User login
As the life expectancy of women in the United States now exceeds 80 years,1 many millions of US women will spend more than one-third to even one-half of their lives beyond menopause. While hormone therapy (HT) can effectively address many of the symptoms of menopause, women who are unwilling or unable to take HT need nonhormonal alternatives for treatment of menopausal symptoms as well as the estrogen-deficiency bone loss that ensues in many women. This article reviews current and experimental nonhormonal therapies for menopausal symptoms and related issues, such as midlife sexual dysfunction and maintenance of bone health.
DEFINING THE TERMINOLOGY OF MENOPAUSE
We begin the discussion of menopausal health with a clarification of some terms.2
Menopause refers to the final menstrual period and simply represents a point in time. Menopause can be diagnosed only a year after it occurs, when it is clear that the last menstrual period was truly the final one.
Perimenopause consists of three components: the period shortly before menopause (when the biological and clinical features of impending menopause begin), menopause itself (final menstrual period), and the year following menopause. Perimenopause is synonymous with menopausal transition.
Postmenopause is the period beginning at the time of the final menstrual period (menopause), although it is recognized only after a year of amenorrhea. The early postmenopausal phase is the first 5 years after menopause, whereas all the time thereafter is referred to as the late menopausal phase.
MENOPAUSAL ASSESSMENT
Symptoms
The primary symptoms of perimenopause are:
- Vasomotor symptoms (eg, hot flashes, night sweats)
- Menstrual cycle changes (ie, oligomenorrhea, amenorrhea)
- Vaginal dryness.
Secondary symptoms include sleep disturbance, low sex drive and/or reduced sexual arousal, stress or urge urinary incontinence, mood changes, and somatic complaints.
Vasomotor symptoms, vaginal dryness, and dyspareunia (painful intercourse) contributing to sexual dysfunction have been correlated with the loss of sex hormones (particularly estrogen) associated with menopause, whereas the other symptoms listed above (sleep disturbance, urinary symptoms, mood changes, somatic symptoms) have not been linked definitively to menopause and may be a function of aging.2
Vasomotor symptoms are the predominant reason that women seek medical treatment around the time of menopause.3 More than 75% of women report hot flashes within the 2 years surrounding menopause. Among these women who have hot flashes, 25% report that these symptoms remain for greater than 5 years, and 10% report that they remain for more than 10 years.3 Vasomotor symptoms may be associated with sleep disturbance, mood swings, cognitive deficits, social impairment, a reduction in productivity, embarrassment, anxiety, and fatigue.
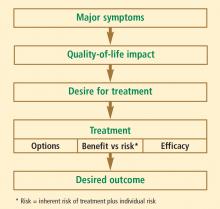
Individualizing the evaluation is imperative
The overall patient must be considered in this assessment, which includes her personal history, family history, social history, and current medication use. Common factors affecting postmenopausal health—such as bone density; vaginal, bladder, and sexual function; cardiovascular health (including lipid profile, blood pressure, and tobacco use); thromboembolic risk; and cancer risk, including breast cancer—should be included in the assessment. For most women under the age of 60 years who have menopausal symptoms, HT remains the gold standard and recommended treatment, according to both the American Association of Clinical Endocrinologists4 and the North American Menopause Society.5 However, for women who cannot or will not take HT, there are other treatment options to consider.
ALTERNATIVE TREATMENTS FOR VASOMOTOR SYMPTOMS: FOCUS ON NONHORMONAL OPTIONS
Options for the treatment of vasomotor symptoms include lifestyle modification, HT, nonhormonal centrally acting agents, and complementary and alternative medicine. Lifestyle modifications to cope with hot flashes include dressing in layers, adjusting room temperature, and deep breathing and relaxation exercises. Complementary and alternative medical approaches to vasomotor symptoms have generally not been evaluated in well-designed studies or have been found ineffective, so they will not be discussed further here. HT was discussed at length in the previous articles in this supplement, and because of its perceived risks, some women are unwilling to use HT. For these women, and particularly for those with contraindications to HT—especially those with breast cancer treated with medications that promote severe vasomotor symptoms—nonhormonal alternatives for vasomotor symptom treatment clearly are needed. Centrally acting agents show the most promise in this regard.
The rationale for a nonhormonal approach
Development of vasomotor symptoms seems to be related to the withdrawal of gonadotropins and the instability of serotonin and norepinephrine in the hypothalamus.6–9 A small increase in core temperature precedes a vasomotor symptom episode in approximately 70% of women. A narrowing of the hypothalamic thermoregulatory set point is followed by an increasing sensation of intense heat and peripheral vasodilation, leading to an exaggerated response (ie, severe sweating and flashing) to the very small rise in core temperature. This pathophysiology of vasomotor symptoms is the basis for the use of alternatives to HT, such as selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs).
SSRIs
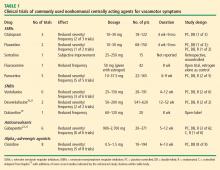
As detailed in Table 1, studies of SSRIs have usually been only weeks in duration, have often been uncontrolled or retrospective in design, and have generally enrolled small numbers of patients, making it difficult to draw valid conclusions from their data.10–13 Overall, the results with SSRIs are mixed with respect to efficacy in reducing the incidence and severity of vasomotor symptoms.
Most studies of SSRIs for this indication have been performed with paroxetine, which has the highest affinity for the norepinephrine receptor among the SSRIs. Fluoxetine and paroxetine have each been studied in randomized controlled trials in menopausal women with vasomotor symptoms, and each has resulted in a reduction in the frequency and severity of those symptoms compared with placebo.11,12 The North American Menopause Society (NAMS), in its 2004 position statement on management of menopause-related vasomotor symptoms,5 and the National Institutes of Health2 have recognized fluoxetine and paroxetine as possible alternatives to HT for the treatment of vasomotor symptoms.
One cautionary note is required with SSRI use in this setting: because SSRIs are strong inhibitors of CYP2D6, an enzyme important in the metabolism of tamoxifen,14 the potential for interactions between SSRIs and tamoxifen must be recognized. In breast cancer patients with the CYP2D6 genotype, paroxetine reduced plasma levels of the active metabolite of tamoxifen.15
SNRIs
Studies of SNRIs have also enrolled few patients, with treatment durations of 4 to 52 weeks (Table 1).10,13
Venlafaxine has been the most widely studied of the SNRIs, but the longest follow-up in studies of venlafaxine has been only 12 weeks.10,13,16–18 It has been shown to reduce the frequency and severity of vasomotor symptoms in several studies, and two of its studies had randomized controlled designs.16,18 In its 2004 position statement, NAMS recognizes low-dose venlafaxine (37.5 to 75.0 mg) as a nonhormonal alternative for the treatment of vasomotor symptoms.5
Duloxetine has been assessed in a single published clinical trial for vasomotor symptoms, a small, 8week, open-label investigation that demonstrated a small reduction in the frequency of vasomotor symptoms with its use.19
Desvenlafaxine succinate, the active metabolite of venlafaxine, was approved by the US Food and Drug Administration (FDA) in February 2008 for the treatment of major depressive disorder. It is currently under FDA review for treatment of menopause-related vasomotor symptoms and is expected to be the first FDA-approved nonhormonal agent for the treatment of menopausal vasomotor symptoms. Among the centrally acting agents studied for treatment of vasomotor symptoms, desvenlafaxine has been assessed in the largest randomized controlled trials to date.
In a randomized trial of 541 menopausal women with hot flashes, both dosages of desvenlafaxine tested (100 and 150 mg/day) were associated with a sustained significant reduction in the incidence of moderate to severe vasomotor symptoms compared with placebo over the 12 to 26 weeks of treatment.20 Withdrawal of desvenlafaxine was associated with a recurrence of symptoms, which the study authors argue is proof that the drug was responsible for the reduced incidence of vasomotor symptoms, despite the large placebo effect observed in the study.
Another randomized trial compared four dosages of desvenlafaxine (50, 100, 150, or 200 mg/day) with placebo in 707 healthy postmenopausal women who experienced at least 50 moderate to severe hot flashes per week.21 Among the 620 evaluable women, the best results overall were seen with the 100-mg dose of desvenlafaxine, which was associated with a 64% reduction from baseline in the average daily number of hot flashes at week 12. Compared with the placebo group, significantly greater percentages of patients achieved a 75% or greater reduction in the number of hot flashes from baseline in the 100-, 150-, and 200mg dose groups at week 4, and in the 100- and 200-mg dose groups at week 12.
The most common side effects associated with desvenlafaxine were nausea, dizziness, and insomnia. The most common symptoms that occurred upon discontinuation were dizziness, nausea, and headache.21 The rate of discontinuation with desvenlafaxine was lowest in the group assigned to 50 mg, which suggests a dose-related effect in terms of side effects. It should be noted that desvenlafaxine in this study was started at full dosage without titration and was discontinued abruptly, practices that are not typical with the use of venlafaxine and may account for the above-mentioned side effects.
SNRIs are weak inhibitors of CYP2D6 and therefore represent a good nonhormonal alternative for vasomotor symptoms in breast cancer patients being treated with tamoxifen.
Anticonvulsants
Gabapentin is an anticonvulsant that has been assessed in several trials for the treatment of vasomotor symptoms, showing superior efficacy to placebo in all placebo-controlled trials (Table 1). Its mechanism of action against hot flashes is uncertain, but it has been theorized that gabapentin may modulate calcium currents.5
In addition to the placebo-controlled trials mentioned above, gabapentin has been assessed in comparison with estrogen22 and in combination with antidepressants.23 One study randomized 60 postmenopausal women with moderate to severe hot flashes to treatment with conjugated estrogens (0.625 mg/day), gabapentin (titrated to 2,400 mg/day), or placebo for 12 weeks.22 Gabapentin and estrogen were similarly effective in reducing the study’s primary outcome measure—hot flash composite score at 12 weeks—and each was significantly superior to placebo in this regard.
Another study assessed gabapentin in combination with antidepressants (mostly venlafaxine or paroxetine) in 118 women with inadequate hot flash control, 91 of whom were evaluable at 5 weeks.23 Three-fourths of the study population had a history of breast cancer, and two-thirds were taking tamoxifen or an aromatase inhibitor at entry. Women were randomized either to remain on their antidepressant and have gabapentin added or to be weaned off their antidepressant and switched to gabapentin monotherapy. Gabapentin alone was associated with a statistically significant 50% median reduction in hot flash frequency from baseline, with no additional efficacy induced by continuation of the antidepressant. Negative mood changes and nervousness by week 2 were noted in the women who discontinued their antidepressants, although there was no change in quality-of-life evaluations.
