” Trial). Am J Cardiol 2013; 111:273–277.
- Kucher N, Boekstegers P, Müller OJ, et al. Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation 2014; 129:479–486.
- Piazza G, Hohlfelder B, Jaff MR, et al; SEATTLE II Investigators. A prospective, single-arm, multicenter trial of ultrasound-facilitated, catheter-directed, low-dose fibrinolysis for acute massive and submassive pulmonary embolism: the SEATTLE II study. JACC Cardiovasc Interv 2015; 8:1382–1392.
- Stein PD, Alnas M, Beemath A, Patel NR. Outcome of pulmonary embolectomy. Am J Cardiol 2007; 99:421–423.
- Keeling WB, Sundt T, Leacche M, et al; SPEAR Working Group. Outcomes after surgical pulmonary embolectomy for acute pulmonary embolus: a multi-institutional study. Ann Thorac Surg 2016; 102:1498–1502.
- Kaufman JA, Kinney TB, Streiff MB, et al. Guidelines for the use of retrievable and convertible vena cava filters: report from the Society of Interventional Radiology multidisciplinary consensus conference. J Vasc Interv Radiol 2006; 17:449–459.
- Kyrle PA, Rosendaal FR, Eichinger S. Risk assessment for recurrent venous thrombosis. Lancet 2010; 376:2032–2039.
- Heit JA. Predicting the risk of venous thromboembolism recurrence. Am J Hematol 2012; 87(suppl 1):S63–S67.
- Becattini C, Agnelli G, Schenone A, et al; WARFASA Investigators. Aspirin for preventing the recurrence of venous thromboembolism. N Engl J Med 2012; 366:1959–1967.
- Brighton TA, Eikelboom JW, Mann K, et al; ASPIRE Investigators. Low-dose aspirin for preventing recurrent venous thromboembolism. N Engl J Med 2012; 367:1979–1987.
- Carrier M, Rodger MA, Wells PS, Righini M, LE Gal G. Residual vein obstruction to predict the risk of recurrent venous thromboembolism in patients with deep vein thrombosis: a systematic review and meta-analysis. J Thromb Haemost 2011; 9:1119–1125.
- Siragusa S, Malato A, Saccullo G, et al. Residual vein thrombosis for assessing duration of anticoagulation after unprovoked deep vein thrombosis of the lower limbs: the extended DACUS study. Am J Hematol 2011; 86:914–917.
- Tosetto A, Iorio A, Marcucci M, et al. Predicting disease recurrence in patients with previous unprovoked venous thromboembolism: a proposed prediction score (DASH). J Thromb Haemost 2012; 10:1019–1025.
- Tosetto A, Testa S, Martinelli I, et al. External validation of the DASH prediction rule: a retrospective cohort study. J Thromb Haemost 2017; 15:1963–1970.
- Rodger MA, Kahn SR, Wells PS, et al. Identifying unprovoked thromboembolism patients at low risk for recurrence who can discontinue anticoagulant therapy. CMAJ 2008; 179:417–426.
- Eichinger S, Heinze G, Jandeck LM, Kyrle PA. Risk assessment of recurrence in patients with unprovoked deep vein thrombosis or pulmonary embolism: the Vienna prediction model. Circulation 2010; 121:1630–1636.
- Heit JA, O’Fallon WM, Petterson TM, et al. Relative impact of risk factors for deep vein thrombosis and pulmonary embolism: a population-based study. Arch Intern Med 2002; 162:1245–1248.
- Dentali F, Douketis JD, Gianni M, Lim W, Crowther MA. Meta-analysis: anticoagulant prophylaxis to prevent symptomatic venous thromboembolism in hospitalized medical patients. Ann Intern Med 2007; 146:278–288.
- Cohen AT, Harrington RA, Goldhaber SZ, et al; APEX Investigators. Extended thromboprophylaxis with betrixaban in acutely ill medical patients. N Engl J Med 2016; 375:534–544.
Venous thromboembolism (VTE) includes both deep vein thrombosis (DVT) and pulmonary embolism (PE). Although the exact incidence of VTE is unknown, an estimated 1 million people in the United States are affected each year, with about a third experiencing a recurrence within 10 years.1 VTE affects hospitalized and nonhospitalized patients, is often overlooked, and results in long-term complications including postthrombotic syndrome (PTS) for DVT, postpulmonary embolism syndrome and chronic thromboembolic pulmonary hypertension for PE, and death.2
TREATMENT
Treatment for VTE should be initiated in the following cases:
- Proximal DVT of the lower extremity
- Symptomatic distal (calf vein) DVT
- Symptomatic upper extremity DVT (axillary-subclavian veins)
- PE
- Subsegmental PE in a patient at risk for recurrence
- Surveillance for subsegmental PE in a patient with no proximal DVT and a low risk of recurrence.
In addition to anticoagulants, other more aggressive therapies for VTE may be appropriate, such as systemic thrombolysis in the case of PE or catheter-directed thrombolytic or pharmacomechnical therapies for DVT or PE, surgical intervention (acute pulmonary embolectomy), or placement of an inferior vena cava (IVC) filter.
This article reviews the management of VTE, highlighting the recent changes in treatment and prevention guidelines from the American College of Chest Physicians (ACCP).3
Risk of bleeding
In assessing a patient’s risk of bleeding for anticoagulation therapy (Table 1), the absence of risk factors is considered low risk for bleeding, the presence of 1 risk factor is considered intermediate risk, and 2 or more risk factors is considered high risk. Compared with low-risk patients, moderate-risk patients have a twofold increased risk of major bleeding and high-risk patients have an eightfold increased risk of major bleeding. This equates to an annualized risk of major bleeding of 0.8% for low-risk patients, 1.6% for moderate-risk patients, and greater than 6.5% for high-risk patients.3
Anticoagulants
Deciding on which anticoagulant to use depends on the indication, the patient’s underlying condition, the patient’s preference, and the patient’s risk of bleeding. Heparin, the LMWHs, fondaparinux and the DOACs (rivaroxaban and apixaban) are the only agents approved by the US Food and Drug Administration (FDA) recommended for the acute treatment phase, while the DOACs and warfarin are anticoagulation options for the long-term and extended treatment phases. The LMWHs should be used for the patient with cancer and during pregnancy.
Unfractionated heparin. UFH is administered parenterally and can be used for the prevention and treatment of VTE. Heparin remains an option for initial treatment of patients with acute VTE and is generally preferred over LMWH for patients who may require advanced therapies, such as for hemodynamically unstable PE or iliofemoral DVT. It is also recommended for patients with renal failure.3 Weight-based dosing (80 U/kg bolus followed by 18 U/kg/hour intravenous infusion) is recommended, targeting an antifactor activated clotting factor (anti-Xa) assay level of 0.3 IU/mL to 0.7 IU/mL. Heparin may also be given subcutaneously in an outpatient setting using an initial bolus of 333 U/kg followed by a subcutaneous dose of 17,500 U twice daily.5
Low-molecular-weight heparin. LMWHs are administered as weight-based subcutaneous injections and have indications for patients with acute VTE and for VTE prophylaxis. LMWHs are used for transitioning to warfarin, dabigatran, or edoxaban for long-term anticoagulation and are recommended over warfarin and DOACs for treatment of VTE in patients with cancer and in pregnant women.3
Enoxaparin (Lovenox), the most commonly used agent in the United States, is given either as a once-daily injection (1.5 mg/kg/day) or a twice-daily injection (1 mg/kg every 12 hours). It is also approved for VTE prophylaxis in patients undergoing hip or knee replacement surgery or abdominal surgery, or in patients with severely restricted mobility during acute illness. LMWH can also be given in patients with renal insufficiency (creatinine clearance [CrCL] < 30 mL/minute) after dose adjustment. No monitoring is required, although it is advised in pediatric patients, pregnant women, obese patients, and patients with renal insufficiency. If monitoring is required, an anti-Xa assay using LMWH as a reference standard should be done 4 hours after subcutaneous injection. The therapeutic range for enoxaparin is 0.5 IU/mL to 1.0 IU/mL for the 12-hour regimen and greater than 1.0 IU/mL for the once-daily dose. Other LMWHs available in the United States include dalteparin (Fragmin) and tinzaparin (Innohep). Each has its own specific indications.
