User login
10 practical, evidence-based recommendations for perioperative antibiotic prophylaxis
Megan O. Schimpf, MD (June 2012)
Update on Menopause
Andrew M. Kaunitz, MD (May 2012)
Urinary tract infections (UTIs) are prevalent among women, afflicting as many as 60% of women during their lifetime.1 Symptoms include urgency, frequency, and dysuria. Although the diagnosis can be made on the basis of symptoms alone in many cases, urinalysis and urine cultures often are helpful in confirming it.2 The differential diagnosis includes infectious or atrophic vaginitis, urethritis from a sexually transmitted infection, urethral diverticulum, painful bladder syndrome, urinary tract calculi, and urinary tract neoplasms. Common risk factors for UTIs are listed in TABLE 1.3
TABLE 1
Risk factors for urinary tract infection in women
Premenopausal women •History of urinary tract infection (UTI) Postmenopausal women |
| SOURCE: Adapted from ACOG3 |
Recurrent UTIs are defined as three infections in 12 months or two infections in 6 months. In this Update, we explore strategies to prevent recurrent UTIs in three groups of women:
- sexually active premenopausal women
- postmenopausal women
- women undergoing pelvic surgery.
In the process, we summarize the results of five trials that explore treatment modalities such as prophylactic antibiotics, vaginal estrogen therapy, cranberry supplementation, and probiotics (TABLE 2).
TABLE 2
Summary of therapeutic strategies for prevention of recurrent urinary tract infections
| Strategy | Dose | Advantages | Disadvantages |
|---|---|---|---|
| Prophylactic antibiotics | Trimethoprim-sulfamethoxazole (Bactrim): 1 double-strength tablet* OR Nitrofurantoin: 50 or 100 mg Either drug can be given daily for 6 months or as one dose postcoitally | Highly effective Inexpensive | Potential for future microbial resistance Caution with nitrofurantoin, particularly in older patients or women who have renal insufficiency In pregnancy, nitrofurantoin is better studied |
| Vaginal estrogen** | Conjugated estrogens (0.625 mg conjugated estrogens/1 g cream [Premarin]). Give 0.5–2.0 g cream twice weekly. Estradiol (100 μg estradiol/1 g cream [Estrace]). Give 1–4 g cream | Highly effective in postmenopausal women, who can be difficult to treat Few true contraindications | Can be expensive Compliance may be an issue |
| Cranberry supplement | Dosing varies among products. Unsweetened natural cranberry juice or cranberry tablets, 1–3 times daily. | Generally well-tolerated Few side effects or contraindications | Can be expensive Compliance may be an issue May not be as effective in postmenopausal patients |
| Probiotics | Dosing varies among products and local availability | Few side effects or contraindications | Limited data Can be expensive |
| * Consider trimethoprim (100 mg) alone if the patient has an allergy to sulfa. ** Creams are preferred to the vaginal ring or tablets because they can be applied to periurethral tissues | |||
Postcoital antibiotic prophylaxis prevents some cases of recurrent UTI
Melekos MD, Asbach HW, Gerharz E, Zarakovitis IE, Weingaertner K, Naber KG. Post-intercourse versus daily ciprofloxacin prophylaxis for recurrent urinary tract infections in premenopausal women. J Urol. 1997;157(3):935–939.
UTIs typically involve fecal flora that colonize the vagina and perineum, most commonly Escherichia coli, Staphylococcus saprophyticus, Klebsiella pneumonia, and Proteus mirabilis. These pathogens ascend to the bladder via the urethra. Sexual intercourse is thought to facilitate this process, and recurrent UTIs in premenopausal women are often postcoital in temporal pattern.
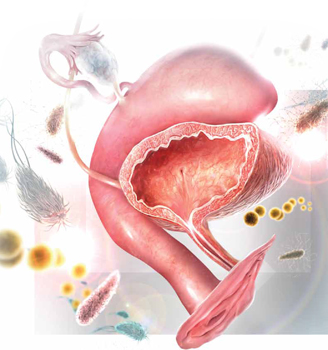
When fecal flora ascend via the urethra from the vagina and perineum to the bladder, the bladder mucosa and urethra may become inflamed, leading to urinary tract infection. The most commonly involved pathogens are Escherichia coli, Staphylococcus saprophyticus, Klebsiella pneumonia, and Proteus mirabilis.Daily antibiotic prophylaxis for 6 to 12 months has proved to be effective in the prevention of recurrent UTIs, reducing the risk of recurrence by 95%, compared with placebo.4
In this trial by Melekos and colleagues, sexually active premenopausal women who had a history of three or more documented UTIs in the preceding 12 months were randomly assigned to:
- oral ciprofloxacin, one dose daily, or
- oral ciprofloxacin, one dose immediately after intercourse.
A total of 135 patients (65 in the daily group and 70 in the postcoital group) were followed for 12 months. The regimens were equally effective at preventing UTIs. The mean number of UTIs in 12 months decreased significantly in both groups—from 3.74 to 0.031 in the daily group and from 3.67 to 0.043 in the postcoital group.
The best antibiotic? Nitrofurantoin or trimethoprim-sulfamethoxazole
This randomized, controlled trial was rigorous and well-executed and included only healthy premenopausal women. However, given the emergence of antibiotic resistance since this trial was conducted, ciprofloxacin is not an ideal antibiotic for prophylaxis.
Both the American Urological Association and the Infectious Disease Society of America recommend that fluoroquinolones be avoided, if possible, in the treatment of uncomplicated UTIs.5 A better therapeutic choice would be nitrofurantoin or trimethoprim-sulfamethoxazole.
Postcoital antibiotic prophylaxis is an effective strategy for the prevention of UTIs associated with sexual intercourse in premenopausal women. Although the optimal duration of such a regimen was not addressed in this study, it would be appropriate to revisit the need for prophylaxis after 1 year.
