User login
The prevalence of overweight and obesity has continued to increase over the past several decades.1,2 Data specific to the veteran population indicates prevalence rates are considerably higher than that of the general population, with overweight or obese veteran women and men at 68.4% and 73%, respectively.3-6
Traditional weight-loss programs (> 1,200 calories per day) fail to produce the degree of weight loss required to reduce surgical risk to a safe level for individuals with a body mass index (BMI) > 35. In contrast, intensive weight-loss programs using very low calorie diets (< 800 calories per day) combined with lifestyle modifications have been effective in generating considerable weight loss. These intensive weight-loss programs have also improved comorbid conditions such as insulin resistance, diabetes, hypertension, hyperlipidemia, and hypertriglyceridemia.7-10 Additionally, these programs have reduced surgical risks by decreasing operative time and reducing hospital length of stay.11,12 Weight loss not only improves surgical risk, but also impacts health care resource allocation.
Related: A Call to Action: Intensive Lifestyle Intervention Against Diabesity
Very low calorie diets have proven to be safe for preoperative weight loss. One prospective study evaluated the safety of a weight-reduction program with 30 patients with morbid obesity and whose elective surgery had been postponed due to patient’s weight status.13 Study participants lost ≥ 15% of their body weight. Subsequently, only 15 patients underwent surgery. Surgery was no longer indicated for 4 participants, 9 did not have surgery for reasons that were unreported, and 2 discontinued the diet. The authors suggested a very low calorie diet program is suitable for preoperative weight reduction in morbid obesity without significant complications.
Most investigations of preoperative very low calorie diets included only those patients awaiting bariatric surgery. These studies confirmed bariatric preoperative weight loss correlates with reduced postoperative complications.11,14,15 Additionally, the National Surgery Quality Improvement Program analysis of bariatric outcomes identified superobesity (defined as > 350 pounds) as a preoperative risk factor associated with postoperative complications.16
Related: Minimally Invasive Surgical Treatments for Obstructive Sleep Apnea
Obesity-related intra- and postoperative complications during elective surgeries are concerning because of the increasing number of obese surgical patients. With a growing aging population and rising rates of obesity, the number of total knee arthroplasties (TKAs) are increasing and now surpass total hip arthoplasties.17 The risk of intra-operative surgical complications is higher in patients with an elevated BMI than in those without, including higher blood transfusion requirements as a result of operative blood loss, difficulty in identifying anatomy leading to iatrogenic damage, or malalignment of the prosthesis.18-20
The risk of postoperative complications in obese patients is reported with rates as high as 32% and is primarily caused by superficial and deep surgical site infections and postoperative venous thromboembolic complications.18,19,21,22 One retrospective study evaluated prevalence, pattern, and severity of 7,721 postoperative complications in obese and nonobese surgical patients occurring within 30 days of surgery.23 Obese patients had significantly higher rates of postoperative myocardial infarction, wound infection, nerve injury, and urinary tract infections. The evidence suggests a higher risk of intra- and postoperative complications of TKA in obese patients, but there remains continued controversy in this area. Furthermore, there is a paucity of data regarding actual postponement or cancellation rate in elective procedures related to obesity. There is a lack of literature evaluating the impact of significant preoperative weight loss by nonsurgical interventions on outcomes of subsequent elective surgery.
The primary aim of this study was to determine whether a medically supervised, very low calorie weight loss program (Optifast, Nestlé Health Science) could safely and effectively produce the weight loss necessary to achieve surgical clearance at the Phoenix VA Health Care System (PVAHCS). The secondary aim was to determine whether a decrease in medication utilization during the diet intervention would offset the cost of the nutrition intervention.
Methods
This was a prospective, theory-based pilot study exploring weight status in response to a very low calorie diet, utilizing a quasi-experimental design. The PVAHCS Institutional Review Board approved the study.
Subjects participated in a medically supervised weight-loss program, including a liquid-meal replacement and weekly education administered by a registered dietitian. Twenty male and female veterans with obesity who had been denied medically indicated nonbariatric elective surgery due to obesity/morbid obesity and who met the study’s inclusion criteria were recruited.
Inclusion criteria included veterans aged 18 to 70 years, BMI > 30, and a nutritional consult for weight loss prior to elective (nonbariatric) surgery. The exclusion criteria included active medical conditions for which weight loss would be contraindicated, active alcohol or substance abuse, and psychological issues that could prevent compliance.