Fondaparinux. Fondaparinux is an indirect factor Xa inhibitor, chemically related to LMWH. It is approved for treatment of patients with acute VTE when used in combination with a VKA (warfarin) or dabigatran or edoxaban. It also has approval for VTE prophylaxis in patients undergoing hip fracture, hip or knee replacement, and abdominal surgery. Fondaparinux is administered as a once-daily subcutaneous injection of 2.5 mg for DVT prophylaxis and a body weight-based dose for the treatment of VTE (5 mg < 50 kg; 7.5 mg 50 to 100 kg; 10 mg > 100 kg).6 Fondaparinux is contraindicated in patients with severe renal impairment (CrCL les 30 mL/min) and bacterial endocarditis.6
Warfarin. Warfarin, a VKA, was the mainstay of therapy for long-term and extended treatment of VTE until the advent of the DOACs. Warfarin must be coadministered with heparin, LMWH, or fondaparinux initially and continued as overlap therapy for a minimum of 5 days until the international normalized ratio [INR] is at least 2.0 for 24 hours.4 Early initiation of a VKA on the first day of parenteral therapy is advised.
Warfarin remains the best option for patients on long-term or extended anticoagulation with liver dysfunction (elevated serum transaminases exceeding twice the upper limits of normal or active liver disease) or renal disease (CrCL < 30 mL/min), as well as patients unable to afford DOACs. Additionally, select patient populations may still be best served by warfarin as these groups were underrepresented or not included in DOAC trials, including pediatric patients, individuals with body weight less than 50 kg or greater than 150 kg, and patients with select types of thrombophilia (eg, antiphospholipid syndrome). Warfarin is also advised for patients with poor compliance, as international normalized ratio of prothrombin time (PT/INR) monitoring is required using a point-of-care testing device or during a visit to an anticoagulation clinic. DOACs do not require monitoring, and noncompliance will not be readily apparent.
Direct oral anticoagulants. The DOACs, which include the factor Xa inhibitors rivaroxaban (Xarelto), apixaban (Eliquis), and edoxaban (Savaysa) and the direct thrombin inhibitor dabigatran (Pradaxa), been studied extensively and shown to be noninferior to VKAs for treatment of VTE.7 DOACs are currently recommended by the ACCP for long-term treatment of VTE, and several have extended treatment recommendations for VTE over the VKAs.3
The advantages of DOACs include no need for PT/INR monitoring, a fixed dosage, shorter half-life, rapid onset of action (for monotherapy), and in most cases, no need for bridging for interventional or surgical procedures. Additional advantages may include a decreased burden of care for the physician and improved quality of life for the patient. DOACs are also the agents of choice for patients who prefer oral therapy (avoiding parenteral therapy), have limited access to an anticoagulation clinic (home bound or geographic inaccessibility for PT/INR monitoring), or have food or drug-drug interactions. Patients at risk of gastrointestinal bleeding or dyspepsia should avoid dabigatran, while apixaban may be preferred if there is a history of gastrointestinal bleeding.8
Rivaroxaban or apixaban can be used as monotherapy for the initial treatment of VTE, while a 5-day course of heparin, LMWH, or fondaparinux is necessary with dabigatran or edoxaban. Rivaroxaban has been approved by the FDA for use in the prevention and treatment of VTE.9,10 For VTE prophylaxis, rivaroxaban is given orally at 10 mg once daily for 35 days for patients undergoing total hip replacement surgery and for 12 days for patients undergoing knee replacement surgery. For the treatment of VTE, rivaroxaban is given orally at 15 mg twice a day for the initial 21 days of treatment, followed by once daily at 20 mg per day for long-term treatment. It is also approved for extended-duration therapy in both 10-mg and 20-mg doses. In a recently published randomized double-blind trial of rivaroxaban compared with aspirin, the risk of a recurrent event was lower with either dose of rivaroxaban compared with aspirin without an increase in bleeding.11 Rivaroxaban is contraindicated in patients with renal insufficiency (CrCL < 30 mL/min). Both the 15-mg and 20-mg tablets must be taken with food.
Apixaban is also approved for monotherapy of VTE and was found to be noninferior to standard therapy of LMWH and warfarin with less bleeding.12 Apixaban is used for VTE prophylaxis in patients undergoing hip or knee replacement surgery, given at 2.5 mg twice daily beginning 12 to 24 hours postoperatively for 35 days (hip) or 12 days (knee). The acute-phase dosage is 10 mg twice daily for 7 days followed by 5 mg twice daily for long-term treatment of VTE. The recommended dose should be reduced to 2.5 mg twice daily in patients that meet 2 of the following criteria: age 80 or older; body weight of 60 kg or less; or with a serum creatinine 1.5 mg/dL or greater. Apixaban is also approved for extended treatment of VTE. In a randomized, double-blind study of 2 doses (2.5 mg and 5 mg, twice daily) of apixaban compared with placebo, apixaban reduced the risk of recurrent VTE without increasing the risk of bleeding.13
Both dabigatran and edoxaban require an initial 5-day overlap with a parenteral anticoagulant.14,15 Dabigatran is given at 150 mg orally twice daily if the CrCL is greater than 30 mL/min for the long-term treatment of VTE. Edoxaban is given orally at 60 mg once daily but reduced to 30 mg once daily if the CrCL is 30 mL/min to 50 mL/min, if body weight is 60 kg or less, or with use of certain P-glycoprotein inhibitors. Dabigatran has been evaluated in 2 double-blind, randomized controlled trials comparing the extended use of dabigatran with warfarin or placebo in patients with VTE.16 Dabigatran carried a lower risk of major or clinically relevant bleeding than warfarin but a higher risk than placebo. Dabigatran was noninferior to warfarin but significantly reduced the rate of recurrence in the placebo group.16
The major side effect observed with all DOACs is bleeding, but they have been proven safer particularly in the terms of major bleeding compared with the standard heparin-LMWH-VKA regimen for treatment of VTE.17–19 The risk of major bleeding, and in particular intracranial bleeding, has been shown to be less with DOACs compared with VKAs in 2 meta-analysis trials.17,18 Of the 4 new DOACs, only dabigatran currently has an anticoagulant-reversing agent (idarucizumab), although an antidote for the other 3 agents is awaiting FDA approval.20
Subsegmental pulmonary embolism
There is debate as to the need for treatment of patients with subsegmental PE. The most recent guidelines advise clinical surveillance over anticoagulation for patients with a low risk for recurrent VTE and no evidence for a proximal DVT.3 However, individuals who are hospitalized, have reduced mobility, have active cancer or are being treated with chemotherapy, or have a low cardiopulmonary reserve should be considered for anticoagulation unless they have a high bleeding risk.