Dieter AA, Amundsen CL, Visco AG, Siddiqui NY. Treatment for urinary tract infection after midurethral sling: a retrospective study comparing patients who receive short-term postoperative catheterization and patients who pass a void trial on the day of surgery. Female Pelvic Med Reconstr Surg. 2012;18(3):175–178.
Urinary tract catheterization and urogynecologic surgery are associated with an increased risk for UTI. The risk of UTI following a midurethral sling procedure, in particular, ranges from 4.1% to 33.6% in the literature.6,7 To further explore the risk of UTI after placement of a midurethral sling, Dieter and colleagues followed 138 women who had undergone the procedure with and without concomitant pelvic surgery. The primary outcome was treatment of UTI within the first 3 weeks postoperatively.
Catheterization increased the risk of UTI
Fifty-eight percent of women required placement of a catheter postoperatively—either an indwelling Foley or intermittent self-catheterization. The duration of catheterization ranged from 1 to 14 days, with a mean of 4 days. The incidence of UTI was significantly higher in the group that was catheterized postoperatively, compared with the group that was not (30.0% vs 5.2%), and catheterization remained an independent risk factor for UTI after adjusting for other confounding factors.
Data may not be applicable to other types of surgery
This large retrospective cohort study of a well-characterized population was based on consistent postoperative data related to catheterization and UTI treatment. Because the study focused on patients who had undergone placement of a midurethral sling, its findings may not be applicable to women undergoing other types of pelvic surgery, including general gynecologic procedures. However, given the significant difference in the rate of UTI between the two groups, the increased risk of UTI may be at least partially attributable to short-term postoperative catheterization rather than urinary tract instrumentation during the procedure.
The risk of UTI is increased with short-term catheterization following placement of a midurethral sling. There may be a role for antibiotic prophylaxis in the setting of short-term postoperative catheterization; however, a prospective, randomized, placebo-controlled study is needed to determine whether the rate of UTI would be reduced.
Vaginal estrogen prevents recurrent UTIs among postmenopausal women
Raz R, Stamm WE. A controlled trial of intravaginal estriol in postmenopausal women with recurrent urinary tract infections. N Engl J Med. 1993;329(11):753–756.
The tissues of the vagina, urethra, bladder, and pelvic floor musculature all express estrogen receptors.8 In postmenopausal women, the effects of decreased estrogen on the urinary tract include a rise in the vaginal pH level and decreased colonization with Lactobacillus. These effects predispose this population to an increased risk for UTI.3 The literature does not support the use of oral estrogen replacement as a therapy for recurrent UTI; however, data suggest that vaginal estrogen replacement may be helpful.9
Raz and Stamm conducted their randomized trial of 93 postmenopausal women with a history of recurrent UTIs to elucidate the effects of vaginal estrogen on the risk of UTI. Fifty women were randomly assigned to treatment with intravaginal estriol cream (0.5 mg nightly for 2 weeks, followed by 0.5 mg twice weekly for 8 months), and 43 women were randomly assigned to placebo (equivalent regimen). Compared with the placebo group, the women treated with estriol experienced a significantly reduced risk of UTI (0.5 vs 5.9 infections per patient-year), increased lactobacilli on vaginal cultures (61% vs 0%), decreased vaginal pH, and a lower rate of colonization with Enterobacteriaceae species.
Although this rigorous double-blind, randomized, placebo-controlled trial was published 20 years ago, its findings remain significant—and have been corroborated in other studies.9
Pros and cons of vaginal estrogen replacement
Raz and Stamm utilized vaginal estriol; the preparations used most commonly today are conjugated estrogens (Premarin) and estradiol (Estrace). Vaginal estrogen formulations can be expensive. Compliance also can wane over time. This study, in particular, showed a discontinuation rate of 28%; mild local reactions were the reason. Although the women who discontinued treatment in this study were included in the final analysis, no subanalysis of these patients was published.
Despite these challenges, local estrogen replacement is generally well-tolerated and, with infrequent dosing (twice weekly), has few contraindications. In fact, local estrogen replacement is one of the most highly effective regimens for UTI prevention among postmenopausal women, who can otherwise be difficult to treat for recurrent UTIs.
Vaginal estrogen is an effective therapy for the prevention of UTIs in postmenopausal women.
Cranberry supplementation may prevent UTIs,
but products vary widely
Stothers L. A randomized trial to evaluate effectiveness and cost-effectiveness of naturopathic cranberry products as prophylaxis against urinary tract infection in women. Can J Urol. 2002;9(3):1558–1562.
Cranberries have been used for many years in various formulations to prevent UTI, but no definitive mechanism has been established. In theory, cranberries keep bacteria from adhering to the urothelium.10 In vitro studies have revealed that Escherichia coli is prevented from adhering to uroepithelial cells by two components of cranberry—fructose and proanthocyanidins.10
In this trial of 150 sexually active women (ages 21–72 years) who had experienced at least two UTIs in the past calendar year, Stothers randomly assigned participants to one of three arms for 12 months:
- placebo tablets and cranberry juice (n = 50)
- cranberry tablets and placebo juice (n = 50)
- placebo tablets and placebo juice (n = 50).
Tablets were taken twice daily, and juice was consumed three times daily. All cranberry juice was organic, unsweetened, and unfiltered and taken in 250-mL servings; cranberry tablets were 1:30 parts concentrated cranberry juice.
The risk of UTI during treatment was reduced significantly in the groups taking a cranberry formulation, compared with placebo. Twenty percent of patients consuming cranberry juice experienced a UTI during treatment, compared with 18% of those taking a cranberry tablet and 32% of those in the placebo group (P<.05). In this study, the annual cost of prophylaxis with cranberry juice was $1,400 per woman, and it was $624 per woman for the cranberry tablets. Compliance was lowest among women consuming cranberry juice, decreasing at times to less than 80%.
Findings are difficult to extrapolate
This randomized, double-blind study demonstrated a significant reduction in the rate of UTI with cranberry supplementation, compared with placebo, among women with a mean age of 40 to 44 years. However, because cranberry preparations, juice, and tablets are not regulated as to the amount and bioavailability of the active ingredient, it is difficult to compare one to another and extrapolate to a particular type of preparation.