The most common side effects of gabapentin in this clinical setting have been somnolence, disorientation, and headache. Notably, the effective dosages of gabapentin studied in women with vasomotor symptoms were higher (900 to 2,700 mg/day) than is sometimes possible to achieve in real-world practice, so the clinical relevance of these studies may be somewhat limited. Nevertheless, the NAMS position statement recognizes gabapentin as an alternative to HT for treating vasomotor symptoms.5
Alpha2-adrenergic receptor agonists
The alpha2-adrenergic receptor agonist clonidine has been used for treatment of hot flashes, but its efficacy has been modest at best in small trials of short duration (Table 1). The total daily doses used ranged from 0.5 mg to 1.5 mg, and side effects of dry mouth and dizziness were reported to cause relatively high discontinuation rates. While clonidine is an option, it should be reserved for patients who are intolerant of the other nonhormonal options discussed above.
Special considerations in breast cancer patients
Women with breast cancer merit special consideration, for several reasons. First, their cancer constitutes a contraindication to HT, so they are leading candidates for nonhormonal approaches to vasomotor symptom control. Second, chemotherapy itself may induce menopausal symptoms. Finally, vasomotor symptoms are often induced by other common (and longer-term) breast cancer therapies, including aromatase inhibitors (ie, anastrozole, exemestane, letrozole) in addition to tamoxifen, as mentioned above. Because SSRIs are strong inhibitors of CYP2D6, which is critical to tamoxifen metabolism, the SNRIs or gabapentin are preferred nonhormonal options in women taking tamoxifen.
Vitamin E: Scant evidence for symptom improvement, but a role in VTE prevention?
Vitamin E was frequently recommended in the past as a possible nonhormonal alternative to treat vasomotor symptoms, but small clinical trials have shown that it is not much more likely than placebo to be effective for this indication. Evidence from the Women’s Health Study indicates, however, that any value of vitamin E supplementation in this population may lie in reducing the risk of venous thromboembolism (VTE).24 In this large placebo-controlled trial, randomization to 600 IU of alpha-tocopherol every other day was associated with modest reductions in VTE overall and more significant reductions among the subgroup of women at highest risk for VTE—ie, those with a history of prior VTE or a prothrombotic mutation.
ALTERNATIVES TO SYSTEMIC ESTROGEN FOR OTHER MENOPAUSAL HEALTH ISSUES
Vaginal atrophy
New research is helping to define just how “low” low-dose topical therapy can go while still providing efficacy. A recent placebo-controlled trial compared 10-µg and 25-µg strengths of estradiol-containing vaginal tablets for vaginal atrophy in 230 postmenopausal women.26 Over the 12-week study, both doses of estradiol significantly improved vaginal maturation, lowered vaginal pH, and reduced the severity of vaginal symptoms compared with placebo. Although improvements were greater with the 25-µg dose, the results suggest that 10-µg topical estradiol is an effective option for women with vaginal atrophy who wish to minimize their exposure to estrogen.
Although there is insufficient evidence to support endometrial surveillance in asymptomatic women using vaginal estrogen, such surveillance may be indicated in women at high risk for endometrial cancer, in those requiring a higher dose for vaginal atrophy relief, and in those with spotting or breakthrough bleeding.25
Sexual dysfunction
Sexual dysfunction is not directly caused by the menopausal transition but is multifactorial, involving physical health, mental health, relationship dynamics, and partner availability, among other factors. The two most common complaints relating to sexual dysfunction in women at midlife are lack of desire and hypoarousal.
A number of therapies are currently under investigation for treatment of female sexual dysfunction at midlife. These include the same low-dose vaginal estrogen preparations used for vaginal atrophy as well as newer approaches currently in clinical testing, such as the melanocortin receptor agonist bremelanotide27,28 and topical alprostadil,29 both of which act by inducing sexual arousal. In a study of premenopausal women with sexual arousal disorder, bremelanotide increased both genital arousal and sexual desire.30
The most widely studied pharmacotherapy approach to sexual dysfunction has been testosterone replacement. A recent Cochrane review assessed the results of 23 trials that evaluated the addition of testosterone to HT (estrogen with or without progestogen) in 1,957 peri- and postmenopausal women.31 It found that adding testosterone to HT has a beneficial effect on sexual function in postmenopausal women but also confers the adverse effect of reducing levels of high-density lipoprotein cholesterol. The authors concluded that the impact of testosterone therapy on other health outcomes in estrogenized postmenopausal women is unclear, as is the existence of a benefit in sexual function for perimenopausal women.
In addition to systemic testosterone, a transdermal testosterone patch has been studied for treatment of sexual dysfunction in postmenopausal women; FDA evaluation of the patch is awaiting the availability of long-term safety data, although the testosterone patch for women is available in Europe.
According to a 2005 NAMS position statement on testosterone therapy,32 postmenopausal women presenting with symptoms of decreased sexual desire that causes personal distress may be candidates for testosterone therapy. The NAMS statement further clarifies that all other identifiable causes of sexual dysfunction should be considered and ruled out as appropriate. Because of a lack of safety and efficacy data on the use of testosterone therapy in unestrogenized women, testosterone therapy alone cannot be recommended in women not taking concomitant estrogen.32
Bone health
Many alternatives to systemic HT exist for maintaining bone health, including calcium and vitamin D supplementation, bisphosphonates, selective estrogen receptor modulators, calcitonin, recombinant human parathyroid hormone, and ultralow-dose transdermal estradiol. Beyond calcium and vitamin D supplementation, the most appropriate options for most women will likely be bisphosphonates or transdermal estradiol.
Ultralow-dose (0.014 mg/day) transdermal estradiol has been shown to significantly increase bone mineral density (BMD) at both the hip and the spine in post-menopausal women compared with placebo.33 However, the only agent that has demonstrated fracture risk reduction in women who do not otherwise have osteoporosis is standard-dose estrogen therapy (eg, 0.625 mg conjugated equine estrogens). Lower doses of estrogen, which may maintain bone density, do not have data on fracture risk reduction. Although lower doses of estrogen have been found to increase BMD, there is a dose-dependent response, with higher doses producing more of an increase.34,35
Bisphosphonates. The oral bisphosphonates, alendronate and risedronate, have proven efficacy in reducing hip fracture rates in women who already have osteoporosis. Risedronate recently gained FDA approval for administration in a regimen involving two 75-mg tablets taken on a monthly (consecutive-day) basis, and a 150-mg monthly risedronate tablet is expected soon.
Zoledronic acid, an injectable bisphosphonate, recently gained FDA approval for administration as a once-yearly intravenous infusion after this regimen was shown in a large 3-year placebo-controlled trial to significantly reduce the risk of morphometric spine, hip, nonvertebral, wrist, and rib fractures in postmenopausal women with osteoporosis.36 In this trial, atrial fibrillation was more common in women treated with zoledronic acid than in those who received placebo. However, any link between zoledronic acid and atrial fibrillation is uncertain, since episodes of atrial fibrillation tended to occur more than 30 days after the infusion and since circulating active levels of zoledronic acid persist for only up to 1 week. (A history of arrhythmia or atrial fibrillation is not listed in FDA-approved labeling as a contraindication to zoledronic acid.) Also, there was no increased risk of jaw osteonecrosis in subjects treated with zoledronic acid.36
Intravenous dosing can be helpful when patients have intolerable gastrointestinal side effects or other contraindications to oral dosing, as well as to ensure adherence.
Ibandronate is another bisphosphonate that has been shown to reduce the risk of vertebral fractures. It is administered as a once-monthly oral dose or as an intravenous injection given every 3 months. Although these less-frequent dosing regimens can be more convenient for patients and the injectable form can eliminate gastrointestinal side effects, widespread use of ibandronate has been limited somewhat by a lack of evidence for reduction of nonvertebral and hip fractures.37
Raloxifene is the only selective estrogen receptor modulator (SERMs, which the FDA recently requested be called “estrogen agonists/estrogen antagonists”) approved for the prevention and treatment of osteoporosis in postmenopausal women. Raloxifene reduced the vertebral fracture rate by 40% to 50% over 2 to 4 years of use but did not reduce nonvertebral fracture rates. Raloxifene also reduces the risk of invasive breast cancer development.38,39 It has not been shown to lower the risk of coronary events or overall stroke risk but was instead associated with an increased risk of VTE and fatal stroke.
Synthetic recombinant human parathyroid hormone (PTH[1–34]; teriparatide) is currently the sole available agent in the new class of bone anabolic agents, although others are on the horizon. PTH(1–34) is given as a once-daily subcutaneous injection for up to 2 years of therapy. It is associated with a reduction in the risk of vertebral and nonvertebral fractures and is indicated for postmenopausal women (and men) with osteoporosis who are at high risk for fracture, as well as those in whom other medications have failed or are not tolerated.
Although rat studies revealed a potential increased risk for osteosarcoma with PTH(1–34) use, this has not been seen in any human studies or in postmarketing surveillance. As the risk was dependent on dose and duration of therapy, use of PTH(1–34) is not recommended for more than 2 years or in patients at increased risk for osteosarcoma.
Concomitant use of PTH(1–34) with a bisphosphonate seems to blunt its effect and is therefore to be avoided. Resumption of bisphosphonate use after 2 years of PTH(1–34) therapy seems to prevent the loss of densitometric gains that ensues upon cessation of PTH(1–34).40
Calcitonin is an older agent administered mainly as a nasal spray. It reduces vertebral fracture risk in postmenopausal women and is FDA-approved for the treatment, but not prevention, of osteoporosis. Calcitonin has questionable mild analgesic effects in compression fracture treatment. Because of its expense and inferior efficacy relative to other therapies, it is generally reserved for patients who cannot tolerate other agents.41
Therapies on the horizon for osteoporosis prevention and/or treatment in postmenopausal women include strontium ranelate, third-generation SERMs or estrogen agonists/antagonists (ie, bazedoxifene and lasofoxifene), and combination estrogen/SERM therapies.
SUMMARY
The risk-benefit assessment for management of vasomotor symptoms and other menopause-related health issues should be tailored to formulate the most efficacious and safe treatment plan for each individual woman. The most appropriate management is guided by the individual patient’s own assessment of her most bothersome symptom(s) and her preferences and comfort level regarding various risks and quality-of-life issues. To best inform these patient choices, physicians must strive to clearly and accurately present the risks and benefits of the various available treatment options.
For most symptomatic menopausal women, HT remains the best treatment. However, for women unable or unwilling to take HT, there are alternatives for the treatment of vasomotor symptoms and bone loss. Low doses of local vaginal estrogen remain an option for treatment of genitourinary atrophy, even in women in whom systemic HT may be contraindicated.
Reassessment of current data and ongoing clinical trials will assist clinicians and patients in decision-making regarding menopausal HT. Nonhormonal therapies for menopausal symptoms should be used to provide effective treatment options for those menopausal patients unwilling or unable to take HT.
- Anderson RN. United States life tables, 1997. Natl Vital Stat Rep 1999; 47:1–37.
- NIH State-of-the-Science Panel. National Institutes of Health State-of-the-Science Conference Statement: management of menopause-related symptoms. Ann Intern Med 2005; 142:1003–1013.
- Utian WH. Psychosocial and socioeconomic burden of vasomotor symptoms in menopause: a comprehensive review. Health Qual Life Outcomes 2005; 3:47.