Thrombolytic therapy
Thrombolytic therapy may be beneficial in select patients with VTE and can be delivered systemically or locally per catheter-directed therapy (CDT). Both routes carry an increased risk of hemorrhage compared with standard anticoagulation. The Catheter-Directed-Venous Thrombolysis (CaVenT) trial and Thrombus Obliteration by Rapid Percutaneous Endovenous Intervention in Deep Venous Occlusion (TORPEDO) trial compared CDT with standard therapy.21,22 In CaVEnT, CDT resulted in increased clinical benefit during the 5-year follow-up but did not result in improved quality of life.21 In the TORPEDO trial, patients with proximal DVT receiving percutaneous endovenous intervention and anticoagulation compared with anticoagulation alone demonstrated superiority in the reduction of PTS at greater than 2 years.22 Early results of the Acute Venous Thrombosis: Thrombus Removal With Adjunctive Catheter-directed Thrombolysis (ATTRACT) trial show that most patients with DVT did not have a long-term benefit from CDT, buy they did have reduced leg pain and swelling and some had reduced risk of moderate-to-severe PTS.23
The 2012 and 2016 ACCP guidelines advise anticoagulant therapy over CDT for patients with acute DVT of the leg but suggest patients who may benefit are those with iliofemoral DVT with symptoms for less than 14 days, good functional status, a life expectancy greater than 1 year, and a low risk of bleeding.3,4 This is in contrast to the 2008 CHEST guidelines that recommended patients who have extensive proximal DVT, who have a high risk of limb gangrene, who are at low risk of bleeding, and who otherwise have good functional status be given CDT if the expertise and resources are available.24 It has been suggested that CDT promotes early recanalization and minimizes the incidence of PTS.
Thrombolytic therapy for acute PE remains controversial because there is no clearly established short-term mortality benefit. In the Pulmonary Embolism Thrombolysis (PEITHO) trial, thrombolysis prevented hemodynamic decompensation but increased the risk of major hemorrhage and stroke.25 A lower dose (50 mg) of thrombolytic therapy was studied in the Moderate Pulmonary Embolism Treated With Thrombolysis (MOPPET) trial and was found to be safe and effective in the treatment of moderate PE.26
CDT has also been shown to be effective in the treatment of PE. The Ultrasound Acceleration Thrombolysis of Pulmonary Embolism (ULTIMA) trial demonstrated that catheter-directed thrombolysis with ultrasonographic guidance in patients with acute intermediate-risk PE was superior in reversing right ventricular dilatation without an increase in bleeding complications compared with UFH.27 The Ultrasound-Facilitated, Catheter-Directed, Low-Dose Fibrinolysis for Acute Massive and Submassive Pulmonary Embolism (SEATTLE II) study found that this approach decreased right ventricular dilation, decreased pulmonary hypertension, decreased anatomic burden, and minimized the risk of intracranial hemorrhage in patients with massive and submassive PE.28
Alteplase (Activase) is a recombinant tissue-type plasminogen activator approved by the FDA for treatment of acute PE. Alteplase is administered as a 100-mg infusion over 2 hours. Because of favorable outcomes with prompt recognition and anticoagulation for PE, the ACCP guidelines recommend systemic thrombolysis for hemodynamically unstable patients (systolic blood pressure < 90 mm Hg) with acute PE and a low risk of bleeding using a peripheral vein.3 These guidelines also recommend thrombolysis for the patient whose condition deteriorates after starting anticoagulant therapy but who have yet to develop hypotension.
If the appropriate expertise is available, CDT is suggested for patients with acute PE if they have hypotension and a high bleeding risk, have failed systemic thrombolysis, or are in shock that is likely to cause death before systemic thrombolysis can take effect.3 An area of ongoing debate is whether there is a benefit for thrombolytic therapy in patients with submassive PE who are hemodynamically stable but have evidence of right ventricular dysfunction on echocardiography or computed tomographic angiography. Bleeding remains the most serious complication of thrombolytic therapy.4
Surgical interventions: Pulmonary embolectomy and IVC filters
Pulmonary embolectomy. According to ACCP guidelines, surgical pulmonary embolectomy for the initial treatment of PE is reserved for patients with massive PE (documented angiographically, if possible), shock despite heparin and resuscitation efforts, and failure of thrombolytic therapy or a contraindication to its use.4 To date, there have been no randomized trials evaluating this procedure. Pooled data published by Stein et al29 reported a 20% operative mortality rate in patients undergoing pulmonary embolectomy between 1985 and 2005 compared with 32% in patients undergoing the procedure before 1985. A more recent retrospective review of 214 patients undergoing surgical embolectomy for massive and submassive PE reported an in-hospital mortality rate of 11.7%, with the highest death rate (32.1%) in patients who had a preoperative cardiac arrest.30 The use of surgical embolectomy has also been reported in patients with intermediate-risk to high-risk conditions (defined as elevated biomarkers and evidence of right heart strain on computed tomographic angiography or echocardiography).19
IVC filters. Current guidelines recommend against routine use of IVC filters for patients with DVT or PE who are able to be treated with anticoagulants.3 Absolute indications for the placement of IVC filters include a contraindication to anticoagulation, complications of anticoagulation, and recurrent thromboembolism despite adequate anticoagulant therapy.4 Relative indications for IVC filters are massive PE, iliocaval DVT, free-floating proximal DVT, cardiac or pulmonary insufficiency, high risk of complications from anticoagulation (frequent falls, ataxia), and poor compliance.
Retrievable filters may be considered for situations in which anticoagulation is temporarily contraindicated or there is a short duration of PE risk.31 The current consensus guidelines advise that indications for placing a retrievable IVC filter are the same as for placing a permanent device.31 An IVC filter alone is not effective therapy for VTE, and resumption of anticoagulation is recommended as soon as possible after placement.
DURATION OF TREATMENT
Current guidelines recommend 3 months of anticoagulation (long-term) for patients with an episode of acute proximal or isolated distal DVT of the leg or PE resulting from surgery or a nonsurgical transient cause.3 Patients who have the antiphospholipid syndrome, who are homozygous for factor V Leiden, or who are doubly heterozygous for factor V Leiden and prothrombin gene mutation should be considered for longer (extended) anticoagulation. Extended anticoagulation is also recommended in patients with active cancer and in patients who have unexplained recurrent VTE (Table 2).3
The duration of treatment for unprovoked VTE remains controversial. In the most recent ACCP guidelines, indefinite or extended anticoagulation is indicated for patients with a low or moderate risk of bleeding for a first (and second) unprovoked VTE.4 Patients with a high risk of bleeding with a first (or second) unprovoked VTE that is a proximal DVT of the leg or PE be treated for 3 months.3,4 Three DOACs (rivaroxaban, apixaban, and dabigatran) have extended-duration indications. The 2016 ACCP guidelines suggest aspirin over no treatment for the patient who has decided to stop anticoagulation therapy, although the guidelines do not consider aspirin a reasonable alternative to anticoagulation.34,35 Use of markers such as residual venous obstruction and D-dimer level in conjunction with the DASH score have been studied in an effort to predict the risk of recurrence and thus the duration of anticoagulation.36,37 Residual venous obstruction appears to be less useful than the D-dimer level as an indicator for recurrence. The D-dimer used in conjunction with the DASH prediction score may help to calculate recurrence risk based on the following predictors: abnormal D-dimer 3 weeks after stopping anticoagulation, age under 50, male sex, and hormone use at the time of the VTE.38 DASH score assessment may help physicians decide whether to continue anticoagulation therapy but it has not been shown to be helpful in men.4 A more recent study confirmed the validity of the DASH score with better prediction in patients under age 65. The recurrence rate was higher in the older population, suggesting that this population should be considered for prolonged treatment if the bleeding risk is acceptable.39 Other prediction tools include the Vienna prediction model and the clinical decision rule “Men continue and HER DOO2”—ie, HER = hyperpigmentation, edema, redness; DOO = D-dimer ≥ 250 μg/L, obesity body mass index ≥ 30 kg/m2, old age (≥ 65); 2 = high risk if more than 2 of these factors.40,41
SCREENING AND PREVENTION
Nearly 60% of all VTE events occur in hospitals and nursing homes.42 Yet anticoagulant prophylaxis is used in only 16% to 33% of at-risk hospitalized medical patients compared with 90% of at-risk hospitalized surgical patients.43 Adequate prophylaxis can reduce the incidence of VTE as demonstrated in a meta-analysis involving 19,958 patients, which revealed a 64% reduction in relative risk (RR) of a fatal PE, 58% reduction in RR of a symptomic PE, and a 53% reduction in RR of a symptomatic DVT.43
The consequences of VTE include symptomatic DVT and PE, fatal PE, the cost of investigating symptomatic patients, the risk and cost of treatment (bleeding), PTS, and chronic thromboembolic pulmonary hypertension. Heparin, enoxaparin, and fondaparinux are approved agents for prophylactic but each agent has specific indications. Factor Xa inhibitors, rivaroxaban, and apixaban are approved for use in patients undergoing total knee or hip replacement. More recently, the factor Xa inhibitor, betrixaban, has been approved for VTE prophylaxis for up to 42 days in adult patients hospitalized for acute medical illness.44 For patients with increased bleeding risk who are unable to receive pharmacologic prophylaxis, intermittent pneumatic compression devices or graduated compression stockings should be used.