This study does highlight the higher rate of noncompliance and cost with cranberry juice, although it was as effective at reducing UTIs as cranberry tablets.
Cranberry supplementation reduced the risk of UTIs in sexually active women; placebo did not. Cranberry use may be an alternative to postcoital antibiotic prophylaxis; a randomized comparison of these therapies is needed.
Can nonhormonal therapy alter vaginal flora?
Stapleton AE, Au-Yeung M, Hooton TM, et al. Randomized, placebo-controlled phase 2 trial of a Lactobacillus crispatus probiotic given intravaginally for prevention of recurrent urinary tract infection. Clin Infect Dis. 2011;52(10):1212–1217.
Probiotics have been used recently in attempts to prevent recurrent UTI, albeit with very little evidence in the literature. Their effectiveness is plausible due to promotion of healthy vaginal flora.
This study by Stapleton and colleagues enrolled premenopausal women (ages 18–40) with a history of one UTI within the past calendar year and a current, active, uncomplicated UTI. Ninety-nine percent of participants were sexually active. All women were treated with a standard antibiotic regimen for UTI. Seven to 10 days later, participants were randomly assigned to:
- Lactobacillus crispatus vaginal suppository [Lactin-V (Osel)], daily for 5 days and then weekly for 10 weeks (n = 50), or
- placebo (same regimen) (n = 50).
The risk of UTI was 15% among women in the probiotic group, compared with 27% in the placebo group—but this difference was only statistically significant for women who had a higher level of Lactobacillus crispatus vaginal colonization in the treatment group.
Vaginal probiotic formulations may be hard to obtain
The use of probiotics to prevent recurrent UTIs is new and innovative. However, vaginal probiotic formulations are not widely available, and most commercially available oral probiotic formulations are marketed for digestive health—an area where the effects have been studied widely.
In this study, the mean age was 21 years. Given that hypoestrogenization is associated with decreased vaginal colonization with Lactobacillus, an interesting area of future study would be the use of probiotics in postmenopausal women.
Continued investigation of probiotics is warranted, as this approach could help in the treatment of women who have intolerance to antibiotics and is generally considered safe and well-tolerated.
Intravaginal probiotic prophylaxis may reduce the risk of recurrent UTIs. However, further studies are needed to confirm early enthusiasm and delineate ideal populations.
We want to hear from you! Tell us what you think.
1. Foxman B, Barolow R, D’Arcy H, Gillespie B, Sobel JD. Urinary tract infection: self-reported incidence and associated costs. Ann Epidemiol. 2000;10(8):509-515.
2. Bent S, Nallamothu BK, Simel DL, Fihn SD, Saint S. Does this woman have an acute uncomplicated urinary tract infection? JAMA. 2002;287(20):2701-2710.
3. ACOG Practice Bulletin #91: Treatment of urinary tract infections in nonpregnant women. Obstet Gynecol. 2008;111(3):785-794.
4. Hooton TM. Recurrent urinary tract infection in women. Int J Antimicrob Agents. 2001;17(4):259-268.
5. Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Disease Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52(5):e103-120.
6. Sutkin G, Alperin M, Meyn L, Wiesenfeld HC, Ellison R, Zyczynski HM. Symptomatic urinary tract infections after surgery for prolapse and/or incontinence. Int Urogynecol J. 2010;21(8):955-961.
7. Dieter AA, Amundsen CL, Visco AG, Siddiqui NY. Treatment for urinary tract infection after midurethral sling: a retrospective study comparing patients who receive short-term postoperative catheterization and patients who pass a void trial on the day of surgery. Female Pelvic Med Reconstr Surg. 2012;18(3):175-178.
8. Robinson D, Cardozo L. Estrogens and the lower urinary tract. Neurourol Urodyn. 2011;30(5):754-757.
9. Perrotta C, Aznar M, Mejia R, Albert X, Ng CW. Oestrogens for preventing recurrent urinary tract infection in postmenopausal women. Cochrane Database Syst Rev. 2008;(2):CD005131.-
10. Jepson RG, Craig JC. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev. 2008;(1):CD001321.-
10 practical, evidence-based recommendations for perioperative antibiotic prophylaxis
Megan O. Schimpf, MD (June 2012)
Update on Menopause
Andrew M. Kaunitz, MD (May 2012)
Urinary tract infections (UTIs) are prevalent among women, afflicting as many as 60% of women during their lifetime.1 Symptoms include urgency, frequency, and dysuria. Although the diagnosis can be made on the basis of symptoms alone in many cases, urinalysis and urine cultures often are helpful in confirming it.2 The differential diagnosis includes infectious or atrophic vaginitis, urethritis from a sexually transmitted infection, urethral diverticulum, painful bladder syndrome, urinary tract calculi, and urinary tract neoplasms. Common risk factors for UTIs are listed in TABLE 1.3
TABLE 1
Risk factors for urinary tract infection in women
Premenopausal women •History of urinary tract infection (UTI) Postmenopausal women |
| SOURCE: Adapted from ACOG3 |
Recurrent UTIs are defined as three infections in 12 months or two infections in 6 months. In this Update, we explore strategies to prevent recurrent UTIs in three groups of women:
- sexually active premenopausal women
- postmenopausal women
- women undergoing pelvic surgery.
In the process, we summarize the results of five trials that explore treatment modalities such as prophylactic antibiotics, vaginal estrogen therapy, cranberry supplementation, and probiotics (TABLE 2).