- American Association of Clinical Endocrinologists (AACE) postion statement on hormone replacement therapy (HRT) and cardiovascular risk. American Association of Clinical Endocrinologists Web site. www.aace.com/pub/pdf/guidelines/HRTCVRISKposition_ statement.pdf. Accessed March 5, 2008.
- North American Menopause Society. Treatment of menopause-associated vasomotor symptoms: position statement of The North American Menopause Society. Menopause 2004; 11:11–33.
- Deecher DC. Physiology of thermoregulatory dysfunction and current approaches to the treatment of vasomotor symptoms. Expert Opin Investig Drugs 2005; 14:435–448.
- Bachmann GA. Menopausal vasomotor symptoms: a review of causes, effects and evidence-based treatment options. J Reprod Med 2005; 50:155–165.
- Freedman RR. Pathophysiology and treatment of menopausal hot flashes. Semin Reprod Med 2005; 23:117–125.
- Berendsen HH. The role of serotonin in hot flashes. Maturitas 2000; 36:155–164.
- Nelson HD, Vesco KK, Haney E, et al. Nonhormonal therapies for menopausal hot flashes: systematic review and meta-analysis. JAMA 2006; 295:2057–2071.
- Loprinzi CL, Sloan JA, Perez EA, et al. Phase III evaluation of fluoxetine for treatment of hot flashes. J Clin Oncol 2002; 20:1578–1583.
- Stearns V, Beebe KL, Iyengar M, Dube E. Paroxetine controlled release in the treatment of menopausal hot flashes: a randomized controlled trial. JAMA 2003; 289:2827–2834.
- Rapkin AJ. Vasomotor symptoms in menopause: physiologic condition and central nervous system approaches to treatment. Am J Obstet Gynecol 2007; 196:97–106.
- Jin Y, Desta Z, Stearns V, et al. CYP2D6 genotype, antidepressant use, and tamoxifen metabolism during adjuvant breast cancer treatment. J Natl Cancer Inst 2005; 97:30–39.
- Stearns V, Johnson MD, Rae JM, et al. Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine. J Natl Cancer Inst 2003; 95:1758–1764.
- Loprinzi CL, Kugler JW, Sloan JA, et al. Venlafaxine in management of hot flashes in survivors of breast cancer: a randomised controlled trial. Lancet 2000; 356:2059–2063.
- Barton D, La VB, Loprinzi C, Novotny P, Wilwerding MB, Sloan J. Venlafaxine for the control of hot flashes: results of a longitudinal continuation study. Oncol Nurs Forum 2002; 29:33–40.
- Evans ML, Pritts E, Vittinghoff E, et al. Management of postmenopausal hot flushes with venlafaxine hydrochloride: a randomized, controlled trial. Obstet Gynecol 2005; 105:161–166.
- Joffe H, Soares CN, Petrillo LF, et al. Treatment of depression and menopause-related symptoms with the serotonin-norepinephrine reuptake inhibitor duloxetine. J Clin Psychiatry 2007; 68:943–950.
- Kagan R, Constantine G, Olivier S. Treatment with desvenlafaxine succinate (DVS) results in a sustained reduction in number of severe hot flushes (HFs) in menopausal women [abstract]. Menopause 2007; 14:1084. Abstract S-13.
- Speroff L, Gass M, Constantine G, Olivier S, for the Study 315 Investigators. Efficacy and tolerability of desvenlafaxine succinate treatment for menopausal vasomotor symptoms: a randomized controlled trial. Obstet Gynecol 2008; 111:77–87.
- Reddy SY, Warner H, Guttuso T Jr, et al. Gabapentin, estrogen, and placebo for treating hot flushes: a randomized controlled trial. Obstet Gynecol 2006; 108:41–48.
- Loprinzi CL, Kugler JW, Barton DL, et al. Phase III trial of gabapentin alone or in conjunction with an antidepressant in the management of hot flashes in women who have inadequate control with an antidepressant alone: NCCTG N03C5. J Clin Oncol 2007; 25:308–312.
- Glynn RJ, Ridker PM, Goldhaber SZ, Zee RY, Buring JE. Effects of random allocation to vitamin E supplementation on the occurrence of venous thromboembolism: report from the Women’s Health Study. Circulation 2007; 116:1497–1503.
- North American Menopause Society. The role of local vaginal estrogen for treatment of vaginal atrophy in postmenopausal women: 2007 position statement of The North American Menopause Society. Menopause 2007; 14:355–369.
- Bachmann G, Lobo RA, Gut R, Nachtigall L, Notelovitz M. Efficacy of low-dose estradiol vaginal tablets in the treatment of atrophic vaginitis: a randomized controlled trial. Obstet Gynecol 2008; 111:67–76.
- Pfaus J, Giuliano F, Gelez H. Bremelanotide: an overview of preclinical CNS effects on female sexual dysfunction. J Sex Med 2007; 4(Suppl 4):269–279.
- Safarinejad MR. Evaluation of the safety and efficacy of bremelanotide, a melanocortin receptor agonist, in female subjects with arousal disorder: a double-blind placebo-controlled, fixed dose, randomized study. J Sex Med 2008; 5:887–897.
- Heiman JR, Gittelman M, Costabile R, et al. Topical alprostadil (PGE1) for the treatment of female sexual arousal disorder: in-clinic evaluation of safety and efficacy. J Psychosom Obstet Gynaecol 2006; 27:31–41.
- Diamond LE, Earle DC, Heiman JR, et al. An effect on the subjective sexual response in premenopausal women with sexual arousal disorder by bremelanotide (PT-141), a melanocortin receptor agonist. J Sex Med 2006; 3:628–638.
- Somboonporn W, Davis S, Seif MW, Bell R. Testosterone for peri- and postmenopausal women. Cochrane Database Syst Rev 2005; (4):CD004509.
- North American Menopause Society. The role of testosterone therapy in postmenopausal women: position statement of The North American Menopause Society. Menopause 2005; 12:497–511.
- Ettinger B, Ensrud KE, Wallace R, et al. Effects of ultralow-dose transdermal estradiol on bone mineral density: a randomized clinical trial. Obstet Gynecol 2004; 104:443–451.
- Genant HK, Lucas J, Weiss S, et al. Low-dose esterified estrogen therapy: effects on bone, plasma estradiol concentrations, endometrium, and lipid levels. Estratab/Osteoporosis Study Group. Arch Intern Med 1997; 157:2609–2615.
- Lindsay R, Gallagher JC, Kleerekoper M, Pickar JH. Effect of lower doses of conjugated equine estrogens with and without medroxyprogesterone acetate on bone in early postmenopausal women. JAMA 2002; 287:2668–2676.
- Black DM, Delmas PD, Eastell R, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 2007; 356:1809–1822.
- MacLean C, Newberry S, Maglione M, et al. Systematic review: comparative effectiveness of treatments to prevent fractures in men and women with low bone density or osteoporosis. Ann Intern Med 2008; 148:197–213.
- Vogel VG, Constantino JP, Wickerham DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA 2006; 295:2727–2741.
- Barrett-Connor E, Mosca L, Collins P, et al. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med 2006; 355:125–137.
- Black DM, Bilezikian JP, Ensrud KE, et al. One year of alendronate after one year of parathyroid hormone (1-84) for osteoporosis. N Engl J Med 2005; 353:555–565.
- Chesnut CH III, Silverman S, Andriano K, et al. A randomized trial of nasal spray salmon calcitonin in postmenopausal women with established osteoporosis: the prevent recurrence of osteoporotic fractures study. PROOF Study Group. Am J Med 2000; 109:267–276.
As the life expectancy of women in the United States now exceeds 80 years,1 many millions of US women will spend more than one-third to even one-half of their lives beyond menopause. While hormone therapy (HT) can effectively address many of the symptoms of menopause, women who are unwilling or unable to take HT need nonhormonal alternatives for treatment of menopausal symptoms as well as the estrogen-deficiency bone loss that ensues in many women. This article reviews current and experimental nonhormonal therapies for menopausal symptoms and related issues, such as midlife sexual dysfunction and maintenance of bone health.
DEFINING THE TERMINOLOGY OF MENOPAUSE
We begin the discussion of menopausal health with a clarification of some terms.2
Menopause refers to the final menstrual period and simply represents a point in time. Menopause can be diagnosed only a year after it occurs, when it is clear that the last menstrual period was truly the final one.
Perimenopause consists of three components: the period shortly before menopause (when the biological and clinical features of impending menopause begin), menopause itself (final menstrual period), and the year following menopause. Perimenopause is synonymous with menopausal transition.
Postmenopause is the period beginning at the time of the final menstrual period (menopause), although it is recognized only after a year of amenorrhea. The early postmenopausal phase is the first 5 years after menopause, whereas all the time thereafter is referred to as the late menopausal phase.
MENOPAUSAL ASSESSMENT
Symptoms
The primary symptoms of perimenopause are:
- Vasomotor symptoms (eg, hot flashes, night sweats)
- Menstrual cycle changes (ie, oligomenorrhea, amenorrhea)
- Vaginal dryness.
Secondary symptoms include sleep disturbance, low sex drive and/or reduced sexual arousal, stress or urge urinary incontinence, mood changes, and somatic complaints.
Vasomotor symptoms, vaginal dryness, and dyspareunia (painful intercourse) contributing to sexual dysfunction have been correlated with the loss of sex hormones (particularly estrogen) associated with menopause, whereas the other symptoms listed above (sleep disturbance, urinary symptoms, mood changes, somatic symptoms) have not been linked definitively to menopause and may be a function of aging.2
Vasomotor symptoms are the predominant reason that women seek medical treatment around the time of menopause.3 More than 75% of women report hot flashes within the 2 years surrounding menopause. Among these women who have hot flashes, 25% report that these symptoms remain for greater than 5 years, and 10% report that they remain for more than 10 years.3 Vasomotor symptoms may be associated with sleep disturbance, mood swings, cognitive deficits, social impairment, a reduction in productivity, embarrassment, anxiety, and fatigue.
Individualizing the evaluation is imperative
The overall patient must be considered in this assessment, which includes her personal history, family history, social history, and current medication use. Common factors affecting postmenopausal health—such as bone density; vaginal, bladder, and sexual function; cardiovascular health (including lipid profile, blood pressure, and tobacco use); thromboembolic risk; and cancer risk, including breast cancer—should be included in the assessment. For most women under the age of 60 years who have menopausal symptoms, HT remains the gold standard and recommended treatment, according to both the American Association of Clinical Endocrinologists4 and the North American Menopause Society.5 However, for women who cannot or will not take HT, there are other treatment options to consider.