Compression stockings
Current ACCP guidelines advise against routine use of compression stockings to prevent PTS in patients who have had a DVT.3 While current evidence suggests compression stockings do not prevent PTS, they reduce symptoms of acute or chronic DVT for some patients.
Venous thromboembolism (VTE) includes both deep vein thrombosis (DVT) and pulmonary embolism (PE). Although the exact incidence of VTE is unknown, an estimated 1 million people in the United States are affected each year, with about a third experiencing a recurrence within 10 years.1 VTE affects hospitalized and nonhospitalized patients, is often overlooked, and results in long-term complications including postthrombotic syndrome (PTS) for DVT, postpulmonary embolism syndrome and chronic thromboembolic pulmonary hypertension for PE, and death.2
TREATMENT
Treatment for VTE should be initiated in the following cases:
- Proximal DVT of the lower extremity
- Symptomatic distal (calf vein) DVT
- Symptomatic upper extremity DVT (axillary-subclavian veins)
- PE
- Subsegmental PE in a patient at risk for recurrence
- Surveillance for subsegmental PE in a patient with no proximal DVT and a low risk of recurrence.
In addition to anticoagulants, other more aggressive therapies for VTE may be appropriate, such as systemic thrombolysis in the case of PE or catheter-directed thrombolytic or pharmacomechnical therapies for DVT or PE, surgical intervention (acute pulmonary embolectomy), or placement of an inferior vena cava (IVC) filter.
This article reviews the management of VTE, highlighting the recent changes in treatment and prevention guidelines from the American College of Chest Physicians (ACCP).3
Risk of bleeding
In assessing a patient’s risk of bleeding for anticoagulation therapy (Table 1), the absence of risk factors is considered low risk for bleeding, the presence of 1 risk factor is considered intermediate risk, and 2 or more risk factors is considered high risk. Compared with low-risk patients, moderate-risk patients have a twofold increased risk of major bleeding and high-risk patients have an eightfold increased risk of major bleeding. This equates to an annualized risk of major bleeding of 0.8% for low-risk patients, 1.6% for moderate-risk patients, and greater than 6.5% for high-risk patients.3
Anticoagulants
Deciding on which anticoagulant to use depends on the indication, the patient’s underlying condition, the patient’s preference, and the patient’s risk of bleeding. Heparin, the LMWHs, fondaparinux and the DOACs (rivaroxaban and apixaban) are the only agents approved by the US Food and Drug Administration (FDA) recommended for the acute treatment phase, while the DOACs and warfarin are anticoagulation options for the long-term and extended treatment phases. The LMWHs should be used for the patient with cancer and during pregnancy.
Unfractionated heparin. UFH is administered parenterally and can be used for the prevention and treatment of VTE. Heparin remains an option for initial treatment of patients with acute VTE and is generally preferred over LMWH for patients who may require advanced therapies, such as for hemodynamically unstable PE or iliofemoral DVT. It is also recommended for patients with renal failure.3 Weight-based dosing (80 U/kg bolus followed by 18 U/kg/hour intravenous infusion) is recommended, targeting an antifactor activated clotting factor (anti-Xa) assay level of 0.3 IU/mL to 0.7 IU/mL. Heparin may also be given subcutaneously in an outpatient setting using an initial bolus of 333 U/kg followed by a subcutaneous dose of 17,500 U twice daily.5
Low-molecular-weight heparin. LMWHs are administered as weight-based subcutaneous injections and have indications for patients with acute VTE and for VTE prophylaxis. LMWHs are used for transitioning to warfarin, dabigatran, or edoxaban for long-term anticoagulation and are recommended over warfarin and DOACs for treatment of VTE in patients with cancer and in pregnant women.3
Enoxaparin (Lovenox), the most commonly used agent in the United States, is given either as a once-daily injection (1.5 mg/kg/day) or a twice-daily injection (1 mg/kg every 12 hours). It is also approved for VTE prophylaxis in patients undergoing hip or knee replacement surgery or abdominal surgery, or in patients with severely restricted mobility during acute illness. LMWH can also be given in patients with renal insufficiency (creatinine clearance [CrCL] < 30 mL/minute) after dose adjustment. No monitoring is required, although it is advised in pediatric patients, pregnant women, obese patients, and patients with renal insufficiency. If monitoring is required, an anti-Xa assay using LMWH as a reference standard should be done 4 hours after subcutaneous injection. The therapeutic range for enoxaparin is 0.5 IU/mL to 1.0 IU/mL for the 12-hour regimen and greater than 1.0 IU/mL for the once-daily dose. Other LMWHs available in the United States include dalteparin (Fragmin) and tinzaparin (Innohep). Each has its own specific indications.
Fondaparinux. Fondaparinux is an indirect factor Xa inhibitor, chemically related to LMWH. It is approved for treatment of patients with acute VTE when used in combination with a VKA (warfarin) or dabigatran or edoxaban. It also has approval for VTE prophylaxis in patients undergoing hip fracture, hip or knee replacement, and abdominal surgery. Fondaparinux is administered as a once-daily subcutaneous injection of 2.5 mg for DVT prophylaxis and a body weight-based dose for the treatment of VTE (5 mg < 50 kg; 7.5 mg 50 to 100 kg; 10 mg > 100 kg).6 Fondaparinux is contraindicated in patients with severe renal impairment (CrCL les 30 mL/min) and bacterial endocarditis.6
Warfarin. Warfarin, a VKA, was the mainstay of therapy for long-term and extended treatment of VTE until the advent of the DOACs. Warfarin must be coadministered with heparin, LMWH, or fondaparinux initially and continued as overlap therapy for a minimum of 5 days until the international normalized ratio [INR] is at least 2.0 for 24 hours.4 Early initiation of a VKA on the first day of parenteral therapy is advised.
Warfarin remains the best option for patients on long-term or extended anticoagulation with liver dysfunction (elevated serum transaminases exceeding twice the upper limits of normal or active liver disease) or renal disease (CrCL < 30 mL/min), as well as patients unable to afford DOACs. Additionally, select patient populations may still be best served by warfarin as these groups were underrepresented or not included in DOAC trials, including pediatric patients, individuals with body weight less than 50 kg or greater than 150 kg, and patients with select types of thrombophilia (eg, antiphospholipid syndrome). Warfarin is also advised for patients with poor compliance, as international normalized ratio of prothrombin time (PT/INR) monitoring is required using a point-of-care testing device or during a visit to an anticoagulation clinic. DOACs do not require monitoring, and noncompliance will not be readily apparent.