TABLE 2
Summary of therapeutic strategies for prevention of recurrent urinary tract infections
| Strategy | Dose | Advantages | Disadvantages |
|---|---|---|---|
| Prophylactic antibiotics | Trimethoprim-sulfamethoxazole (Bactrim): 1 double-strength tablet* OR Nitrofurantoin: 50 or 100 mg Either drug can be given daily for 6 months or as one dose postcoitally | Highly effective Inexpensive | Potential for future microbial resistance Caution with nitrofurantoin, particularly in older patients or women who have renal insufficiency In pregnancy, nitrofurantoin is better studied |
| Vaginal estrogen** | Conjugated estrogens (0.625 mg conjugated estrogens/1 g cream [Premarin]). Give 0.5–2.0 g cream twice weekly. Estradiol (100 μg estradiol/1 g cream [Estrace]). Give 1–4 g cream | Highly effective in postmenopausal women, who can be difficult to treat Few true contraindications | Can be expensive Compliance may be an issue |
| Cranberry supplement | Dosing varies among products. Unsweetened natural cranberry juice or cranberry tablets, 1–3 times daily. | Generally well-tolerated Few side effects or contraindications | Can be expensive Compliance may be an issue May not be as effective in postmenopausal patients |
| Probiotics | Dosing varies among products and local availability | Few side effects or contraindications | Limited data Can be expensive |
| * Consider trimethoprim (100 mg) alone if the patient has an allergy to sulfa. ** Creams are preferred to the vaginal ring or tablets because they can be applied to periurethral tissues | |||
Postcoital antibiotic prophylaxis prevents some cases of recurrent UTI
Melekos MD, Asbach HW, Gerharz E, Zarakovitis IE, Weingaertner K, Naber KG. Post-intercourse versus daily ciprofloxacin prophylaxis for recurrent urinary tract infections in premenopausal women. J Urol. 1997;157(3):935–939.
UTIs typically involve fecal flora that colonize the vagina and perineum, most commonly Escherichia coli, Staphylococcus saprophyticus, Klebsiella pneumonia, and Proteus mirabilis. These pathogens ascend to the bladder via the urethra. Sexual intercourse is thought to facilitate this process, and recurrent UTIs in premenopausal women are often postcoital in temporal pattern.

When fecal flora ascend via the urethra from the vagina and perineum to the bladder, the bladder mucosa and urethra may become inflamed, leading to urinary tract infection. The most commonly involved pathogens are Escherichia coli, Staphylococcus saprophyticus, Klebsiella pneumonia, and Proteus mirabilis.Daily antibiotic prophylaxis for 6 to 12 months has proved to be effective in the prevention of recurrent UTIs, reducing the risk of recurrence by 95%, compared with placebo.4
In this trial by Melekos and colleagues, sexually active premenopausal women who had a history of three or more documented UTIs in the preceding 12 months were randomly assigned to:
- oral ciprofloxacin, one dose daily, or
- oral ciprofloxacin, one dose immediately after intercourse.
A total of 135 patients (65 in the daily group and 70 in the postcoital group) were followed for 12 months. The regimens were equally effective at preventing UTIs. The mean number of UTIs in 12 months decreased significantly in both groups—from 3.74 to 0.031 in the daily group and from 3.67 to 0.043 in the postcoital group.
The best antibiotic? Nitrofurantoin or trimethoprim-sulfamethoxazole
This randomized, controlled trial was rigorous and well-executed and included only healthy premenopausal women. However, given the emergence of antibiotic resistance since this trial was conducted, ciprofloxacin is not an ideal antibiotic for prophylaxis.
Both the American Urological Association and the Infectious Disease Society of America recommend that fluoroquinolones be avoided, if possible, in the treatment of uncomplicated UTIs.5 A better therapeutic choice would be nitrofurantoin or trimethoprim-sulfamethoxazole.
Postcoital antibiotic prophylaxis is an effective strategy for the prevention of UTIs associated with sexual intercourse in premenopausal women. Although the optimal duration of such a regimen was not addressed in this study, it would be appropriate to revisit the need for prophylaxis after 1 year.
Dieter AA, Amundsen CL, Visco AG, Siddiqui NY. Treatment for urinary tract infection after midurethral sling: a retrospective study comparing patients who receive short-term postoperative catheterization and patients who pass a void trial on the day of surgery. Female Pelvic Med Reconstr Surg. 2012;18(3):175–178.
Urinary tract catheterization and urogynecologic surgery are associated with an increased risk for UTI. The risk of UTI following a midurethral sling procedure, in particular, ranges from 4.1% to 33.6% in the literature.6,7 To further explore the risk of UTI after placement of a midurethral sling, Dieter and colleagues followed 138 women who had undergone the procedure with and without concomitant pelvic surgery. The primary outcome was treatment of UTI within the first 3 weeks postoperatively.
Catheterization increased the risk of UTI
Fifty-eight percent of women required placement of a catheter postoperatively—either an indwelling Foley or intermittent self-catheterization. The duration of catheterization ranged from 1 to 14 days, with a mean of 4 days. The incidence of UTI was significantly higher in the group that was catheterized postoperatively, compared with the group that was not (30.0% vs 5.2%), and catheterization remained an independent risk factor for UTI after adjusting for other confounding factors.
Data may not be applicable to other types of surgery
This large retrospective cohort study of a well-characterized population was based on consistent postoperative data related to catheterization and UTI treatment. Because the study focused on patients who had undergone placement of a midurethral sling, its findings may not be applicable to women undergoing other types of pelvic surgery, including general gynecologic procedures. However, given the significant difference in the rate of UTI between the two groups, the increased risk of UTI may be at least partially attributable to short-term postoperative catheterization rather than urinary tract instrumentation during the procedure.
The risk of UTI is increased with short-term catheterization following placement of a midurethral sling. There may be a role for antibiotic prophylaxis in the setting of short-term postoperative catheterization; however, a prospective, randomized, placebo-controlled study is needed to determine whether the rate of UTI would be reduced.
Vaginal estrogen prevents recurrent UTIs among postmenopausal women
Raz R, Stamm WE. A controlled trial of intravaginal estriol in postmenopausal women with recurrent urinary tract infections. N Engl J Med. 1993;329(11):753–756.