ALTERNATIVE TREATMENTS FOR VASOMOTOR SYMPTOMS: FOCUS ON NONHORMONAL OPTIONS
Options for the treatment of vasomotor symptoms include lifestyle modification, HT, nonhormonal centrally acting agents, and complementary and alternative medicine. Lifestyle modifications to cope with hot flashes include dressing in layers, adjusting room temperature, and deep breathing and relaxation exercises. Complementary and alternative medical approaches to vasomotor symptoms have generally not been evaluated in well-designed studies or have been found ineffective, so they will not be discussed further here. HT was discussed at length in the previous articles in this supplement, and because of its perceived risks, some women are unwilling to use HT. For these women, and particularly for those with contraindications to HT—especially those with breast cancer treated with medications that promote severe vasomotor symptoms—nonhormonal alternatives for vasomotor symptom treatment clearly are needed. Centrally acting agents show the most promise in this regard.
The rationale for a nonhormonal approach
Development of vasomotor symptoms seems to be related to the withdrawal of gonadotropins and the instability of serotonin and norepinephrine in the hypothalamus.6–9 A small increase in core temperature precedes a vasomotor symptom episode in approximately 70% of women. A narrowing of the hypothalamic thermoregulatory set point is followed by an increasing sensation of intense heat and peripheral vasodilation, leading to an exaggerated response (ie, severe sweating and flashing) to the very small rise in core temperature. This pathophysiology of vasomotor symptoms is the basis for the use of alternatives to HT, such as selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs).
SSRIs
As detailed in Table 1, studies of SSRIs have usually been only weeks in duration, have often been uncontrolled or retrospective in design, and have generally enrolled small numbers of patients, making it difficult to draw valid conclusions from their data.10–13 Overall, the results with SSRIs are mixed with respect to efficacy in reducing the incidence and severity of vasomotor symptoms.
Most studies of SSRIs for this indication have been performed with paroxetine, which has the highest affinity for the norepinephrine receptor among the SSRIs. Fluoxetine and paroxetine have each been studied in randomized controlled trials in menopausal women with vasomotor symptoms, and each has resulted in a reduction in the frequency and severity of those symptoms compared with placebo.11,12 The North American Menopause Society (NAMS), in its 2004 position statement on management of menopause-related vasomotor symptoms,5 and the National Institutes of Health2 have recognized fluoxetine and paroxetine as possible alternatives to HT for the treatment of vasomotor symptoms.
One cautionary note is required with SSRI use in this setting: because SSRIs are strong inhibitors of CYP2D6, an enzyme important in the metabolism of tamoxifen,14 the potential for interactions between SSRIs and tamoxifen must be recognized. In breast cancer patients with the CYP2D6 genotype, paroxetine reduced plasma levels of the active metabolite of tamoxifen.15
SNRIs
Studies of SNRIs have also enrolled few patients, with treatment durations of 4 to 52 weeks (Table 1).10,13
Venlafaxine has been the most widely studied of the SNRIs, but the longest follow-up in studies of venlafaxine has been only 12 weeks.10,13,16–18 It has been shown to reduce the frequency and severity of vasomotor symptoms in several studies, and two of its studies had randomized controlled designs.16,18 In its 2004 position statement, NAMS recognizes low-dose venlafaxine (37.5 to 75.0 mg) as a nonhormonal alternative for the treatment of vasomotor symptoms.5
Duloxetine has been assessed in a single published clinical trial for vasomotor symptoms, a small, 8week, open-label investigation that demonstrated a small reduction in the frequency of vasomotor symptoms with its use.19
Desvenlafaxine succinate, the active metabolite of venlafaxine, was approved by the US Food and Drug Administration (FDA) in February 2008 for the treatment of major depressive disorder. It is currently under FDA review for treatment of menopause-related vasomotor symptoms and is expected to be the first FDA-approved nonhormonal agent for the treatment of menopausal vasomotor symptoms. Among the centrally acting agents studied for treatment of vasomotor symptoms, desvenlafaxine has been assessed in the largest randomized controlled trials to date.
In a randomized trial of 541 menopausal women with hot flashes, both dosages of desvenlafaxine tested (100 and 150 mg/day) were associated with a sustained significant reduction in the incidence of moderate to severe vasomotor symptoms compared with placebo over the 12 to 26 weeks of treatment.20 Withdrawal of desvenlafaxine was associated with a recurrence of symptoms, which the study authors argue is proof that the drug was responsible for the reduced incidence of vasomotor symptoms, despite the large placebo effect observed in the study.
Another randomized trial compared four dosages of desvenlafaxine (50, 100, 150, or 200 mg/day) with placebo in 707 healthy postmenopausal women who experienced at least 50 moderate to severe hot flashes per week.21 Among the 620 evaluable women, the best results overall were seen with the 100-mg dose of desvenlafaxine, which was associated with a 64% reduction from baseline in the average daily number of hot flashes at week 12. Compared with the placebo group, significantly greater percentages of patients achieved a 75% or greater reduction in the number of hot flashes from baseline in the 100-, 150-, and 200mg dose groups at week 4, and in the 100- and 200-mg dose groups at week 12.
The most common side effects associated with desvenlafaxine were nausea, dizziness, and insomnia. The most common symptoms that occurred upon discontinuation were dizziness, nausea, and headache.21 The rate of discontinuation with desvenlafaxine was lowest in the group assigned to 50 mg, which suggests a dose-related effect in terms of side effects. It should be noted that desvenlafaxine in this study was started at full dosage without titration and was discontinued abruptly, practices that are not typical with the use of venlafaxine and may account for the above-mentioned side effects.
SNRIs are weak inhibitors of CYP2D6 and therefore represent a good nonhormonal alternative for vasomotor symptoms in breast cancer patients being treated with tamoxifen.
Anticonvulsants
Gabapentin is an anticonvulsant that has been assessed in several trials for the treatment of vasomotor symptoms, showing superior efficacy to placebo in all placebo-controlled trials (Table 1). Its mechanism of action against hot flashes is uncertain, but it has been theorized that gabapentin may modulate calcium currents.5
In addition to the placebo-controlled trials mentioned above, gabapentin has been assessed in comparison with estrogen22 and in combination with antidepressants.23 One study randomized 60 postmenopausal women with moderate to severe hot flashes to treatment with conjugated estrogens (0.625 mg/day), gabapentin (titrated to 2,400 mg/day), or placebo for 12 weeks.22 Gabapentin and estrogen were similarly effective in reducing the study’s primary outcome measure—hot flash composite score at 12 weeks—and each was significantly superior to placebo in this regard.
Another study assessed gabapentin in combination with antidepressants (mostly venlafaxine or paroxetine) in 118 women with inadequate hot flash control, 91 of whom were evaluable at 5 weeks.23 Three-fourths of the study population had a history of breast cancer, and two-thirds were taking tamoxifen or an aromatase inhibitor at entry. Women were randomized either to remain on their antidepressant and have gabapentin added or to be weaned off their antidepressant and switched to gabapentin monotherapy. Gabapentin alone was associated with a statistically significant 50% median reduction in hot flash frequency from baseline, with no additional efficacy induced by continuation of the antidepressant. Negative mood changes and nervousness by week 2 were noted in the women who discontinued their antidepressants, although there was no change in quality-of-life evaluations.
The most common side effects of gabapentin in this clinical setting have been somnolence, disorientation, and headache. Notably, the effective dosages of gabapentin studied in women with vasomotor symptoms were higher (900 to 2,700 mg/day) than is sometimes possible to achieve in real-world practice, so the clinical relevance of these studies may be somewhat limited. Nevertheless, the NAMS position statement recognizes gabapentin as an alternative to HT for treating vasomotor symptoms.5
Alpha2-adrenergic receptor agonists
The alpha2-adrenergic receptor agonist clonidine has been used for treatment of hot flashes, but its efficacy has been modest at best in small trials of short duration (Table 1). The total daily doses used ranged from 0.5 mg to 1.5 mg, and side effects of dry mouth and dizziness were reported to cause relatively high discontinuation rates. While clonidine is an option, it should be reserved for patients who are intolerant of the other nonhormonal options discussed above.
Special considerations in breast cancer patients
Women with breast cancer merit special consideration, for several reasons. First, their cancer constitutes a contraindication to HT, so they are leading candidates for nonhormonal approaches to vasomotor symptom control. Second, chemotherapy itself may induce menopausal symptoms. Finally, vasomotor symptoms are often induced by other common (and longer-term) breast cancer therapies, including aromatase inhibitors (ie, anastrozole, exemestane, letrozole) in addition to tamoxifen, as mentioned above. Because SSRIs are strong inhibitors of CYP2D6, which is critical to tamoxifen metabolism, the SNRIs or gabapentin are preferred nonhormonal options in women taking tamoxifen.
Vitamin E: Scant evidence for symptom improvement, but a role in VTE prevention?
Vitamin E was frequently recommended in the past as a possible nonhormonal alternative to treat vasomotor symptoms, but small clinical trials have shown that it is not much more likely than placebo to be effective for this indication. Evidence from the Women’s Health Study indicates, however, that any value of vitamin E supplementation in this population may lie in reducing the risk of venous thromboembolism (VTE).24 In this large placebo-controlled trial, randomization to 600 IU of alpha-tocopherol every other day was associated with modest reductions in VTE overall and more significant reductions among the subgroup of women at highest risk for VTE—ie, those with a history of prior VTE or a prothrombotic mutation.
ALTERNATIVES TO SYSTEMIC ESTROGEN FOR OTHER MENOPAUSAL HEALTH ISSUES
Vaginal atrophy
New research is helping to define just how “low” low-dose topical therapy can go while still providing efficacy. A recent placebo-controlled trial compared 10-µg and 25-µg strengths of estradiol-containing vaginal tablets for vaginal atrophy in 230 postmenopausal women.26 Over the 12-week study, both doses of estradiol significantly improved vaginal maturation, lowered vaginal pH, and reduced the severity of vaginal symptoms compared with placebo. Although improvements were greater with the 25-µg dose, the results suggest that 10-µg topical estradiol is an effective option for women with vaginal atrophy who wish to minimize their exposure to estrogen.
Although there is insufficient evidence to support endometrial surveillance in asymptomatic women using vaginal estrogen, such surveillance may be indicated in women at high risk for endometrial cancer, in those requiring a higher dose for vaginal atrophy relief, and in those with spotting or breakthrough bleeding.25
Sexual dysfunction
Sexual dysfunction is not directly caused by the menopausal transition but is multifactorial, involving physical health, mental health, relationship dynamics, and partner availability, among other factors. The two most common complaints relating to sexual dysfunction in women at midlife are lack of desire and hypoarousal.
A number of therapies are currently under investigation for treatment of female sexual dysfunction at midlife. These include the same low-dose vaginal estrogen preparations used for vaginal atrophy as well as newer approaches currently in clinical testing, such as the melanocortin receptor agonist bremelanotide27,28 and topical alprostadil,29 both of which act by inducing sexual arousal. In a study of premenopausal women with sexual arousal disorder, bremelanotide increased both genital arousal and sexual desire.30
The most widely studied pharmacotherapy approach to sexual dysfunction has been testosterone replacement. A recent Cochrane review assessed the results of 23 trials that evaluated the addition of testosterone to HT (estrogen with or without progestogen) in 1,957 peri- and postmenopausal women.31 It found that adding testosterone to HT has a beneficial effect on sexual function in postmenopausal women but also confers the adverse effect of reducing levels of high-density lipoprotein cholesterol. The authors concluded that the impact of testosterone therapy on other health outcomes in estrogenized postmenopausal women is unclear, as is the existence of a benefit in sexual function for perimenopausal women.