Direct oral anticoagulants. The DOACs, which include the factor Xa inhibitors rivaroxaban (Xarelto), apixaban (Eliquis), and edoxaban (Savaysa) and the direct thrombin inhibitor dabigatran (Pradaxa), been studied extensively and shown to be noninferior to VKAs for treatment of VTE.7 DOACs are currently recommended by the ACCP for long-term treatment of VTE, and several have extended treatment recommendations for VTE over the VKAs.3
The advantages of DOACs include no need for PT/INR monitoring, a fixed dosage, shorter half-life, rapid onset of action (for monotherapy), and in most cases, no need for bridging for interventional or surgical procedures. Additional advantages may include a decreased burden of care for the physician and improved quality of life for the patient. DOACs are also the agents of choice for patients who prefer oral therapy (avoiding parenteral therapy), have limited access to an anticoagulation clinic (home bound or geographic inaccessibility for PT/INR monitoring), or have food or drug-drug interactions. Patients at risk of gastrointestinal bleeding or dyspepsia should avoid dabigatran, while apixaban may be preferred if there is a history of gastrointestinal bleeding.8
Rivaroxaban or apixaban can be used as monotherapy for the initial treatment of VTE, while a 5-day course of heparin, LMWH, or fondaparinux is necessary with dabigatran or edoxaban. Rivaroxaban has been approved by the FDA for use in the prevention and treatment of VTE.9,10 For VTE prophylaxis, rivaroxaban is given orally at 10 mg once daily for 35 days for patients undergoing total hip replacement surgery and for 12 days for patients undergoing knee replacement surgery. For the treatment of VTE, rivaroxaban is given orally at 15 mg twice a day for the initial 21 days of treatment, followed by once daily at 20 mg per day for long-term treatment. It is also approved for extended-duration therapy in both 10-mg and 20-mg doses. In a recently published randomized double-blind trial of rivaroxaban compared with aspirin, the risk of a recurrent event was lower with either dose of rivaroxaban compared with aspirin without an increase in bleeding.11 Rivaroxaban is contraindicated in patients with renal insufficiency (CrCL < 30 mL/min). Both the 15-mg and 20-mg tablets must be taken with food.
Apixaban is also approved for monotherapy of VTE and was found to be noninferior to standard therapy of LMWH and warfarin with less bleeding.12 Apixaban is used for VTE prophylaxis in patients undergoing hip or knee replacement surgery, given at 2.5 mg twice daily beginning 12 to 24 hours postoperatively for 35 days (hip) or 12 days (knee). The acute-phase dosage is 10 mg twice daily for 7 days followed by 5 mg twice daily for long-term treatment of VTE. The recommended dose should be reduced to 2.5 mg twice daily in patients that meet 2 of the following criteria: age 80 or older; body weight of 60 kg or less; or with a serum creatinine 1.5 mg/dL or greater. Apixaban is also approved for extended treatment of VTE. In a randomized, double-blind study of 2 doses (2.5 mg and 5 mg, twice daily) of apixaban compared with placebo, apixaban reduced the risk of recurrent VTE without increasing the risk of bleeding.13
Both dabigatran and edoxaban require an initial 5-day overlap with a parenteral anticoagulant.14,15 Dabigatran is given at 150 mg orally twice daily if the CrCL is greater than 30 mL/min for the long-term treatment of VTE. Edoxaban is given orally at 60 mg once daily but reduced to 30 mg once daily if the CrCL is 30 mL/min to 50 mL/min, if body weight is 60 kg or less, or with use of certain P-glycoprotein inhibitors. Dabigatran has been evaluated in 2 double-blind, randomized controlled trials comparing the extended use of dabigatran with warfarin or placebo in patients with VTE.16 Dabigatran carried a lower risk of major or clinically relevant bleeding than warfarin but a higher risk than placebo. Dabigatran was noninferior to warfarin but significantly reduced the rate of recurrence in the placebo group.16
The major side effect observed with all DOACs is bleeding, but they have been proven safer particularly in the terms of major bleeding compared with the standard heparin-LMWH-VKA regimen for treatment of VTE.17–19 The risk of major bleeding, and in particular intracranial bleeding, has been shown to be less with DOACs compared with VKAs in 2 meta-analysis trials.17,18 Of the 4 new DOACs, only dabigatran currently has an anticoagulant-reversing agent (idarucizumab), although an antidote for the other 3 agents is awaiting FDA approval.20
Subsegmental pulmonary embolism
There is debate as to the need for treatment of patients with subsegmental PE. The most recent guidelines advise clinical surveillance over anticoagulation for patients with a low risk for recurrent VTE and no evidence for a proximal DVT.3 However, individuals who are hospitalized, have reduced mobility, have active cancer or are being treated with chemotherapy, or have a low cardiopulmonary reserve should be considered for anticoagulation unless they have a high bleeding risk.
Thrombolytic therapy
Thrombolytic therapy may be beneficial in select patients with VTE and can be delivered systemically or locally per catheter-directed therapy (CDT). Both routes carry an increased risk of hemorrhage compared with standard anticoagulation. The Catheter-Directed-Venous Thrombolysis (CaVenT) trial and Thrombus Obliteration by Rapid Percutaneous Endovenous Intervention in Deep Venous Occlusion (TORPEDO) trial compared CDT with standard therapy.21,22 In CaVEnT, CDT resulted in increased clinical benefit during the 5-year follow-up but did not result in improved quality of life.21 In the TORPEDO trial, patients with proximal DVT receiving percutaneous endovenous intervention and anticoagulation compared with anticoagulation alone demonstrated superiority in the reduction of PTS at greater than 2 years.22 Early results of the Acute Venous Thrombosis: Thrombus Removal With Adjunctive Catheter-directed Thrombolysis (ATTRACT) trial show that most patients with DVT did not have a long-term benefit from CDT, buy they did have reduced leg pain and swelling and some had reduced risk of moderate-to-severe PTS.23
The 2012 and 2016 ACCP guidelines advise anticoagulant therapy over CDT for patients with acute DVT of the leg but suggest patients who may benefit are those with iliofemoral DVT with symptoms for less than 14 days, good functional status, a life expectancy greater than 1 year, and a low risk of bleeding.3,4 This is in contrast to the 2008 CHEST guidelines that recommended patients who have extensive proximal DVT, who have a high risk of limb gangrene, who are at low risk of bleeding, and who otherwise have good functional status be given CDT if the expertise and resources are available.24 It has been suggested that CDT promotes early recanalization and minimizes the incidence of PTS.
Thrombolytic therapy for acute PE remains controversial because there is no clearly established short-term mortality benefit. In the Pulmonary Embolism Thrombolysis (PEITHO) trial, thrombolysis prevented hemodynamic decompensation but increased the risk of major hemorrhage and stroke.25 A lower dose (50 mg) of thrombolytic therapy was studied in the Moderate Pulmonary Embolism Treated With Thrombolysis (MOPPET) trial and was found to be safe and effective in the treatment of moderate PE.26
CDT has also been shown to be effective in the treatment of PE. The Ultrasound Acceleration Thrombolysis of Pulmonary Embolism (ULTIMA) trial demonstrated that catheter-directed thrombolysis with ultrasonographic guidance in patients with acute intermediate-risk PE was superior in reversing right ventricular dilatation without an increase in bleeding complications compared with UFH.27 The Ultrasound-Facilitated, Catheter-Directed, Low-Dose Fibrinolysis for Acute Massive and Submassive Pulmonary Embolism (SEATTLE II) study found that this approach decreased right ventricular dilation, decreased pulmonary hypertension, decreased anatomic burden, and minimized the risk of intracranial hemorrhage in patients with massive and submassive PE.28
Alteplase (Activase) is a recombinant tissue-type plasminogen activator approved by the FDA for treatment of acute PE. Alteplase is administered as a 100-mg infusion over 2 hours. Because of favorable outcomes with prompt recognition and anticoagulation for PE, the ACCP guidelines recommend systemic thrombolysis for hemodynamically unstable patients (systolic blood pressure < 90 mm Hg) with acute PE and a low risk of bleeding using a peripheral vein.3 These guidelines also recommend thrombolysis for the patient whose condition deteriorates after starting anticoagulant therapy but who have yet to develop hypotension.