Screening Measures
A complete metabolic panel and prealbumin levels were assessed at baseline and used as indicators of overall electrolyte, hydration, and nutritional status. A complete blood count and thyroid stimulating test were used to rule out anemia, infections, and thyroid disorders. Because rapid weight loss may precipitate serious ventricular arrhythmias, an electrocardiogram was performed at baseline and after each 50 pounds of weight loss.
Intervention
Subjects consumed 5 Optifast packets per day (each mixed with 6-10 ounces of water), providing 800 calories per day (34% protein, 49% carbohydrate, and 17% fat; with 100% of the Dietary Reference Intake for vitamins and minerals). Participants were enrolled in the program for a minimum of 6 weeks and a maximum of 16 weeks.
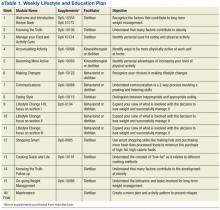
The research dietitian provided participants with weekly modules focused on lifestyle and education plans developed by Nestlé (eTable 1). Concentrating initially on behavior modification techniques and later introducing concepts dealing with food minimized distracting stimuli for participants. Subjects were required to consume an additional 2 quarts of noncaloric liquid to maintain hydration and were educated not to consume any liquids or solids containing calories. Subjects were required to maintain a diary on timing of Optifast and fluid consumption. Caffeine intake was limited (< 200 mg per day) because of its effects on fluid loss, cardiac stimulation, and irritation to the gastric mucosa. Participants served as their own controls.
Three weeks prior to completing the liquid diet, patients were instructed on a 3-week dietary transition plan, incorporating solid foods into their meal plan. Transition guidelines used the plate method, based on recommendations from the Dietary Guidelines for Americans to assist individuals in making healthy food choices, as patients were transitioned from the liquid to solid food.24 During transition week 1, subjects consumed 4 shakes per day and 1 meal (885 kcal per day); the second transition week consisted of 3 shakes and 2 meals (1,030 kcal per day); and the final transition week included 1 shake and 3 meals (1,080 kcal per day).
Outcome Measurements
Subjects were weighed weekly. To assess dietary compliance, participants were given a log to record daily intake of the liquid diet, additional liquids consumed, and physical activity. Bioelectrical impedance analysis was used pre- and postintervention to determine body composition, including body fat percentile.
Biochemical outcome measures affected by very low calorie diets (lipids, hemoglobin A1c, fasting glucose) were measured at baseline and every 4 weeks, and clinical outcomes were measured weekly. A BodyGem hand-held indirect calorimeter measured resting energy expenditure (REE) to monitor caloric needs during weight loss and to guide the transition to solid food. Medication use related to obesity was recorded weekly, and the total medication costs were calculated pre- and postintervention.
Medication Management
Blood pressure was monitored weekly. If a patient was prescribed warfarin, the primary care provider and pharmacist were alerted, because it was anticipated that dosages would change with weight loss. Patients on insulin had a 50% reduction on week 1, and subsequent adjustments were made at the discretion of the provider based on glucose monitoring. Oral hypoglycemic agent adjustments were also made based on glucose monitoring.
All patients were prescribed ursodeoxycholic acid 300 mg twice a day to reduce the risk of gallstone formation.25 Psyllium was provided to prevent constipation, a commonly reported adverse event (AE) of Optifast. Over-the-counter lactase additives were recommended for patients with known lactose intolerance. As recommended by the Optifast program, patients were instructed to avoid nonsteroidal anti-inflammatory drugs, aspirin and laxatives, amphetamines/stimulants, pseudoephedrine, and sugar-containing medications. Medications were adjusted according to clinical practices.
Statistical Analysis
Distributions of continuous measurements at the beginning (baseline) and end (follow-up) of the study and changes in these measurements (follow-up minus baseline) were tested for normality using the Shapiro-Wilk test. Where both baseline and follow-up values of a given measurement were distributed normally, both baseline and follow-up values are shown as mean ± SD (Table 1). If ≥ 1 baseline and follow-up measurements were not normally distributed, both baseline and follow-up measurements are shown as median with interquartile range. Changes in measurements are either shown as mean ± SD or median and interquartile range as appropriate. Significance of the former changes was evaluated with a paired t test; whereas the latter changes were evaluated with a Wilcoxon signed rank test.