In addition to systemic testosterone, a transdermal testosterone patch has been studied for treatment of sexual dysfunction in postmenopausal women; FDA evaluation of the patch is awaiting the availability of long-term safety data, although the testosterone patch for women is available in Europe.
According to a 2005 NAMS position statement on testosterone therapy,32 postmenopausal women presenting with symptoms of decreased sexual desire that causes personal distress may be candidates for testosterone therapy. The NAMS statement further clarifies that all other identifiable causes of sexual dysfunction should be considered and ruled out as appropriate. Because of a lack of safety and efficacy data on the use of testosterone therapy in unestrogenized women, testosterone therapy alone cannot be recommended in women not taking concomitant estrogen.32
Bone health
Many alternatives to systemic HT exist for maintaining bone health, including calcium and vitamin D supplementation, bisphosphonates, selective estrogen receptor modulators, calcitonin, recombinant human parathyroid hormone, and ultralow-dose transdermal estradiol. Beyond calcium and vitamin D supplementation, the most appropriate options for most women will likely be bisphosphonates or transdermal estradiol.
Ultralow-dose (0.014 mg/day) transdermal estradiol has been shown to significantly increase bone mineral density (BMD) at both the hip and the spine in post-menopausal women compared with placebo.33 However, the only agent that has demonstrated fracture risk reduction in women who do not otherwise have osteoporosis is standard-dose estrogen therapy (eg, 0.625 mg conjugated equine estrogens). Lower doses of estrogen, which may maintain bone density, do not have data on fracture risk reduction. Although lower doses of estrogen have been found to increase BMD, there is a dose-dependent response, with higher doses producing more of an increase.34,35
Bisphosphonates. The oral bisphosphonates, alendronate and risedronate, have proven efficacy in reducing hip fracture rates in women who already have osteoporosis. Risedronate recently gained FDA approval for administration in a regimen involving two 75-mg tablets taken on a monthly (consecutive-day) basis, and a 150-mg monthly risedronate tablet is expected soon.
Zoledronic acid, an injectable bisphosphonate, recently gained FDA approval for administration as a once-yearly intravenous infusion after this regimen was shown in a large 3-year placebo-controlled trial to significantly reduce the risk of morphometric spine, hip, nonvertebral, wrist, and rib fractures in postmenopausal women with osteoporosis.36 In this trial, atrial fibrillation was more common in women treated with zoledronic acid than in those who received placebo. However, any link between zoledronic acid and atrial fibrillation is uncertain, since episodes of atrial fibrillation tended to occur more than 30 days after the infusion and since circulating active levels of zoledronic acid persist for only up to 1 week. (A history of arrhythmia or atrial fibrillation is not listed in FDA-approved labeling as a contraindication to zoledronic acid.) Also, there was no increased risk of jaw osteonecrosis in subjects treated with zoledronic acid.36
Intravenous dosing can be helpful when patients have intolerable gastrointestinal side effects or other contraindications to oral dosing, as well as to ensure adherence.
Ibandronate is another bisphosphonate that has been shown to reduce the risk of vertebral fractures. It is administered as a once-monthly oral dose or as an intravenous injection given every 3 months. Although these less-frequent dosing regimens can be more convenient for patients and the injectable form can eliminate gastrointestinal side effects, widespread use of ibandronate has been limited somewhat by a lack of evidence for reduction of nonvertebral and hip fractures.37
Raloxifene is the only selective estrogen receptor modulator (SERMs, which the FDA recently requested be called “estrogen agonists/estrogen antagonists”) approved for the prevention and treatment of osteoporosis in postmenopausal women. Raloxifene reduced the vertebral fracture rate by 40% to 50% over 2 to 4 years of use but did not reduce nonvertebral fracture rates. Raloxifene also reduces the risk of invasive breast cancer development.38,39 It has not been shown to lower the risk of coronary events or overall stroke risk but was instead associated with an increased risk of VTE and fatal stroke.
Synthetic recombinant human parathyroid hormone (PTH[1–34]; teriparatide) is currently the sole available agent in the new class of bone anabolic agents, although others are on the horizon. PTH(1–34) is given as a once-daily subcutaneous injection for up to 2 years of therapy. It is associated with a reduction in the risk of vertebral and nonvertebral fractures and is indicated for postmenopausal women (and men) with osteoporosis who are at high risk for fracture, as well as those in whom other medications have failed or are not tolerated.
Although rat studies revealed a potential increased risk for osteosarcoma with PTH(1–34) use, this has not been seen in any human studies or in postmarketing surveillance. As the risk was dependent on dose and duration of therapy, use of PTH(1–34) is not recommended for more than 2 years or in patients at increased risk for osteosarcoma.
Concomitant use of PTH(1–34) with a bisphosphonate seems to blunt its effect and is therefore to be avoided. Resumption of bisphosphonate use after 2 years of PTH(1–34) therapy seems to prevent the loss of densitometric gains that ensues upon cessation of PTH(1–34).40
Calcitonin is an older agent administered mainly as a nasal spray. It reduces vertebral fracture risk in postmenopausal women and is FDA-approved for the treatment, but not prevention, of osteoporosis. Calcitonin has questionable mild analgesic effects in compression fracture treatment. Because of its expense and inferior efficacy relative to other therapies, it is generally reserved for patients who cannot tolerate other agents.41
Therapies on the horizon for osteoporosis prevention and/or treatment in postmenopausal women include strontium ranelate, third-generation SERMs or estrogen agonists/antagonists (ie, bazedoxifene and lasofoxifene), and combination estrogen/SERM therapies.
SUMMARY
The risk-benefit assessment for management of vasomotor symptoms and other menopause-related health issues should be tailored to formulate the most efficacious and safe treatment plan for each individual woman. The most appropriate management is guided by the individual patient’s own assessment of her most bothersome symptom(s) and her preferences and comfort level regarding various risks and quality-of-life issues. To best inform these patient choices, physicians must strive to clearly and accurately present the risks and benefits of the various available treatment options.
For most symptomatic menopausal women, HT remains the best treatment. However, for women unable or unwilling to take HT, there are alternatives for the treatment of vasomotor symptoms and bone loss. Low doses of local vaginal estrogen remain an option for treatment of genitourinary atrophy, even in women in whom systemic HT may be contraindicated.
Reassessment of current data and ongoing clinical trials will assist clinicians and patients in decision-making regarding menopausal HT. Nonhormonal therapies for menopausal symptoms should be used to provide effective treatment options for those menopausal patients unwilling or unable to take HT.
As the life expectancy of women in the United States now exceeds 80 years,1 many millions of US women will spend more than one-third to even one-half of their lives beyond menopause. While hormone therapy (HT) can effectively address many of the symptoms of menopause, women who are unwilling or unable to take HT need nonhormonal alternatives for treatment of menopausal symptoms as well as the estrogen-deficiency bone loss that ensues in many women. This article reviews current and experimental nonhormonal therapies for menopausal symptoms and related issues, such as midlife sexual dysfunction and maintenance of bone health.
DEFINING THE TERMINOLOGY OF MENOPAUSE
We begin the discussion of menopausal health with a clarification of some terms.2
Menopause refers to the final menstrual period and simply represents a point in time. Menopause can be diagnosed only a year after it occurs, when it is clear that the last menstrual period was truly the final one.
Perimenopause consists of three components: the period shortly before menopause (when the biological and clinical features of impending menopause begin), menopause itself (final menstrual period), and the year following menopause. Perimenopause is synonymous with menopausal transition.
Postmenopause is the period beginning at the time of the final menstrual period (menopause), although it is recognized only after a year of amenorrhea. The early postmenopausal phase is the first 5 years after menopause, whereas all the time thereafter is referred to as the late menopausal phase.
MENOPAUSAL ASSESSMENT
Symptoms
The primary symptoms of perimenopause are:
- Vasomotor symptoms (eg, hot flashes, night sweats)
- Menstrual cycle changes (ie, oligomenorrhea, amenorrhea)
- Vaginal dryness.
Secondary symptoms include sleep disturbance, low sex drive and/or reduced sexual arousal, stress or urge urinary incontinence, mood changes, and somatic complaints.
Vasomotor symptoms, vaginal dryness, and dyspareunia (painful intercourse) contributing to sexual dysfunction have been correlated with the loss of sex hormones (particularly estrogen) associated with menopause, whereas the other symptoms listed above (sleep disturbance, urinary symptoms, mood changes, somatic symptoms) have not been linked definitively to menopause and may be a function of aging.2
Vasomotor symptoms are the predominant reason that women seek medical treatment around the time of menopause.3 More than 75% of women report hot flashes within the 2 years surrounding menopause. Among these women who have hot flashes, 25% report that these symptoms remain for greater than 5 years, and 10% report that they remain for more than 10 years.3 Vasomotor symptoms may be associated with sleep disturbance, mood swings, cognitive deficits, social impairment, a reduction in productivity, embarrassment, anxiety, and fatigue.
Individualizing the evaluation is imperative
The overall patient must be considered in this assessment, which includes her personal history, family history, social history, and current medication use. Common factors affecting postmenopausal health—such as bone density; vaginal, bladder, and sexual function; cardiovascular health (including lipid profile, blood pressure, and tobacco use); thromboembolic risk; and cancer risk, including breast cancer—should be included in the assessment. For most women under the age of 60 years who have menopausal symptoms, HT remains the gold standard and recommended treatment, according to both the American Association of Clinical Endocrinologists4 and the North American Menopause Society.5 However, for women who cannot or will not take HT, there are other treatment options to consider.
ALTERNATIVE TREATMENTS FOR VASOMOTOR SYMPTOMS: FOCUS ON NONHORMONAL OPTIONS
Options for the treatment of vasomotor symptoms include lifestyle modification, HT, nonhormonal centrally acting agents, and complementary and alternative medicine. Lifestyle modifications to cope with hot flashes include dressing in layers, adjusting room temperature, and deep breathing and relaxation exercises. Complementary and alternative medical approaches to vasomotor symptoms have generally not been evaluated in well-designed studies or have been found ineffective, so they will not be discussed further here. HT was discussed at length in the previous articles in this supplement, and because of its perceived risks, some women are unwilling to use HT. For these women, and particularly for those with contraindications to HT—especially those with breast cancer treated with medications that promote severe vasomotor symptoms—nonhormonal alternatives for vasomotor symptom treatment clearly are needed. Centrally acting agents show the most promise in this regard.