The tissues of the vagina, urethra, bladder, and pelvic floor musculature all express estrogen receptors.8 In postmenopausal women, the effects of decreased estrogen on the urinary tract include a rise in the vaginal pH level and decreased colonization with Lactobacillus. These effects predispose this population to an increased risk for UTI.3 The literature does not support the use of oral estrogen replacement as a therapy for recurrent UTI; however, data suggest that vaginal estrogen replacement may be helpful.9
Raz and Stamm conducted their randomized trial of 93 postmenopausal women with a history of recurrent UTIs to elucidate the effects of vaginal estrogen on the risk of UTI. Fifty women were randomly assigned to treatment with intravaginal estriol cream (0.5 mg nightly for 2 weeks, followed by 0.5 mg twice weekly for 8 months), and 43 women were randomly assigned to placebo (equivalent regimen). Compared with the placebo group, the women treated with estriol experienced a significantly reduced risk of UTI (0.5 vs 5.9 infections per patient-year), increased lactobacilli on vaginal cultures (61% vs 0%), decreased vaginal pH, and a lower rate of colonization with Enterobacteriaceae species.
Although this rigorous double-blind, randomized, placebo-controlled trial was published 20 years ago, its findings remain significant—and have been corroborated in other studies.9
Pros and cons of vaginal estrogen replacement
Raz and Stamm utilized vaginal estriol; the preparations used most commonly today are conjugated estrogens (Premarin) and estradiol (Estrace). Vaginal estrogen formulations can be expensive. Compliance also can wane over time. This study, in particular, showed a discontinuation rate of 28%; mild local reactions were the reason. Although the women who discontinued treatment in this study were included in the final analysis, no subanalysis of these patients was published.
Despite these challenges, local estrogen replacement is generally well-tolerated and, with infrequent dosing (twice weekly), has few contraindications. In fact, local estrogen replacement is one of the most highly effective regimens for UTI prevention among postmenopausal women, who can otherwise be difficult to treat for recurrent UTIs.
Vaginal estrogen is an effective therapy for the prevention of UTIs in postmenopausal women.
Cranberry supplementation may prevent UTIs,
but products vary widely
Stothers L. A randomized trial to evaluate effectiveness and cost-effectiveness of naturopathic cranberry products as prophylaxis against urinary tract infection in women. Can J Urol. 2002;9(3):1558–1562.
Cranberries have been used for many years in various formulations to prevent UTI, but no definitive mechanism has been established. In theory, cranberries keep bacteria from adhering to the urothelium.10 In vitro studies have revealed that Escherichia coli is prevented from adhering to uroepithelial cells by two components of cranberry—fructose and proanthocyanidins.10
In this trial of 150 sexually active women (ages 21–72 years) who had experienced at least two UTIs in the past calendar year, Stothers randomly assigned participants to one of three arms for 12 months:
- placebo tablets and cranberry juice (n = 50)
- cranberry tablets and placebo juice (n = 50)
- placebo tablets and placebo juice (n = 50).
Tablets were taken twice daily, and juice was consumed three times daily. All cranberry juice was organic, unsweetened, and unfiltered and taken in 250-mL servings; cranberry tablets were 1:30 parts concentrated cranberry juice.
The risk of UTI during treatment was reduced significantly in the groups taking a cranberry formulation, compared with placebo. Twenty percent of patients consuming cranberry juice experienced a UTI during treatment, compared with 18% of those taking a cranberry tablet and 32% of those in the placebo group (P<.05). In this study, the annual cost of prophylaxis with cranberry juice was $1,400 per woman, and it was $624 per woman for the cranberry tablets. Compliance was lowest among women consuming cranberry juice, decreasing at times to less than 80%.
Findings are difficult to extrapolate
This randomized, double-blind study demonstrated a significant reduction in the rate of UTI with cranberry supplementation, compared with placebo, among women with a mean age of 40 to 44 years. However, because cranberry preparations, juice, and tablets are not regulated as to the amount and bioavailability of the active ingredient, it is difficult to compare one to another and extrapolate to a particular type of preparation.
This study does highlight the higher rate of noncompliance and cost with cranberry juice, although it was as effective at reducing UTIs as cranberry tablets.
Cranberry supplementation reduced the risk of UTIs in sexually active women; placebo did not. Cranberry use may be an alternative to postcoital antibiotic prophylaxis; a randomized comparison of these therapies is needed.
Can nonhormonal therapy alter vaginal flora?
Stapleton AE, Au-Yeung M, Hooton TM, et al. Randomized, placebo-controlled phase 2 trial of a Lactobacillus crispatus probiotic given intravaginally for prevention of recurrent urinary tract infection. Clin Infect Dis. 2011;52(10):1212–1217.
Probiotics have been used recently in attempts to prevent recurrent UTI, albeit with very little evidence in the literature. Their effectiveness is plausible due to promotion of healthy vaginal flora.
This study by Stapleton and colleagues enrolled premenopausal women (ages 18–40) with a history of one UTI within the past calendar year and a current, active, uncomplicated UTI. Ninety-nine percent of participants were sexually active. All women were treated with a standard antibiotic regimen for UTI. Seven to 10 days later, participants were randomly assigned to:
- Lactobacillus crispatus vaginal suppository [Lactin-V (Osel)], daily for 5 days and then weekly for 10 weeks (n = 50), or
- placebo (same regimen) (n = 50).
The risk of UTI was 15% among women in the probiotic group, compared with 27% in the placebo group—but this difference was only statistically significant for women who had a higher level of Lactobacillus crispatus vaginal colonization in the treatment group.
Vaginal probiotic formulations may be hard to obtain
The use of probiotics to prevent recurrent UTIs is new and innovative. However, vaginal probiotic formulations are not widely available, and most commercially available oral probiotic formulations are marketed for digestive health—an area where the effects have been studied widely.
In this study, the mean age was 21 years. Given that hypoestrogenization is associated with decreased vaginal colonization with Lactobacillus, an interesting area of future study would be the use of probiotics in postmenopausal women.