If the appropriate expertise is available, CDT is suggested for patients with acute PE if they have hypotension and a high bleeding risk, have failed systemic thrombolysis, or are in shock that is likely to cause death before systemic thrombolysis can take effect.3 An area of ongoing debate is whether there is a benefit for thrombolytic therapy in patients with submassive PE who are hemodynamically stable but have evidence of right ventricular dysfunction on echocardiography or computed tomographic angiography. Bleeding remains the most serious complication of thrombolytic therapy.4
Surgical interventions: Pulmonary embolectomy and IVC filters
Pulmonary embolectomy. According to ACCP guidelines, surgical pulmonary embolectomy for the initial treatment of PE is reserved for patients with massive PE (documented angiographically, if possible), shock despite heparin and resuscitation efforts, and failure of thrombolytic therapy or a contraindication to its use.4 To date, there have been no randomized trials evaluating this procedure. Pooled data published by Stein et al29 reported a 20% operative mortality rate in patients undergoing pulmonary embolectomy between 1985 and 2005 compared with 32% in patients undergoing the procedure before 1985. A more recent retrospective review of 214 patients undergoing surgical embolectomy for massive and submassive PE reported an in-hospital mortality rate of 11.7%, with the highest death rate (32.1%) in patients who had a preoperative cardiac arrest.30 The use of surgical embolectomy has also been reported in patients with intermediate-risk to high-risk conditions (defined as elevated biomarkers and evidence of right heart strain on computed tomographic angiography or echocardiography).19
IVC filters. Current guidelines recommend against routine use of IVC filters for patients with DVT or PE who are able to be treated with anticoagulants.3 Absolute indications for the placement of IVC filters include a contraindication to anticoagulation, complications of anticoagulation, and recurrent thromboembolism despite adequate anticoagulant therapy.4 Relative indications for IVC filters are massive PE, iliocaval DVT, free-floating proximal DVT, cardiac or pulmonary insufficiency, high risk of complications from anticoagulation (frequent falls, ataxia), and poor compliance.
Retrievable filters may be considered for situations in which anticoagulation is temporarily contraindicated or there is a short duration of PE risk.31 The current consensus guidelines advise that indications for placing a retrievable IVC filter are the same as for placing a permanent device.31 An IVC filter alone is not effective therapy for VTE, and resumption of anticoagulation is recommended as soon as possible after placement.
DURATION OF TREATMENT
Current guidelines recommend 3 months of anticoagulation (long-term) for patients with an episode of acute proximal or isolated distal DVT of the leg or PE resulting from surgery or a nonsurgical transient cause.3 Patients who have the antiphospholipid syndrome, who are homozygous for factor V Leiden, or who are doubly heterozygous for factor V Leiden and prothrombin gene mutation should be considered for longer (extended) anticoagulation. Extended anticoagulation is also recommended in patients with active cancer and in patients who have unexplained recurrent VTE (Table 2).3
The duration of treatment for unprovoked VTE remains controversial. In the most recent ACCP guidelines, indefinite or extended anticoagulation is indicated for patients with a low or moderate risk of bleeding for a first (and second) unprovoked VTE.4 Patients with a high risk of bleeding with a first (or second) unprovoked VTE that is a proximal DVT of the leg or PE be treated for 3 months.3,4 Three DOACs (rivaroxaban, apixaban, and dabigatran) have extended-duration indications. The 2016 ACCP guidelines suggest aspirin over no treatment for the patient who has decided to stop anticoagulation therapy, although the guidelines do not consider aspirin a reasonable alternative to anticoagulation.34,35 Use of markers such as residual venous obstruction and D-dimer level in conjunction with the DASH score have been studied in an effort to predict the risk of recurrence and thus the duration of anticoagulation.36,37 Residual venous obstruction appears to be less useful than the D-dimer level as an indicator for recurrence. The D-dimer used in conjunction with the DASH prediction score may help to calculate recurrence risk based on the following predictors: abnormal D-dimer 3 weeks after stopping anticoagulation, age under 50, male sex, and hormone use at the time of the VTE.38 DASH score assessment may help physicians decide whether to continue anticoagulation therapy but it has not been shown to be helpful in men.4 A more recent study confirmed the validity of the DASH score with better prediction in patients under age 65. The recurrence rate was higher in the older population, suggesting that this population should be considered for prolonged treatment if the bleeding risk is acceptable.39 Other prediction tools include the Vienna prediction model and the clinical decision rule “Men continue and HER DOO2”—ie, HER = hyperpigmentation, edema, redness; DOO = D-dimer ≥ 250 μg/L, obesity body mass index ≥ 30 kg/m2, old age (≥ 65); 2 = high risk if more than 2 of these factors.40,41
SCREENING AND PREVENTION
Nearly 60% of all VTE events occur in hospitals and nursing homes.42 Yet anticoagulant prophylaxis is used in only 16% to 33% of at-risk hospitalized medical patients compared with 90% of at-risk hospitalized surgical patients.43 Adequate prophylaxis can reduce the incidence of VTE as demonstrated in a meta-analysis involving 19,958 patients, which revealed a 64% reduction in relative risk (RR) of a fatal PE, 58% reduction in RR of a symptomic PE, and a 53% reduction in RR of a symptomatic DVT.43
The consequences of VTE include symptomatic DVT and PE, fatal PE, the cost of investigating symptomatic patients, the risk and cost of treatment (bleeding), PTS, and chronic thromboembolic pulmonary hypertension. Heparin, enoxaparin, and fondaparinux are approved agents for prophylactic but each agent has specific indications. Factor Xa inhibitors, rivaroxaban, and apixaban are approved for use in patients undergoing total knee or hip replacement. More recently, the factor Xa inhibitor, betrixaban, has been approved for VTE prophylaxis for up to 42 days in adult patients hospitalized for acute medical illness.44 For patients with increased bleeding risk who are unable to receive pharmacologic prophylaxis, intermittent pneumatic compression devices or graduated compression stockings should be used.
Compression stockings
Current ACCP guidelines advise against routine use of compression stockings to prevent PTS in patients who have had a DVT.3 While current evidence suggests compression stockings do not prevent PTS, they reduce symptoms of acute or chronic DVT for some patients.
- Centers for Disease Control and Prevention. Venous thromboembolism (blood clots). https://www.cdc.gov/ncbddd/dvt/data.html. Updated June 22, 2015. Reviewed April 6, 2017. Accessed October 24, 2017.
- Klok FA, van der Hulle T, den Exter PL, Lankeit M, Huisman MV, Konstantinides S. The post-PE syndrome: A new concept for chronic complications of pulmonary embolism. Blood Rev 2014; 28:221–226.
- Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest 2016; 149:315–352.
- Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2012; 141(suppl 2):e419S–494S.
- Kearon C, Ginsberg JS, Julian JA, et al; Fixed-Dose Heparin (FIDO) Investigators. Comparison of fixed-dose weight-adjusted unfractionated heparin and low-molecular-weight heparin for acute treatment of venous thromboembolism. JAMA 2006; 296:935–942.
- Arixtra [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2010. https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/021345s023lbl.pdf. Accessed October 24, 2017.
- Adam SS, McDuffie JR, Ortel TL, Williams Jr JW. Comparative effectiveness of warfarin and new oral anticoagulants for the management of atrial fibrillation and venous thromboembolism: a systematic review. Ann Intern Med 2012; 157:796–807.
- Yeh CH, Gross PL, Weitz JI. Evolving use of new oral anticoagulants for treatment of venous thromboembolism. Blood 2014; 124:1020–1028.
- EINSTEIN–PE Investigators; Büller HR, Prins MH, Lensin AW, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med 2012; 366:1287–1297.
- EINSTEIN Investigators; Bauersachs R, Berkowitz SD, Brenner B, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010; 363:2499–2510.
- Weitz JI, Lensing AWA, Prins MH, et al; EINSTEIN CHOICE Investigators. Rivaroxaban or aspirin for extended treatment of venous thromboembolism. N Engl J Med 2017; 376:1211–1222.
- Agnelli G, Buller HR, Cohen A, et al; AMPLIFY Investigators. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med 2013; 369:799–808.