The rationale for a nonhormonal approach
Development of vasomotor symptoms seems to be related to the withdrawal of gonadotropins and the instability of serotonin and norepinephrine in the hypothalamus.6–9 A small increase in core temperature precedes a vasomotor symptom episode in approximately 70% of women. A narrowing of the hypothalamic thermoregulatory set point is followed by an increasing sensation of intense heat and peripheral vasodilation, leading to an exaggerated response (ie, severe sweating and flashing) to the very small rise in core temperature. This pathophysiology of vasomotor symptoms is the basis for the use of alternatives to HT, such as selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs).
SSRIs
As detailed in Table 1, studies of SSRIs have usually been only weeks in duration, have often been uncontrolled or retrospective in design, and have generally enrolled small numbers of patients, making it difficult to draw valid conclusions from their data.10–13 Overall, the results with SSRIs are mixed with respect to efficacy in reducing the incidence and severity of vasomotor symptoms.
Most studies of SSRIs for this indication have been performed with paroxetine, which has the highest affinity for the norepinephrine receptor among the SSRIs. Fluoxetine and paroxetine have each been studied in randomized controlled trials in menopausal women with vasomotor symptoms, and each has resulted in a reduction in the frequency and severity of those symptoms compared with placebo.11,12 The North American Menopause Society (NAMS), in its 2004 position statement on management of menopause-related vasomotor symptoms,5 and the National Institutes of Health2 have recognized fluoxetine and paroxetine as possible alternatives to HT for the treatment of vasomotor symptoms.
One cautionary note is required with SSRI use in this setting: because SSRIs are strong inhibitors of CYP2D6, an enzyme important in the metabolism of tamoxifen,14 the potential for interactions between SSRIs and tamoxifen must be recognized. In breast cancer patients with the CYP2D6 genotype, paroxetine reduced plasma levels of the active metabolite of tamoxifen.15
SNRIs
Studies of SNRIs have also enrolled few patients, with treatment durations of 4 to 52 weeks (Table 1).10,13
Venlafaxine has been the most widely studied of the SNRIs, but the longest follow-up in studies of venlafaxine has been only 12 weeks.10,13,16–18 It has been shown to reduce the frequency and severity of vasomotor symptoms in several studies, and two of its studies had randomized controlled designs.16,18 In its 2004 position statement, NAMS recognizes low-dose venlafaxine (37.5 to 75.0 mg) as a nonhormonal alternative for the treatment of vasomotor symptoms.5
Duloxetine has been assessed in a single published clinical trial for vasomotor symptoms, a small, 8week, open-label investigation that demonstrated a small reduction in the frequency of vasomotor symptoms with its use.19
Desvenlafaxine succinate, the active metabolite of venlafaxine, was approved by the US Food and Drug Administration (FDA) in February 2008 for the treatment of major depressive disorder. It is currently under FDA review for treatment of menopause-related vasomotor symptoms and is expected to be the first FDA-approved nonhormonal agent for the treatment of menopausal vasomotor symptoms. Among the centrally acting agents studied for treatment of vasomotor symptoms, desvenlafaxine has been assessed in the largest randomized controlled trials to date.
In a randomized trial of 541 menopausal women with hot flashes, both dosages of desvenlafaxine tested (100 and 150 mg/day) were associated with a sustained significant reduction in the incidence of moderate to severe vasomotor symptoms compared with placebo over the 12 to 26 weeks of treatment.20 Withdrawal of desvenlafaxine was associated with a recurrence of symptoms, which the study authors argue is proof that the drug was responsible for the reduced incidence of vasomotor symptoms, despite the large placebo effect observed in the study.
Another randomized trial compared four dosages of desvenlafaxine (50, 100, 150, or 200 mg/day) with placebo in 707 healthy postmenopausal women who experienced at least 50 moderate to severe hot flashes per week.21 Among the 620 evaluable women, the best results overall were seen with the 100-mg dose of desvenlafaxine, which was associated with a 64% reduction from baseline in the average daily number of hot flashes at week 12. Compared with the placebo group, significantly greater percentages of patients achieved a 75% or greater reduction in the number of hot flashes from baseline in the 100-, 150-, and 200mg dose groups at week 4, and in the 100- and 200-mg dose groups at week 12.
The most common side effects associated with desvenlafaxine were nausea, dizziness, and insomnia. The most common symptoms that occurred upon discontinuation were dizziness, nausea, and headache.21 The rate of discontinuation with desvenlafaxine was lowest in the group assigned to 50 mg, which suggests a dose-related effect in terms of side effects. It should be noted that desvenlafaxine in this study was started at full dosage without titration and was discontinued abruptly, practices that are not typical with the use of venlafaxine and may account for the above-mentioned side effects.
SNRIs are weak inhibitors of CYP2D6 and therefore represent a good nonhormonal alternative for vasomotor symptoms in breast cancer patients being treated with tamoxifen.
Anticonvulsants
Gabapentin is an anticonvulsant that has been assessed in several trials for the treatment of vasomotor symptoms, showing superior efficacy to placebo in all placebo-controlled trials (Table 1). Its mechanism of action against hot flashes is uncertain, but it has been theorized that gabapentin may modulate calcium currents.5
In addition to the placebo-controlled trials mentioned above, gabapentin has been assessed in comparison with estrogen22 and in combination with antidepressants.23 One study randomized 60 postmenopausal women with moderate to severe hot flashes to treatment with conjugated estrogens (0.625 mg/day), gabapentin (titrated to 2,400 mg/day), or placebo for 12 weeks.22 Gabapentin and estrogen were similarly effective in reducing the study’s primary outcome measure—hot flash composite score at 12 weeks—and each was significantly superior to placebo in this regard.
Another study assessed gabapentin in combination with antidepressants (mostly venlafaxine or paroxetine) in 118 women with inadequate hot flash control, 91 of whom were evaluable at 5 weeks.23 Three-fourths of the study population had a history of breast cancer, and two-thirds were taking tamoxifen or an aromatase inhibitor at entry. Women were randomized either to remain on their antidepressant and have gabapentin added or to be weaned off their antidepressant and switched to gabapentin monotherapy. Gabapentin alone was associated with a statistically significant 50% median reduction in hot flash frequency from baseline, with no additional efficacy induced by continuation of the antidepressant. Negative mood changes and nervousness by week 2 were noted in the women who discontinued their antidepressants, although there was no change in quality-of-life evaluations.
The most common side effects of gabapentin in this clinical setting have been somnolence, disorientation, and headache. Notably, the effective dosages of gabapentin studied in women with vasomotor symptoms were higher (900 to 2,700 mg/day) than is sometimes possible to achieve in real-world practice, so the clinical relevance of these studies may be somewhat limited. Nevertheless, the NAMS position statement recognizes gabapentin as an alternative to HT for treating vasomotor symptoms.5
Alpha2-adrenergic receptor agonists
The alpha2-adrenergic receptor agonist clonidine has been used for treatment of hot flashes, but its efficacy has been modest at best in small trials of short duration (Table 1). The total daily doses used ranged from 0.5 mg to 1.5 mg, and side effects of dry mouth and dizziness were reported to cause relatively high discontinuation rates. While clonidine is an option, it should be reserved for patients who are intolerant of the other nonhormonal options discussed above.
Special considerations in breast cancer patients
Women with breast cancer merit special consideration, for several reasons. First, their cancer constitutes a contraindication to HT, so they are leading candidates for nonhormonal approaches to vasomotor symptom control. Second, chemotherapy itself may induce menopausal symptoms. Finally, vasomotor symptoms are often induced by other common (and longer-term) breast cancer therapies, including aromatase inhibitors (ie, anastrozole, exemestane, letrozole) in addition to tamoxifen, as mentioned above. Because SSRIs are strong inhibitors of CYP2D6, which is critical to tamoxifen metabolism, the SNRIs or gabapentin are preferred nonhormonal options in women taking tamoxifen.
Vitamin E: Scant evidence for symptom improvement, but a role in VTE prevention?
Vitamin E was frequently recommended in the past as a possible nonhormonal alternative to treat vasomotor symptoms, but small clinical trials have shown that it is not much more likely than placebo to be effective for this indication. Evidence from the Women’s Health Study indicates, however, that any value of vitamin E supplementation in this population may lie in reducing the risk of venous thromboembolism (VTE).24 In this large placebo-controlled trial, randomization to 600 IU of alpha-tocopherol every other day was associated with modest reductions in VTE overall and more significant reductions among the subgroup of women at highest risk for VTE—ie, those with a history of prior VTE or a prothrombotic mutation.
ALTERNATIVES TO SYSTEMIC ESTROGEN FOR OTHER MENOPAUSAL HEALTH ISSUES
Vaginal atrophy
New research is helping to define just how “low” low-dose topical therapy can go while still providing efficacy. A recent placebo-controlled trial compared 10-µg and 25-µg strengths of estradiol-containing vaginal tablets for vaginal atrophy in 230 postmenopausal women.26 Over the 12-week study, both doses of estradiol significantly improved vaginal maturation, lowered vaginal pH, and reduced the severity of vaginal symptoms compared with placebo. Although improvements were greater with the 25-µg dose, the results suggest that 10-µg topical estradiol is an effective option for women with vaginal atrophy who wish to minimize their exposure to estrogen.
Although there is insufficient evidence to support endometrial surveillance in asymptomatic women using vaginal estrogen, such surveillance may be indicated in women at high risk for endometrial cancer, in those requiring a higher dose for vaginal atrophy relief, and in those with spotting or breakthrough bleeding.25
Sexual dysfunction
Sexual dysfunction is not directly caused by the menopausal transition but is multifactorial, involving physical health, mental health, relationship dynamics, and partner availability, among other factors. The two most common complaints relating to sexual dysfunction in women at midlife are lack of desire and hypoarousal.
A number of therapies are currently under investigation for treatment of female sexual dysfunction at midlife. These include the same low-dose vaginal estrogen preparations used for vaginal atrophy as well as newer approaches currently in clinical testing, such as the melanocortin receptor agonist bremelanotide27,28 and topical alprostadil,29 both of which act by inducing sexual arousal. In a study of premenopausal women with sexual arousal disorder, bremelanotide increased both genital arousal and sexual desire.30
The most widely studied pharmacotherapy approach to sexual dysfunction has been testosterone replacement. A recent Cochrane review assessed the results of 23 trials that evaluated the addition of testosterone to HT (estrogen with or without progestogen) in 1,957 peri- and postmenopausal women.31 It found that adding testosterone to HT has a beneficial effect on sexual function in postmenopausal women but also confers the adverse effect of reducing levels of high-density lipoprotein cholesterol. The authors concluded that the impact of testosterone therapy on other health outcomes in estrogenized postmenopausal women is unclear, as is the existence of a benefit in sexual function for perimenopausal women.
In addition to systemic testosterone, a transdermal testosterone patch has been studied for treatment of sexual dysfunction in postmenopausal women; FDA evaluation of the patch is awaiting the availability of long-term safety data, although the testosterone patch for women is available in Europe.