Continued investigation of probiotics is warranted, as this approach could help in the treatment of women who have intolerance to antibiotics and is generally considered safe and well-tolerated.
Intravaginal probiotic prophylaxis may reduce the risk of recurrent UTIs. However, further studies are needed to confirm early enthusiasm and delineate ideal populations.
We want to hear from you! Tell us what you think.
10 practical, evidence-based recommendations for perioperative antibiotic prophylaxis
Megan O. Schimpf, MD (June 2012)
Update on Menopause
Andrew M. Kaunitz, MD (May 2012)
Urinary tract infections (UTIs) are prevalent among women, afflicting as many as 60% of women during their lifetime.1 Symptoms include urgency, frequency, and dysuria. Although the diagnosis can be made on the basis of symptoms alone in many cases, urinalysis and urine cultures often are helpful in confirming it.2 The differential diagnosis includes infectious or atrophic vaginitis, urethritis from a sexually transmitted infection, urethral diverticulum, painful bladder syndrome, urinary tract calculi, and urinary tract neoplasms. Common risk factors for UTIs are listed in TABLE 1.3
TABLE 1
Risk factors for urinary tract infection in women
Premenopausal women •History of urinary tract infection (UTI) Postmenopausal women |
| SOURCE: Adapted from ACOG3 |
Recurrent UTIs are defined as three infections in 12 months or two infections in 6 months. In this Update, we explore strategies to prevent recurrent UTIs in three groups of women:
- sexually active premenopausal women
- postmenopausal women
- women undergoing pelvic surgery.
In the process, we summarize the results of five trials that explore treatment modalities such as prophylactic antibiotics, vaginal estrogen therapy, cranberry supplementation, and probiotics (TABLE 2).
TABLE 2
Summary of therapeutic strategies for prevention of recurrent urinary tract infections
| Strategy | Dose | Advantages | Disadvantages |
|---|---|---|---|
| Prophylactic antibiotics | Trimethoprim-sulfamethoxazole (Bactrim): 1 double-strength tablet* OR Nitrofurantoin: 50 or 100 mg Either drug can be given daily for 6 months or as one dose postcoitally | Highly effective Inexpensive | Potential for future microbial resistance Caution with nitrofurantoin, particularly in older patients or women who have renal insufficiency In pregnancy, nitrofurantoin is better studied |
| Vaginal estrogen** | Conjugated estrogens (0.625 mg conjugated estrogens/1 g cream [Premarin]). Give 0.5–2.0 g cream twice weekly. Estradiol (100 μg estradiol/1 g cream [Estrace]). Give 1–4 g cream | Highly effective in postmenopausal women, who can be difficult to treat Few true contraindications | Can be expensive Compliance may be an issue |
| Cranberry supplement | Dosing varies among products. Unsweetened natural cranberry juice or cranberry tablets, 1–3 times daily. | Generally well-tolerated Few side effects or contraindications | Can be expensive Compliance may be an issue May not be as effective in postmenopausal patients |
| Probiotics | Dosing varies among products and local availability | Few side effects or contraindications | Limited data Can be expensive |
| * Consider trimethoprim (100 mg) alone if the patient has an allergy to sulfa. ** Creams are preferred to the vaginal ring or tablets because they can be applied to periurethral tissues | |||
Postcoital antibiotic prophylaxis prevents some cases of recurrent UTI
Melekos MD, Asbach HW, Gerharz E, Zarakovitis IE, Weingaertner K, Naber KG. Post-intercourse versus daily ciprofloxacin prophylaxis for recurrent urinary tract infections in premenopausal women. J Urol. 1997;157(3):935–939.
UTIs typically involve fecal flora that colonize the vagina and perineum, most commonly Escherichia coli, Staphylococcus saprophyticus, Klebsiella pneumonia, and Proteus mirabilis. These pathogens ascend to the bladder via the urethra. Sexual intercourse is thought to facilitate this process, and recurrent UTIs in premenopausal women are often postcoital in temporal pattern.

When fecal flora ascend via the urethra from the vagina and perineum to the bladder, the bladder mucosa and urethra may become inflamed, leading to urinary tract infection. The most commonly involved pathogens are Escherichia coli, Staphylococcus saprophyticus, Klebsiella pneumonia, and Proteus mirabilis.Daily antibiotic prophylaxis for 6 to 12 months has proved to be effective in the prevention of recurrent UTIs, reducing the risk of recurrence by 95%, compared with placebo.4
In this trial by Melekos and colleagues, sexually active premenopausal women who had a history of three or more documented UTIs in the preceding 12 months were randomly assigned to:
- oral ciprofloxacin, one dose daily, or
- oral ciprofloxacin, one dose immediately after intercourse.
A total of 135 patients (65 in the daily group and 70 in the postcoital group) were followed for 12 months. The regimens were equally effective at preventing UTIs. The mean number of UTIs in 12 months decreased significantly in both groups—from 3.74 to 0.031 in the daily group and from 3.67 to 0.043 in the postcoital group.
The best antibiotic? Nitrofurantoin or trimethoprim-sulfamethoxazole
This randomized, controlled trial was rigorous and well-executed and included only healthy premenopausal women. However, given the emergence of antibiotic resistance since this trial was conducted, ciprofloxacin is not an ideal antibiotic for prophylaxis.
Both the American Urological Association and the Infectious Disease Society of America recommend that fluoroquinolones be avoided, if possible, in the treatment of uncomplicated UTIs.5 A better therapeutic choice would be nitrofurantoin or trimethoprim-sulfamethoxazole.
Postcoital antibiotic prophylaxis is an effective strategy for the prevention of UTIs associated with sexual intercourse in premenopausal women. Although the optimal duration of such a regimen was not addressed in this study, it would be appropriate to revisit the need for prophylaxis after 1 year.