Results
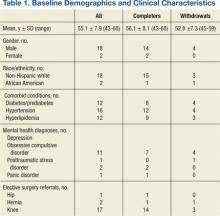
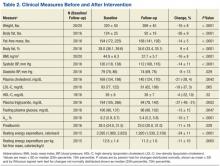
A total of 65 veterans were referred to the program. Eighteen male and 2 female veterans ranging from ages 43 to 68 years, with a mean age of 55 years (SD ± 7.9) consented to participate; 16 (80%) completed the study. Four subjects dropped out; 1 due to lactose intolerance uncontrolled by lactase, 1 due to exacerbation of obsessive compulsive disorder, 1 moved out of state, and 1 opted out before beginning the dietary intervention. Comorbidities included psychiatric diagnoses (80%), hypertension (80%), diabetes (60%), and hyperlipidemia (60%). Baseline characteristics were not different between those who withdrew and those who completed the study (Table 1). Study outcomes based on intent-to-treat analysis are presented in Table 2.
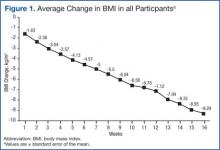
BMI decreased linearly during the intervention (Figure 1). In 10 subjects, the change in BMI postintervention was both statistically (-16 ± 8%, P < .0001) and clinically significant and sufficient for surgical clearance. Eight (40%) had surgery and 2 (10%) no longer needed surgery due to self-reported improved quality of life and decreased pain. Despite the clinically and statistically significant weight loss, 14.5% of the weight lost was fat-free mass; decrease in body fat was 9% ± 4% (P < .0001).
All study subjects consumed 5 Optifast packets per day for at least 10 weeks and no longer than 16 weeks. Of the participants who completed the intervention, the majority elected to continue the intervention time to 16 weeks; however 1 participant went to week 10 and 2 participants completed through week 13. Nonadherence in this protocol was defined as > 2 weeks of weight gain. Two participants gained weight for 6 and 7 weeks, respectively.
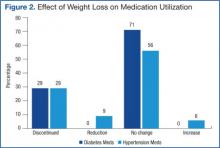
Mean systolic blood pressure, plasma triglyceride and fasting glucose levels, A1c, and REE levels decreased significantly postintervention. Additionally, patients experienced either dose reduction or discontinuation of diabetes or hypertension medication use postintervention (Figure 2). Discontinued diabetes medications included rosiglitazone (n = 1), glyburide (n = 1), and metformin (n = 2). Discontinued or reduced antihypertensives included furosemide (n = 1), thiazides (n = 3), beta blockers (n = 1), angiotensin-converting enzyme inhibitors (n = 4), calcium channel blockers (n = 2), and angiotensin II receptor antagonists (n = 2).
Discussion
To the authors’ knowledge, this was the first study using a low calorie liquid diet to achieve weight loss to qualify for nonbariatric elective surgery. This diet provides an alternative intervention for individuals who would otherwise be denied elective surgery due to extreme obesity. Eighty percent of participants completed 10 to 16 weeks of the 800 calorie liquid diet plan with significant weight loss of 16 BMI ± 8%. The intervention was well tolerated without significant AEs.
It is difficult to compare these results to prior studies, as the target populations differ. Previous studies utilizing calorie levels < 800 calories per day included mostly women and consequently, their preintervention weights were lower than in the current study population.10 This study population was predominately older males with a high prevalence of comorbid medical and psychiatric conditions. Despite these demographic and clinical differences, improvements in biochemistries were similar to those demonstrated previously.8-10 The observations for beneficial changes in cardiovascular and glycemic risk factors and reduced medication use related to weight loss and calorie control are consistent with previous results.8-10
Related: Moral Questions Surrounding Bariatric Surgery
To the authors’ knowledge, REE has not been reported in earlier investigations of very low calorie diet interventions. This study found significant decreases in REE, which was measured pre- and postintervention. Participants were given postintervention REE value and individualized meal plans were developed from this number. An interesting and unexpected finding was that this number seemed to provide useful reinforcement for patients as they transitioned to solid food. This may have helped improve adherence to meal plans. Despite concerns regarding possible weight gain, the weight loss continued at a similar rate during the transition, demonstrating that continued weight loss can occur with a combination of food and liquid diet.