- Agnelli G, Buller HR, Cohen A, et al; AMPLIFY-EXT Investigators. Apixaban for extended treatment of venous thromboembolism. N Engl J Med 2013; 368:699–708.
- Schulman S, Kearon C, Kakkar AK, et al; RE-COVER Study Group. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009; 361:2342–2352.
- The Hokusai-VTE Investigators; Büller HR, Décousus H, Grosso MA, et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med 2013; 369:1406–1415.
- Schulman S, Kearon C, Kakkar AK, et al; RE-MEDY Trial Investigators; RE-SONATE Trial Investigators. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med 2013; 368:709–718.
- van Es N, Coppens M, Schulman S, Middeldorp S, Büller HR. Direct oral anticoagulants compared with vitamin K antagonists for acute venous thromboembolism: evidence from phase 3 trials. Blood 2014; 124:1968–1975.
- Chai-Adisaksopha C, Crowther M, Isayama T, Lim W. The impact of bleeding complications in patients receiving target-specific oral anticoagulants: a systematic review and meta-analysis. Blood 2014; 124:2450–2458.
- Konstantinides SV, Torbicki A, Agnelli G, et al; Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 2014; 35:3033–3069, 3069a–3069k.
- Pollack CV Jr, Reilly PA, Eikelboom J, et al. Idarucizumab for dabigatran reversal. N Engl J Med 2015; 373:511–520.
- Haig Y, Enden T, Grøtta O, et al; CaVenT Study Group. Post-thrombotic syndrome after catheter-directed thrombolysis for deep vein thrombosis (CaVenT): 5-year follow-up results of an open-label, randomised controlled trial. Lancet Haematol 2016; 3:e64–e71.
- Sharifi M, Bay C, Mehdipour M, Sharifi J; TORPEDO Investigators. Thrombus obliteration by rapid percutaneous endovenous intervention in deep venous occlusion (TORPEDO) trial: midterm results. J Endovasc Ther 2012; 19:273–280.
- Society of Interventional Radiology. Pivotal study of minimally invasive therapy improves the care of patients with deep vein thrombosis [news release]. https://www.sirweb.org/advocacy-and-outreach/media/news-release-archive/news-release-ATTRACT-Trial. Published March 6, 2017. Accessed November 28, 2017.
- Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians evidence-based clinical practice guidelines (8th ed). Chest 2008; 133(suppl 6):454S–545S.
- Meyer G, Vicaut E, Danays T, et al; PEITHO Investigators. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med 2014; 370:1402–1411.
- Sharifi M, Bay C, Skrocki L, Rahimi F, Mehdipour M; “MOPETT” Investigators. Moderate pulmonary embolism treated with thrombolysis (from the “MOPETT” Trial). Am J Cardiol 2013; 111:273–277.
- Kucher N, Boekstegers P, Müller OJ, et al. Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation 2014; 129:479–486.
- Piazza G, Hohlfelder B, Jaff MR, et al; SEATTLE II Investigators. A prospective, single-arm, multicenter trial of ultrasound-facilitated, catheter-directed, low-dose fibrinolysis for acute massive and submassive pulmonary embolism: the SEATTLE II study. JACC Cardiovasc Interv 2015; 8:1382–1392.
- Stein PD, Alnas M, Beemath A, Patel NR. Outcome of pulmonary embolectomy. Am J Cardiol 2007; 99:421–423.
- Keeling WB, Sundt T, Leacche M, et al; SPEAR Working Group. Outcomes after surgical pulmonary embolectomy for acute pulmonary embolus: a multi-institutional study. Ann Thorac Surg 2016; 102:1498–1502.
- Kaufman JA, Kinney TB, Streiff MB, et al. Guidelines for the use of retrievable and convertible vena cava filters: report from the Society of Interventional Radiology multidisciplinary consensus conference. J Vasc Interv Radiol 2006; 17:449–459.
- Kyrle PA, Rosendaal FR, Eichinger S. Risk assessment for recurrent venous thrombosis. Lancet 2010; 376:2032–2039.
- Heit JA. Predicting the risk of venous thromboembolism recurrence. Am J Hematol 2012; 87(suppl 1):S63–S67.
- Becattini C, Agnelli G, Schenone A, et al; WARFASA Investigators. Aspirin for preventing the recurrence of venous thromboembolism. N Engl J Med 2012; 366:1959–1967.
- Brighton TA, Eikelboom JW, Mann K, et al; ASPIRE Investigators. Low-dose aspirin for preventing recurrent venous thromboembolism. N Engl J Med 2012; 367:1979–1987.
- Carrier M, Rodger MA, Wells PS, Righini M, LE Gal G. Residual vein obstruction to predict the risk of recurrent venous thromboembolism in patients with deep vein thrombosis: a systematic review and meta-analysis. J Thromb Haemost 2011; 9:1119–1125.
- Siragusa S, Malato A, Saccullo G, et al. Residual vein thrombosis for assessing duration of anticoagulation after unprovoked deep vein thrombosis of the lower limbs: the extended DACUS study. Am J Hematol 2011; 86:914–917.
- Tosetto A, Iorio A, Marcucci M, et al. Predicting disease recurrence in patients with previous unprovoked venous thromboembolism: a proposed prediction score (DASH). J Thromb Haemost 2012; 10:1019–1025.
- Tosetto A, Testa S, Martinelli I, et al. External validation of the DASH prediction rule: a retrospective cohort study. J Thromb Haemost 2017; 15:1963–1970.
- Rodger MA, Kahn SR, Wells PS, et al. Identifying unprovoked thromboembolism patients at low risk for recurrence who can discontinue anticoagulant therapy. CMAJ 2008; 179:417–426.
- Eichinger S, Heinze G, Jandeck LM, Kyrle PA. Risk assessment of recurrence in patients with unprovoked deep vein thrombosis or pulmonary embolism: the Vienna prediction model. Circulation 2010; 121:1630–1636.
- Heit JA, O’Fallon WM, Petterson TM, et al. Relative impact of risk factors for deep vein thrombosis and pulmonary embolism: a population-based study. Arch Intern Med 2002; 162:1245–1248.
- Dentali F, Douketis JD, Gianni M, Lim W, Crowther MA. Meta-analysis: anticoagulant prophylaxis to prevent symptomatic venous thromboembolism in hospitalized medical patients. Ann Intern Med 2007; 146:278–288.
- Cohen AT, Harrington RA, Goldhaber SZ, et al; APEX Investigators. Extended thromboprophylaxis with betrixaban in acutely ill medical patients. N Engl J Med 2016; 375:534–544.
- Centers for Disease Control and Prevention. Venous thromboembolism (blood clots). https://www.cdc.gov/ncbddd/dvt/data.html. Updated June 22, 2015. Reviewed April 6, 2017. Accessed October 24, 2017.
- Klok FA, van der Hulle T, den Exter PL, Lankeit M, Huisman MV, Konstantinides S. The post-PE syndrome: A new concept for chronic complications of pulmonary embolism. Blood Rev 2014; 28:221–226.
- Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest 2016; 149:315–352.
- Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2012; 141(suppl 2):e419S–494S.
- Kearon C, Ginsberg JS, Julian JA, et al; Fixed-Dose Heparin (FIDO) Investigators. Comparison of fixed-dose weight-adjusted unfractionated heparin and low-molecular-weight heparin for acute treatment of venous thromboembolism. JAMA 2006; 296:935–942.
- Arixtra [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2010. https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/021345s023lbl.pdf. Accessed October 24, 2017.
- Adam SS, McDuffie JR, Ortel TL, Williams Jr JW. Comparative effectiveness of warfarin and new oral anticoagulants for the management of atrial fibrillation and venous thromboembolism: a systematic review. Ann Intern Med 2012; 157:796–807.
- Yeh CH, Gross PL, Weitz JI. Evolving use of new oral anticoagulants for treatment of venous thromboembolism. Blood 2014; 124:1020–1028.