Dieter AA, Amundsen CL, Visco AG, Siddiqui NY. Treatment for urinary tract infection after midurethral sling: a retrospective study comparing patients who receive short-term postoperative catheterization and patients who pass a void trial on the day of surgery. Female Pelvic Med Reconstr Surg. 2012;18(3):175–178.
Urinary tract catheterization and urogynecologic surgery are associated with an increased risk for UTI. The risk of UTI following a midurethral sling procedure, in particular, ranges from 4.1% to 33.6% in the literature.6,7 To further explore the risk of UTI after placement of a midurethral sling, Dieter and colleagues followed 138 women who had undergone the procedure with and without concomitant pelvic surgery. The primary outcome was treatment of UTI within the first 3 weeks postoperatively.
Catheterization increased the risk of UTI
Fifty-eight percent of women required placement of a catheter postoperatively—either an indwelling Foley or intermittent self-catheterization. The duration of catheterization ranged from 1 to 14 days, with a mean of 4 days. The incidence of UTI was significantly higher in the group that was catheterized postoperatively, compared with the group that was not (30.0% vs 5.2%), and catheterization remained an independent risk factor for UTI after adjusting for other confounding factors.
Data may not be applicable to other types of surgery
This large retrospective cohort study of a well-characterized population was based on consistent postoperative data related to catheterization and UTI treatment. Because the study focused on patients who had undergone placement of a midurethral sling, its findings may not be applicable to women undergoing other types of pelvic surgery, including general gynecologic procedures. However, given the significant difference in the rate of UTI between the two groups, the increased risk of UTI may be at least partially attributable to short-term postoperative catheterization rather than urinary tract instrumentation during the procedure.
The risk of UTI is increased with short-term catheterization following placement of a midurethral sling. There may be a role for antibiotic prophylaxis in the setting of short-term postoperative catheterization; however, a prospective, randomized, placebo-controlled study is needed to determine whether the rate of UTI would be reduced.
Vaginal estrogen prevents recurrent UTIs among postmenopausal women
Raz R, Stamm WE. A controlled trial of intravaginal estriol in postmenopausal women with recurrent urinary tract infections. N Engl J Med. 1993;329(11):753–756.
The tissues of the vagina, urethra, bladder, and pelvic floor musculature all express estrogen receptors.8 In postmenopausal women, the effects of decreased estrogen on the urinary tract include a rise in the vaginal pH level and decreased colonization with Lactobacillus. These effects predispose this population to an increased risk for UTI.3 The literature does not support the use of oral estrogen replacement as a therapy for recurrent UTI; however, data suggest that vaginal estrogen replacement may be helpful.9
Raz and Stamm conducted their randomized trial of 93 postmenopausal women with a history of recurrent UTIs to elucidate the effects of vaginal estrogen on the risk of UTI. Fifty women were randomly assigned to treatment with intravaginal estriol cream (0.5 mg nightly for 2 weeks, followed by 0.5 mg twice weekly for 8 months), and 43 women were randomly assigned to placebo (equivalent regimen). Compared with the placebo group, the women treated with estriol experienced a significantly reduced risk of UTI (0.5 vs 5.9 infections per patient-year), increased lactobacilli on vaginal cultures (61% vs 0%), decreased vaginal pH, and a lower rate of colonization with Enterobacteriaceae species.
Although this rigorous double-blind, randomized, placebo-controlled trial was published 20 years ago, its findings remain significant—and have been corroborated in other studies.9
Pros and cons of vaginal estrogen replacement
Raz and Stamm utilized vaginal estriol; the preparations used most commonly today are conjugated estrogens (Premarin) and estradiol (Estrace). Vaginal estrogen formulations can be expensive. Compliance also can wane over time. This study, in particular, showed a discontinuation rate of 28%; mild local reactions were the reason. Although the women who discontinued treatment in this study were included in the final analysis, no subanalysis of these patients was published.
Despite these challenges, local estrogen replacement is generally well-tolerated and, with infrequent dosing (twice weekly), has few contraindications. In fact, local estrogen replacement is one of the most highly effective regimens for UTI prevention among postmenopausal women, who can otherwise be difficult to treat for recurrent UTIs.
Vaginal estrogen is an effective therapy for the prevention of UTIs in postmenopausal women.
Cranberry supplementation may prevent UTIs,
but products vary widely
Stothers L. A randomized trial to evaluate effectiveness and cost-effectiveness of naturopathic cranberry products as prophylaxis against urinary tract infection in women. Can J Urol. 2002;9(3):1558–1562.
Cranberries have been used for many years in various formulations to prevent UTI, but no definitive mechanism has been established. In theory, cranberries keep bacteria from adhering to the urothelium.10 In vitro studies have revealed that Escherichia coli is prevented from adhering to uroepithelial cells by two components of cranberry—fructose and proanthocyanidins.10
In this trial of 150 sexually active women (ages 21–72 years) who had experienced at least two UTIs in the past calendar year, Stothers randomly assigned participants to one of three arms for 12 months:
- placebo tablets and cranberry juice (n = 50)
- cranberry tablets and placebo juice (n = 50)
- placebo tablets and placebo juice (n = 50).
Tablets were taken twice daily, and juice was consumed three times daily. All cranberry juice was organic, unsweetened, and unfiltered and taken in 250-mL servings; cranberry tablets were 1:30 parts concentrated cranberry juice.
The risk of UTI during treatment was reduced significantly in the groups taking a cranberry formulation, compared with placebo. Twenty percent of patients consuming cranberry juice experienced a UTI during treatment, compared with 18% of those taking a cranberry tablet and 32% of those in the placebo group (P<.05). In this study, the annual cost of prophylaxis with cranberry juice was $1,400 per woman, and it was $624 per woman for the cranberry tablets. Compliance was lowest among women consuming cranberry juice, decreasing at times to less than 80%.
Findings are difficult to extrapolate
This randomized, double-blind study demonstrated a significant reduction in the rate of UTI with cranberry supplementation, compared with placebo, among women with a mean age of 40 to 44 years. However, because cranberry preparations, juice, and tablets are not regulated as to the amount and bioavailability of the active ingredient, it is difficult to compare one to another and extrapolate to a particular type of preparation.