According to a 2005 NAMS position statement on testosterone therapy,32 postmenopausal women presenting with symptoms of decreased sexual desire that causes personal distress may be candidates for testosterone therapy. The NAMS statement further clarifies that all other identifiable causes of sexual dysfunction should be considered and ruled out as appropriate. Because of a lack of safety and efficacy data on the use of testosterone therapy in unestrogenized women, testosterone therapy alone cannot be recommended in women not taking concomitant estrogen.32
Bone health
Many alternatives to systemic HT exist for maintaining bone health, including calcium and vitamin D supplementation, bisphosphonates, selective estrogen receptor modulators, calcitonin, recombinant human parathyroid hormone, and ultralow-dose transdermal estradiol. Beyond calcium and vitamin D supplementation, the most appropriate options for most women will likely be bisphosphonates or transdermal estradiol.
Ultralow-dose (0.014 mg/day) transdermal estradiol has been shown to significantly increase bone mineral density (BMD) at both the hip and the spine in post-menopausal women compared with placebo.33 However, the only agent that has demonstrated fracture risk reduction in women who do not otherwise have osteoporosis is standard-dose estrogen therapy (eg, 0.625 mg conjugated equine estrogens). Lower doses of estrogen, which may maintain bone density, do not have data on fracture risk reduction. Although lower doses of estrogen have been found to increase BMD, there is a dose-dependent response, with higher doses producing more of an increase.34,35
Bisphosphonates. The oral bisphosphonates, alendronate and risedronate, have proven efficacy in reducing hip fracture rates in women who already have osteoporosis. Risedronate recently gained FDA approval for administration in a regimen involving two 75-mg tablets taken on a monthly (consecutive-day) basis, and a 150-mg monthly risedronate tablet is expected soon.
Zoledronic acid, an injectable bisphosphonate, recently gained FDA approval for administration as a once-yearly intravenous infusion after this regimen was shown in a large 3-year placebo-controlled trial to significantly reduce the risk of morphometric spine, hip, nonvertebral, wrist, and rib fractures in postmenopausal women with osteoporosis.36 In this trial, atrial fibrillation was more common in women treated with zoledronic acid than in those who received placebo. However, any link between zoledronic acid and atrial fibrillation is uncertain, since episodes of atrial fibrillation tended to occur more than 30 days after the infusion and since circulating active levels of zoledronic acid persist for only up to 1 week. (A history of arrhythmia or atrial fibrillation is not listed in FDA-approved labeling as a contraindication to zoledronic acid.) Also, there was no increased risk of jaw osteonecrosis in subjects treated with zoledronic acid.36
Intravenous dosing can be helpful when patients have intolerable gastrointestinal side effects or other contraindications to oral dosing, as well as to ensure adherence.
Ibandronate is another bisphosphonate that has been shown to reduce the risk of vertebral fractures. It is administered as a once-monthly oral dose or as an intravenous injection given every 3 months. Although these less-frequent dosing regimens can be more convenient for patients and the injectable form can eliminate gastrointestinal side effects, widespread use of ibandronate has been limited somewhat by a lack of evidence for reduction of nonvertebral and hip fractures.37
Raloxifene is the only selective estrogen receptor modulator (SERMs, which the FDA recently requested be called “estrogen agonists/estrogen antagonists”) approved for the prevention and treatment of osteoporosis in postmenopausal women. Raloxifene reduced the vertebral fracture rate by 40% to 50% over 2 to 4 years of use but did not reduce nonvertebral fracture rates. Raloxifene also reduces the risk of invasive breast cancer development.38,39 It has not been shown to lower the risk of coronary events or overall stroke risk but was instead associated with an increased risk of VTE and fatal stroke.
Synthetic recombinant human parathyroid hormone (PTH[1–34]; teriparatide) is currently the sole available agent in the new class of bone anabolic agents, although others are on the horizon. PTH(1–34) is given as a once-daily subcutaneous injection for up to 2 years of therapy. It is associated with a reduction in the risk of vertebral and nonvertebral fractures and is indicated for postmenopausal women (and men) with osteoporosis who are at high risk for fracture, as well as those in whom other medications have failed or are not tolerated.
Although rat studies revealed a potential increased risk for osteosarcoma with PTH(1–34) use, this has not been seen in any human studies or in postmarketing surveillance. As the risk was dependent on dose and duration of therapy, use of PTH(1–34) is not recommended for more than 2 years or in patients at increased risk for osteosarcoma.
Concomitant use of PTH(1–34) with a bisphosphonate seems to blunt its effect and is therefore to be avoided. Resumption of bisphosphonate use after 2 years of PTH(1–34) therapy seems to prevent the loss of densitometric gains that ensues upon cessation of PTH(1–34).40
Calcitonin is an older agent administered mainly as a nasal spray. It reduces vertebral fracture risk in postmenopausal women and is FDA-approved for the treatment, but not prevention, of osteoporosis. Calcitonin has questionable mild analgesic effects in compression fracture treatment. Because of its expense and inferior efficacy relative to other therapies, it is generally reserved for patients who cannot tolerate other agents.41
Therapies on the horizon for osteoporosis prevention and/or treatment in postmenopausal women include strontium ranelate, third-generation SERMs or estrogen agonists/antagonists (ie, bazedoxifene and lasofoxifene), and combination estrogen/SERM therapies.
SUMMARY
The risk-benefit assessment for management of vasomotor symptoms and other menopause-related health issues should be tailored to formulate the most efficacious and safe treatment plan for each individual woman. The most appropriate management is guided by the individual patient’s own assessment of her most bothersome symptom(s) and her preferences and comfort level regarding various risks and quality-of-life issues. To best inform these patient choices, physicians must strive to clearly and accurately present the risks and benefits of the various available treatment options.
For most symptomatic menopausal women, HT remains the best treatment. However, for women unable or unwilling to take HT, there are alternatives for the treatment of vasomotor symptoms and bone loss. Low doses of local vaginal estrogen remain an option for treatment of genitourinary atrophy, even in women in whom systemic HT may be contraindicated.
Reassessment of current data and ongoing clinical trials will assist clinicians and patients in decision-making regarding menopausal HT. Nonhormonal therapies for menopausal symptoms should be used to provide effective treatment options for those menopausal patients unwilling or unable to take HT.
- Anderson RN. United States life tables, 1997. Natl Vital Stat Rep 1999; 47:1–37.
- NIH State-of-the-Science Panel. National Institutes of Health State-of-the-Science Conference Statement: management of menopause-related symptoms. Ann Intern Med 2005; 142:1003–1013.
- Utian WH. Psychosocial and socioeconomic burden of vasomotor symptoms in menopause: a comprehensive review. Health Qual Life Outcomes 2005; 3:47.
- American Association of Clinical Endocrinologists (AACE) postion statement on hormone replacement therapy (HRT) and cardiovascular risk. American Association of Clinical Endocrinologists Web site. www.aace.com/pub/pdf/guidelines/HRTCVRISKposition_ statement.pdf. Accessed March 5, 2008.
- North American Menopause Society. Treatment of menopause-associated vasomotor symptoms: position statement of The North American Menopause Society. Menopause 2004; 11:11–33.
- Deecher DC. Physiology of thermoregulatory dysfunction and current approaches to the treatment of vasomotor symptoms. Expert Opin Investig Drugs 2005; 14:435–448.
- Bachmann GA. Menopausal vasomotor symptoms: a review of causes, effects and evidence-based treatment options. J Reprod Med 2005; 50:155–165.
- Freedman RR. Pathophysiology and treatment of menopausal hot flashes. Semin Reprod Med 2005; 23:117–125.
- Berendsen HH. The role of serotonin in hot flashes. Maturitas 2000; 36:155–164.
- Nelson HD, Vesco KK, Haney E, et al. Nonhormonal therapies for menopausal hot flashes: systematic review and meta-analysis. JAMA 2006; 295:2057–2071.
- Loprinzi CL, Sloan JA, Perez EA, et al. Phase III evaluation of fluoxetine for treatment of hot flashes. J Clin Oncol 2002; 20:1578–1583.
- Stearns V, Beebe KL, Iyengar M, Dube E. Paroxetine controlled release in the treatment of menopausal hot flashes: a randomized controlled trial. JAMA 2003; 289:2827–2834.
- Rapkin AJ. Vasomotor symptoms in menopause: physiologic condition and central nervous system approaches to treatment. Am J Obstet Gynecol 2007; 196:97–106.
- Jin Y, Desta Z, Stearns V, et al. CYP2D6 genotype, antidepressant use, and tamoxifen metabolism during adjuvant breast cancer treatment. J Natl Cancer Inst 2005; 97:30–39.
- Stearns V, Johnson MD, Rae JM, et al. Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine. J Natl Cancer Inst 2003; 95:1758–1764.
- Loprinzi CL, Kugler JW, Sloan JA, et al. Venlafaxine in management of hot flashes in survivors of breast cancer: a randomised controlled trial. Lancet 2000; 356:2059–2063.
- Barton D, La VB, Loprinzi C, Novotny P, Wilwerding MB, Sloan J. Venlafaxine for the control of hot flashes: results of a longitudinal continuation study. Oncol Nurs Forum 2002; 29:33–40.
- Evans ML, Pritts E, Vittinghoff E, et al. Management of postmenopausal hot flushes with venlafaxine hydrochloride: a randomized, controlled trial. Obstet Gynecol 2005; 105:161–166.
- Joffe H, Soares CN, Petrillo LF, et al. Treatment of depression and menopause-related symptoms with the serotonin-norepinephrine reuptake inhibitor duloxetine. J Clin Psychiatry 2007; 68:943–950.
- Kagan R, Constantine G, Olivier S. Treatment with desvenlafaxine succinate (DVS) results in a sustained reduction in number of severe hot flushes (HFs) in menopausal women [abstract]. Menopause 2007; 14:1084. Abstract S-13.
- Speroff L, Gass M, Constantine G, Olivier S, for the Study 315 Investigators. Efficacy and tolerability of desvenlafaxine succinate treatment for menopausal vasomotor symptoms: a randomized controlled trial. Obstet Gynecol 2008; 111:77–87.
- Reddy SY, Warner H, Guttuso T Jr, et al. Gabapentin, estrogen, and placebo for treating hot flushes: a randomized controlled trial. Obstet Gynecol 2006; 108:41–48.
- Loprinzi CL, Kugler JW, Barton DL, et al. Phase III trial of gabapentin alone or in conjunction with an antidepressant in the management of hot flashes in women who have inadequate control with an antidepressant alone: NCCTG N03C5. J Clin Oncol 2007; 25:308–312.
- Glynn RJ, Ridker PM, Goldhaber SZ, Zee RY, Buring JE. Effects of random allocation to vitamin E supplementation on the occurrence of venous thromboembolism: report from the Women’s Health Study. Circulation 2007; 116:1497–1503.
- North American Menopause Society. The role of local vaginal estrogen for treatment of vaginal atrophy in postmenopausal women: 2007 position statement of The North American Menopause Society. Menopause 2007; 14:355–369.
- Bachmann G, Lobo RA, Gut R, Nachtigall L, Notelovitz M. Efficacy of low-dose estradiol vaginal tablets in the treatment of atrophic vaginitis: a randomized controlled trial. Obstet Gynecol 2008; 111:67–76.
