CHICAGO – The RET tyrosine kinase inhibitor vandetanib shows marked yet variable antitumor activity and toxicity in patients whose RET-positive non–small cell lung cancer (NSCLC) was unsuccessfully treated with chemotherapy, according to results of two small phase II trials presented at the annual meeting of the American Society of Clinical Oncology.
The overall response rates were 53% and 61% in two independent trials conducted by Dr. Takashi Seto of the National Kyushu Cancer Center, Japan, and by Dr. Se-Hoon Lee of Sungkyunkwan University, South Korea, respectively.
Compared to previous studies with other RET inhibitors, though all had small cohorts, there were many similarities in response rates. Progression-free survival was the most variable, but was much higher in the cabozantinib data reported at the 2015 ASCO annual meeting, according to moderator Dr. Karen Reckamp of the City of Hope Comprehensive Cancer Center, Duarte, Calif. “Vandetanib may have lower response rates than some of the others,” she said.
RET is a tyrosine kinase domain that fuses to and undergoes rearrangements with KIF5B and CCDC6 genes. This fusion and subsequent rearrangement results in ligand-dependent dimerization, which causes tumor growth. RET rearrangements were first identified in thyroid cancers.
“RET fusions were identified as new driver oncogenes of NSCLC in 2012 and observed in 1%-2% of all NSCLC,” Dr. Seto said. “Non–small cell lung cancer with RET rearrangement is regarded as a unique entity in terms of pathogenesis,” said Dr. Lee. There are currently multiple RET inhibitors in various stages of development.
In the trial headed by Dr. Seto, the Japanese genetic screening network was utilized to identify 34 NSCLC patients with RET rearrangements. Among the 34 patients, 17 met the eligibility requirements of having failed at least one prior chemotherapy treatment. Of those 17 patients, the median age was 59 years, 74% were female, all had adenocarcinomas, and 68% were nonsmokers.
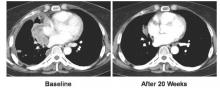
All 17 patients received vandetanib at a dose of 300 mg once daily. The overall response rate was 53% (90% confidence interval, 31-74), and the disease control rate was 88%. The median progression-free survival was 4.7 months (90% CI, 2.8-8.3).
There was a marked difference in overall response rate and progression-free survival among RET fusion subtypes. For CCDC6-RET, the overall response was 83% and the progression-free survival was 8.3 months. For KIF5B-RET, the overall response was 20% with a median progression-free survival of 2.9 months.
Dr. Seto noted that there was no known biological explanation for the observed discrepancy in response rate or survival.
Dr. Seto reported that the safety profile was similar to previous reports. Four patients ended treatment due to adverse events while 16 experienced dose interruptions due to treatment-related toxicities. The most common grade three and four toxicities were hypertension (58%), rash (16%), and diarrhea (11%).
In the trial headed by Dr. Lee, 18 patients with RET rearrangements (confirmed by fluorescent in situ hybridization) met the eligibility requirement of having failed platinum-based chemotherapy. The median age of the cohort was 55 years, and 33% were female.
Similar to Dr. Seto’s study, all 18 patients received vandetanib at 300 mg once daily. Of the 18 patient cohort, 17% achieved partial remission and 44% achieved stable disease. Seven patients had no remission or stabilization. There were no treatment-related mortalities or grade 4 adverse events. Two grade 3 adverse events were reported.
“Looking at these two studies together, I think the important thing about the characteristics you see [is] that the age range is very similar [with a] median in the 50’s,” commented Dr. Reckamp. “The male to female ratio is actually opposite in both so [this] can occur in both men and women. The smoking status, interestingly is similar in both, where about a third of patients were former smokers. Most of the patients had adenocarcinoma. Many of these patients were highly previously treated. Only the Seto group looked at RET fusion partner, which may be important in looking at efficacy for these agents.” Vandetanib is a “challenging drug to tolerate,” Dr. Reckamp also noted.
“Is there a preferred RET inhibitor in small cell lung cancer?” Dr. Reckamp asked. “There are many RET inhibitors approved for other cancer types at this point, and they are multitargeted tyrosine kinases. In small studies they have similar efficacy. Toxicities vary because of the off-target effects, and most of the [treatment] decisions were made based on potential toxicities rather than differing efficacy. So none is really differentiated as the best choice, and it is unlikely that we are going to have the trials to evaluate them head to head.”