This study does highlight the higher rate of noncompliance and cost with cranberry juice, although it was as effective at reducing UTIs as cranberry tablets.
Cranberry supplementation reduced the risk of UTIs in sexually active women; placebo did not. Cranberry use may be an alternative to postcoital antibiotic prophylaxis; a randomized comparison of these therapies is needed.
Can nonhormonal therapy alter vaginal flora?
Stapleton AE, Au-Yeung M, Hooton TM, et al. Randomized, placebo-controlled phase 2 trial of a Lactobacillus crispatus probiotic given intravaginally for prevention of recurrent urinary tract infection. Clin Infect Dis. 2011;52(10):1212–1217.
Probiotics have been used recently in attempts to prevent recurrent UTI, albeit with very little evidence in the literature. Their effectiveness is plausible due to promotion of healthy vaginal flora.
This study by Stapleton and colleagues enrolled premenopausal women (ages 18–40) with a history of one UTI within the past calendar year and a current, active, uncomplicated UTI. Ninety-nine percent of participants were sexually active. All women were treated with a standard antibiotic regimen for UTI. Seven to 10 days later, participants were randomly assigned to:
- Lactobacillus crispatus vaginal suppository [Lactin-V (Osel)], daily for 5 days and then weekly for 10 weeks (n = 50), or
- placebo (same regimen) (n = 50).
The risk of UTI was 15% among women in the probiotic group, compared with 27% in the placebo group—but this difference was only statistically significant for women who had a higher level of Lactobacillus crispatus vaginal colonization in the treatment group.
Vaginal probiotic formulations may be hard to obtain
The use of probiotics to prevent recurrent UTIs is new and innovative. However, vaginal probiotic formulations are not widely available, and most commercially available oral probiotic formulations are marketed for digestive health—an area where the effects have been studied widely.
In this study, the mean age was 21 years. Given that hypoestrogenization is associated with decreased vaginal colonization with Lactobacillus, an interesting area of future study would be the use of probiotics in postmenopausal women.
Continued investigation of probiotics is warranted, as this approach could help in the treatment of women who have intolerance to antibiotics and is generally considered safe and well-tolerated.
Intravaginal probiotic prophylaxis may reduce the risk of recurrent UTIs. However, further studies are needed to confirm early enthusiasm and delineate ideal populations.
We want to hear from you! Tell us what you think.
1. Foxman B, Barolow R, D’Arcy H, Gillespie B, Sobel JD. Urinary tract infection: self-reported incidence and associated costs. Ann Epidemiol. 2000;10(8):509-515.
2. Bent S, Nallamothu BK, Simel DL, Fihn SD, Saint S. Does this woman have an acute uncomplicated urinary tract infection? JAMA. 2002;287(20):2701-2710.
3. ACOG Practice Bulletin #91: Treatment of urinary tract infections in nonpregnant women. Obstet Gynecol. 2008;111(3):785-794.
4. Hooton TM. Recurrent urinary tract infection in women. Int J Antimicrob Agents. 2001;17(4):259-268.
5. Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Disease Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52(5):e103-120.
6. Sutkin G, Alperin M, Meyn L, Wiesenfeld HC, Ellison R, Zyczynski HM. Symptomatic urinary tract infections after surgery for prolapse and/or incontinence. Int Urogynecol J. 2010;21(8):955-961.
7. Dieter AA, Amundsen CL, Visco AG, Siddiqui NY. Treatment for urinary tract infection after midurethral sling: a retrospective study comparing patients who receive short-term postoperative catheterization and patients who pass a void trial on the day of surgery. Female Pelvic Med Reconstr Surg. 2012;18(3):175-178.
8. Robinson D, Cardozo L. Estrogens and the lower urinary tract. Neurourol Urodyn. 2011;30(5):754-757.
9. Perrotta C, Aznar M, Mejia R, Albert X, Ng CW. Oestrogens for preventing recurrent urinary tract infection in postmenopausal women. Cochrane Database Syst Rev. 2008;(2):CD005131.-
10. Jepson RG, Craig JC. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev. 2008;(1):CD001321.-
1. Foxman B, Barolow R, D’Arcy H, Gillespie B, Sobel JD. Urinary tract infection: self-reported incidence and associated costs. Ann Epidemiol. 2000;10(8):509-515.
2. Bent S, Nallamothu BK, Simel DL, Fihn SD, Saint S. Does this woman have an acute uncomplicated urinary tract infection? JAMA. 2002;287(20):2701-2710.
3. ACOG Practice Bulletin #91: Treatment of urinary tract infections in nonpregnant women. Obstet Gynecol. 2008;111(3):785-794.
4. Hooton TM. Recurrent urinary tract infection in women. Int J Antimicrob Agents. 2001;17(4):259-268.
5. Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Disease Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52(5):e103-120.
6. Sutkin G, Alperin M, Meyn L, Wiesenfeld HC, Ellison R, Zyczynski HM. Symptomatic urinary tract infections after surgery for prolapse and/or incontinence. Int Urogynecol J. 2010;21(8):955-961.
7. Dieter AA, Amundsen CL, Visco AG, Siddiqui NY. Treatment for urinary tract infection after midurethral sling: a retrospective study comparing patients who receive short-term postoperative catheterization and patients who pass a void trial on the day of surgery. Female Pelvic Med Reconstr Surg. 2012;18(3):175-178.
8. Robinson D, Cardozo L. Estrogens and the lower urinary tract. Neurourol Urodyn. 2011;30(5):754-757.
9. Perrotta C, Aznar M, Mejia R, Albert X, Ng CW. Oestrogens for preventing recurrent urinary tract infection in postmenopausal women. Cochrane Database Syst Rev. 2008;(2):CD005131.-
10. Jepson RG, Craig JC. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev. 2008;(1):CD001321.-