- Pfaus J, Giuliano F, Gelez H. Bremelanotide: an overview of preclinical CNS effects on female sexual dysfunction. J Sex Med 2007; 4(Suppl 4):269–279.
- Safarinejad MR. Evaluation of the safety and efficacy of bremelanotide, a melanocortin receptor agonist, in female subjects with arousal disorder: a double-blind placebo-controlled, fixed dose, randomized study. J Sex Med 2008; 5:887–897.
- Heiman JR, Gittelman M, Costabile R, et al. Topical alprostadil (PGE1) for the treatment of female sexual arousal disorder: in-clinic evaluation of safety and efficacy. J Psychosom Obstet Gynaecol 2006; 27:31–41.
- Diamond LE, Earle DC, Heiman JR, et al. An effect on the subjective sexual response in premenopausal women with sexual arousal disorder by bremelanotide (PT-141), a melanocortin receptor agonist. J Sex Med 2006; 3:628–638.
- Somboonporn W, Davis S, Seif MW, Bell R. Testosterone for peri- and postmenopausal women. Cochrane Database Syst Rev 2005; (4):CD004509.
- North American Menopause Society. The role of testosterone therapy in postmenopausal women: position statement of The North American Menopause Society. Menopause 2005; 12:497–511.
- Ettinger B, Ensrud KE, Wallace R, et al. Effects of ultralow-dose transdermal estradiol on bone mineral density: a randomized clinical trial. Obstet Gynecol 2004; 104:443–451.
- Genant HK, Lucas J, Weiss S, et al. Low-dose esterified estrogen therapy: effects on bone, plasma estradiol concentrations, endometrium, and lipid levels. Estratab/Osteoporosis Study Group. Arch Intern Med 1997; 157:2609–2615.
- Lindsay R, Gallagher JC, Kleerekoper M, Pickar JH. Effect of lower doses of conjugated equine estrogens with and without medroxyprogesterone acetate on bone in early postmenopausal women. JAMA 2002; 287:2668–2676.
- Black DM, Delmas PD, Eastell R, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 2007; 356:1809–1822.
- MacLean C, Newberry S, Maglione M, et al. Systematic review: comparative effectiveness of treatments to prevent fractures in men and women with low bone density or osteoporosis. Ann Intern Med 2008; 148:197–213.
- Vogel VG, Constantino JP, Wickerham DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA 2006; 295:2727–2741.
- Barrett-Connor E, Mosca L, Collins P, et al. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med 2006; 355:125–137.
- Black DM, Bilezikian JP, Ensrud KE, et al. One year of alendronate after one year of parathyroid hormone (1-84) for osteoporosis. N Engl J Med 2005; 353:555–565.
- Chesnut CH III, Silverman S, Andriano K, et al. A randomized trial of nasal spray salmon calcitonin in postmenopausal women with established osteoporosis: the prevent recurrence of osteoporotic fractures study. PROOF Study Group. Am J Med 2000; 109:267–276.
- Anderson RN. United States life tables, 1997. Natl Vital Stat Rep 1999; 47:1–37.
- NIH State-of-the-Science Panel. National Institutes of Health State-of-the-Science Conference Statement: management of menopause-related symptoms. Ann Intern Med 2005; 142:1003–1013.
- Utian WH. Psychosocial and socioeconomic burden of vasomotor symptoms in menopause: a comprehensive review. Health Qual Life Outcomes 2005; 3:47.
- American Association of Clinical Endocrinologists (AACE) postion statement on hormone replacement therapy (HRT) and cardiovascular risk. American Association of Clinical Endocrinologists Web site. www.aace.com/pub/pdf/guidelines/HRTCVRISKposition_ statement.pdf. Accessed March 5, 2008.
- North American Menopause Society. Treatment of menopause-associated vasomotor symptoms: position statement of The North American Menopause Society. Menopause 2004; 11:11–33.
- Deecher DC. Physiology of thermoregulatory dysfunction and current approaches to the treatment of vasomotor symptoms. Expert Opin Investig Drugs 2005; 14:435–448.
- Bachmann GA. Menopausal vasomotor symptoms: a review of causes, effects and evidence-based treatment options. J Reprod Med 2005; 50:155–165.
- Freedman RR. Pathophysiology and treatment of menopausal hot flashes. Semin Reprod Med 2005; 23:117–125.
- Berendsen HH. The role of serotonin in hot flashes. Maturitas 2000; 36:155–164.
- Nelson HD, Vesco KK, Haney E, et al. Nonhormonal therapies for menopausal hot flashes: systematic review and meta-analysis. JAMA 2006; 295:2057–2071.
- Loprinzi CL, Sloan JA, Perez EA, et al. Phase III evaluation of fluoxetine for treatment of hot flashes. J Clin Oncol 2002; 20:1578–1583.
- Stearns V, Beebe KL, Iyengar M, Dube E. Paroxetine controlled release in the treatment of menopausal hot flashes: a randomized controlled trial. JAMA 2003; 289:2827–2834.
- Rapkin AJ. Vasomotor symptoms in menopause: physiologic condition and central nervous system approaches to treatment. Am J Obstet Gynecol 2007; 196:97–106.
- Jin Y, Desta Z, Stearns V, et al. CYP2D6 genotype, antidepressant use, and tamoxifen metabolism during adjuvant breast cancer treatment. J Natl Cancer Inst 2005; 97:30–39.
- Stearns V, Johnson MD, Rae JM, et al. Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine. J Natl Cancer Inst 2003; 95:1758–1764.
- Loprinzi CL, Kugler JW, Sloan JA, et al. Venlafaxine in management of hot flashes in survivors of breast cancer: a randomised controlled trial. Lancet 2000; 356:2059–2063.
- Barton D, La VB, Loprinzi C, Novotny P, Wilwerding MB, Sloan J. Venlafaxine for the control of hot flashes: results of a longitudinal continuation study. Oncol Nurs Forum 2002; 29:33–40.
- Evans ML, Pritts E, Vittinghoff E, et al. Management of postmenopausal hot flushes with venlafaxine hydrochloride: a randomized, controlled trial. Obstet Gynecol 2005; 105:161–166.
- Joffe H, Soares CN, Petrillo LF, et al. Treatment of depression and menopause-related symptoms with the serotonin-norepinephrine reuptake inhibitor duloxetine. J Clin Psychiatry 2007; 68:943–950.
- Kagan R, Constantine G, Olivier S. Treatment with desvenlafaxine succinate (DVS) results in a sustained reduction in number of severe hot flushes (HFs) in menopausal women [abstract]. Menopause 2007; 14:1084. Abstract S-13.
- Speroff L, Gass M, Constantine G, Olivier S, for the Study 315 Investigators. Efficacy and tolerability of desvenlafaxine succinate treatment for menopausal vasomotor symptoms: a randomized controlled trial. Obstet Gynecol 2008; 111:77–87.
- Reddy SY, Warner H, Guttuso T Jr, et al. Gabapentin, estrogen, and placebo for treating hot flushes: a randomized controlled trial. Obstet Gynecol 2006; 108:41–48.
- Loprinzi CL, Kugler JW, Barton DL, et al. Phase III trial of gabapentin alone or in conjunction with an antidepressant in the management of hot flashes in women who have inadequate control with an antidepressant alone: NCCTG N03C5. J Clin Oncol 2007; 25:308–312.
- Glynn RJ, Ridker PM, Goldhaber SZ, Zee RY, Buring JE. Effects of random allocation to vitamin E supplementation on the occurrence of venous thromboembolism: report from the Women’s Health Study. Circulation 2007; 116:1497–1503.
- North American Menopause Society. The role of local vaginal estrogen for treatment of vaginal atrophy in postmenopausal women: 2007 position statement of The North American Menopause Society. Menopause 2007; 14:355–369.
- Bachmann G, Lobo RA, Gut R, Nachtigall L, Notelovitz M. Efficacy of low-dose estradiol vaginal tablets in the treatment of atrophic vaginitis: a randomized controlled trial. Obstet Gynecol 2008; 111:67–76.
- Pfaus J, Giuliano F, Gelez H. Bremelanotide: an overview of preclinical CNS effects on female sexual dysfunction. J Sex Med 2007; 4(Suppl 4):269–279.
- Safarinejad MR. Evaluation of the safety and efficacy of bremelanotide, a melanocortin receptor agonist, in female subjects with arousal disorder: a double-blind placebo-controlled, fixed dose, randomized study. J Sex Med 2008; 5:887–897.
- Heiman JR, Gittelman M, Costabile R, et al. Topical alprostadil (PGE1) for the treatment of female sexual arousal disorder: in-clinic evaluation of safety and efficacy. J Psychosom Obstet Gynaecol 2006; 27:31–41.
- Diamond LE, Earle DC, Heiman JR, et al. An effect on the subjective sexual response in premenopausal women with sexual arousal disorder by bremelanotide (PT-141), a melanocortin receptor agonist. J Sex Med 2006; 3:628–638.
- Somboonporn W, Davis S, Seif MW, Bell R. Testosterone for peri- and postmenopausal women. Cochrane Database Syst Rev 2005; (4):CD004509.
- North American Menopause Society. The role of testosterone therapy in postmenopausal women: position statement of The North American Menopause Society. Menopause 2005; 12:497–511.
- Ettinger B, Ensrud KE, Wallace R, et al. Effects of ultralow-dose transdermal estradiol on bone mineral density: a randomized clinical trial. Obstet Gynecol 2004; 104:443–451.
- Genant HK, Lucas J, Weiss S, et al. Low-dose esterified estrogen therapy: effects on bone, plasma estradiol concentrations, endometrium, and lipid levels. Estratab/Osteoporosis Study Group. Arch Intern Med 1997; 157:2609–2615.
- Lindsay R, Gallagher JC, Kleerekoper M, Pickar JH. Effect of lower doses of conjugated equine estrogens with and without medroxyprogesterone acetate on bone in early postmenopausal women. JAMA 2002; 287:2668–2676.
- Black DM, Delmas PD, Eastell R, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 2007; 356:1809–1822.
- MacLean C, Newberry S, Maglione M, et al. Systematic review: comparative effectiveness of treatments to prevent fractures in men and women with low bone density or osteoporosis. Ann Intern Med 2008; 148:197–213.
- Vogel VG, Constantino JP, Wickerham DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA 2006; 295:2727–2741.
- Barrett-Connor E, Mosca L, Collins P, et al. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med 2006; 355:125–137.
- Black DM, Bilezikian JP, Ensrud KE, et al. One year of alendronate after one year of parathyroid hormone (1-84) for osteoporosis. N Engl J Med 2005; 353:555–565.
- Chesnut CH III, Silverman S, Andriano K, et al. A randomized trial of nasal spray salmon calcitonin in postmenopausal women with established osteoporosis: the prevent recurrence of osteoporotic fractures study. PROOF Study Group. Am J Med 2000; 109:267–276.