- EINSTEIN–PE Investigators; Büller HR, Prins MH, Lensin AW, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med 2012; 366:1287–1297.
- EINSTEIN Investigators; Bauersachs R, Berkowitz SD, Brenner B, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010; 363:2499–2510.
- Weitz JI, Lensing AWA, Prins MH, et al; EINSTEIN CHOICE Investigators. Rivaroxaban or aspirin for extended treatment of venous thromboembolism. N Engl J Med 2017; 376:1211–1222.
- Agnelli G, Buller HR, Cohen A, et al; AMPLIFY Investigators. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med 2013; 369:799–808.
- Agnelli G, Buller HR, Cohen A, et al; AMPLIFY-EXT Investigators. Apixaban for extended treatment of venous thromboembolism. N Engl J Med 2013; 368:699–708.
- Schulman S, Kearon C, Kakkar AK, et al; RE-COVER Study Group. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009; 361:2342–2352.
- The Hokusai-VTE Investigators; Büller HR, Décousus H, Grosso MA, et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med 2013; 369:1406–1415.
- Schulman S, Kearon C, Kakkar AK, et al; RE-MEDY Trial Investigators; RE-SONATE Trial Investigators. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med 2013; 368:709–718.
- van Es N, Coppens M, Schulman S, Middeldorp S, Büller HR. Direct oral anticoagulants compared with vitamin K antagonists for acute venous thromboembolism: evidence from phase 3 trials. Blood 2014; 124:1968–1975.
- Chai-Adisaksopha C, Crowther M, Isayama T, Lim W. The impact of bleeding complications in patients receiving target-specific oral anticoagulants: a systematic review and meta-analysis. Blood 2014; 124:2450–2458.
- Konstantinides SV, Torbicki A, Agnelli G, et al; Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 2014; 35:3033–3069, 3069a–3069k.
- Pollack CV Jr, Reilly PA, Eikelboom J, et al. Idarucizumab for dabigatran reversal. N Engl J Med 2015; 373:511–520.
- Haig Y, Enden T, Grøtta O, et al; CaVenT Study Group. Post-thrombotic syndrome after catheter-directed thrombolysis for deep vein thrombosis (CaVenT): 5-year follow-up results of an open-label, randomised controlled trial. Lancet Haematol 2016; 3:e64–e71.
- Sharifi M, Bay C, Mehdipour M, Sharifi J; TORPEDO Investigators. Thrombus obliteration by rapid percutaneous endovenous intervention in deep venous occlusion (TORPEDO) trial: midterm results. J Endovasc Ther 2012; 19:273–280.
- Society of Interventional Radiology. Pivotal study of minimally invasive therapy improves the care of patients with deep vein thrombosis [news release]. https://www.sirweb.org/advocacy-and-outreach/media/news-release-archive/news-release-ATTRACT-Trial. Published March 6, 2017. Accessed November 28, 2017.
- Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians evidence-based clinical practice guidelines (8th ed). Chest 2008; 133(suppl 6):454S–545S.
- Meyer G, Vicaut E, Danays T, et al; PEITHO Investigators. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med 2014; 370:1402–1411.
- Sharifi M, Bay C, Skrocki L, Rahimi F, Mehdipour M; “MOPETT” Investigators. Moderate pulmonary embolism treated with thrombolysis (from the “MOPETT” Trial). Am J Cardiol 2013; 111:273–277.
- Kucher N, Boekstegers P, Müller OJ, et al. Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation 2014; 129:479–486.
- Piazza G, Hohlfelder B, Jaff MR, et al; SEATTLE II Investigators. A prospective, single-arm, multicenter trial of ultrasound-facilitated, catheter-directed, low-dose fibrinolysis for acute massive and submassive pulmonary embolism: the SEATTLE II study. JACC Cardiovasc Interv 2015; 8:1382–1392.
- Stein PD, Alnas M, Beemath A, Patel NR. Outcome of pulmonary embolectomy. Am J Cardiol 2007; 99:421–423.
- Keeling WB, Sundt T, Leacche M, et al; SPEAR Working Group. Outcomes after surgical pulmonary embolectomy for acute pulmonary embolus: a multi-institutional study. Ann Thorac Surg 2016; 102:1498–1502.
- Kaufman JA, Kinney TB, Streiff MB, et al. Guidelines for the use of retrievable and convertible vena cava filters: report from the Society of Interventional Radiology multidisciplinary consensus conference. J Vasc Interv Radiol 2006; 17:449–459.
- Kyrle PA, Rosendaal FR, Eichinger S. Risk assessment for recurrent venous thrombosis. Lancet 2010; 376:2032–2039.
- Heit JA. Predicting the risk of venous thromboembolism recurrence. Am J Hematol 2012; 87(suppl 1):S63–S67.
- Becattini C, Agnelli G, Schenone A, et al; WARFASA Investigators. Aspirin for preventing the recurrence of venous thromboembolism. N Engl J Med 2012; 366:1959–1967.
- Brighton TA, Eikelboom JW, Mann K, et al; ASPIRE Investigators. Low-dose aspirin for preventing recurrent venous thromboembolism. N Engl J Med 2012; 367:1979–1987.
- Carrier M, Rodger MA, Wells PS, Righini M, LE Gal G. Residual vein obstruction to predict the risk of recurrent venous thromboembolism in patients with deep vein thrombosis: a systematic review and meta-analysis. J Thromb Haemost 2011; 9:1119–1125.
- Siragusa S, Malato A, Saccullo G, et al. Residual vein thrombosis for assessing duration of anticoagulation after unprovoked deep vein thrombosis of the lower limbs: the extended DACUS study. Am J Hematol 2011; 86:914–917.
- Tosetto A, Iorio A, Marcucci M, et al. Predicting disease recurrence in patients with previous unprovoked venous thromboembolism: a proposed prediction score (DASH). J Thromb Haemost 2012; 10:1019–1025.
- Tosetto A, Testa S, Martinelli I, et al. External validation of the DASH prediction rule: a retrospective cohort study. J Thromb Haemost 2017; 15:1963–1970.
- Rodger MA, Kahn SR, Wells PS, et al. Identifying unprovoked thromboembolism patients at low risk for recurrence who can discontinue anticoagulant therapy. CMAJ 2008; 179:417–426.
- Eichinger S, Heinze G, Jandeck LM, Kyrle PA. Risk assessment of recurrence in patients with unprovoked deep vein thrombosis or pulmonary embolism: the Vienna prediction model. Circulation 2010; 121:1630–1636.
- Heit JA, O’Fallon WM, Petterson TM, et al. Relative impact of risk factors for deep vein thrombosis and pulmonary embolism: a population-based study. Arch Intern Med 2002; 162:1245–1248.
- Dentali F, Douketis JD, Gianni M, Lim W, Crowther MA. Meta-analysis: anticoagulant prophylaxis to prevent symptomatic venous thromboembolism in hospitalized medical patients. Ann Intern Med 2007; 146:278–288.
- Cohen AT, Harrington RA, Goldhaber SZ, et al; APEX Investigators. Extended thromboprophylaxis with betrixaban in acutely ill medical patients. N Engl J Med 2016; 375:534–544.
KEY POINTS
- VTE treatment should begin immediately with heparin, low-molecular-weight heparin (LMWH), fondaparinux, or the DOACs (rivaroxaban or apixaban) in patients deemed appropriate based on a risk assessment for bleeding.
- For patients with VTE and no cancer, long-term treatment with dabigatran, rivaroxaban, apixaban, or edoxaban is recommended over the vitamin K antagonists (VKA).
- LMWH is recommended for the long-term treatment of VTE in patients with cancer.
- For extended-duration anticoagulation, the DOACs (dabigatran, rivaroxaban and apixaban) and the VKA antagonists are options.
- Compression stockings are no longer recommended for prevention of PTS in patients with acute DVT but may be beneficial symptomatically.