The need for elective surgery may have increased motivation to adhere to this weight-loss program. The dropout rate was 20%; lower than previous studies using very low calorie diets and substantially better than traditional weight-loss programs.8,9
An unexpected finding was that 10% of participants who qualified for knee replacement surgery chose to postpone surgery due to decreased pain and improved quality of life. Over the past 20 years, the estimated cost of 1 TKA was $15,000 with an estimated $9 billion spent annually for this procedure in the U.S.26 Importantly, obesity increases the risk of TKA revision surgeries, which are both expensive (average cost of Medicare-covered TKA revision surgeries is $73,696) and projected to increase 66% over the next 25 years.27 Weight loss prior to surgery not only may decrease risk for revisions of TKA, but in some cases also may delay or eliminate the need for surgery.
Although there are significant costs associated with certain weight loss programs, the savings associated with reducing the need for surgery would be substantially greater than that associated with the dietary intervention. The estimated private sector cost of an 18-week weight-loss program (12-week liquid with 6-week transition) is $3,500 per participant. This study program was estimated to cost $2,400 per participant for the 16-week (13-week liquid diet and 3-week transition) program. Patients with obesity awaiting orthopedic, gastrointestinal, or neurosurgery were often referred for bariatric surgery to obtain weight loss. Bariatric surgery averages $17,000 to $26,000, which is more expensive than this diet program.28
The majority of AEs observed in this intervention were expected and similar to other studies.10 Among the 20 participants, 18 experienced a total of 60 AEs, of which 38 (63%) were considered to be study-related. Although constipation was a known AE, 25% of participants subjectively complained of decrease in frequency of bowel movements. The 2 most frequent and unanticipated AEs were increased blood urea nitrogen/ creatinine (n = 9) and reduced sodium (n = 7).
Nonadherence was often related but not limited to the following: inappropriate social cues for eating, lack of social support, sabotage by family or peers, filling an emotional void with food, and/or psychological eating related to depression and posttraumatic stress disorder. Prior to starting a similar intervention, a complete mental health assessment for individuals with known or suspected mental health diagnoses seems warranted.
Conclusion
The study limitations are its small and predominantly male sample size and lack of a randomized control. Nonetheless, this study demonstrated the feasibility of the medically supervised weight loss program to obtain the necessary weight loss in 50% of the veterans (with higher comorbidities and more advanced age). Because of the results of this investigation, the authors have initiated a randomized controlled trial utilizing this intervention. The Optifast program had a high success rate, was cost-effective, and may obviate the need for surgery.
Acknowledgements
This work was supported by pilot funding from the Department Veterans Affairs awarded on a competitive basis to Julie Kurtz. Additional support was provided by the Department of Veterans Affairs. The authors would like to acknowledge the contribution of Julie Stoneroad-Vedda, PA-C, MPAS, Northern Arizona VA Health Care System in Prescott.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999-2000. JAMA. 2002;288(14):1723-1727.
2. Ogden CL, Flegal KM, Carroll MD, Johnson CL. Prevalence and trends in overweight among US children and adolescents, 1999-2000. JAMA. 2002;288(14):1728-1732.
3. Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. JAMA. 2010;303(3):235-241.
4. Ogden CL, Carroll MD, Curtin LR, Lamb MM, Flegal KM. Prevalence of high body mass index in US children and adolescents, 2007-2008. JAMA. 2010;303(3):242-249.
5. Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA. 2012;307(5):491-497.
6. Das SR, Kinsinger LS, Yancy WS Jr, et al. Obesity prevalence among veterans at Veterans Affairs medical facilities. Am J Prev Med. 2005;28(3):291-294.
7. Drawert S, Bedford K, Largent D. Change in glucose, blood pressure, and cholesterol with weight loss in medically obese patients. Obes Res. 1996;4(suppl 1):67S.
8. Kirschner MA, Schneider G, Ertel NH, Gorman J. An eight-year experience with very-low-calorie formula diet for control of major obesity. Int J Obes. 1988;12(1):69-80.
9. Wadden TA, Foster GD, Letizia KA, Stunkard AJ. A multicenter evaluation of a proprietary weight reduction program for the treatment of marked obesity. Arch Intern Med. 1992;152(5):961-966.
10. Anderson JW, Brinkman-Kaplan VL, Lee H, Wood CL. Relationship of weight loss to cardiovascular risk factors in morbidly obese individuals. J Am Coll Nutr. 1994;13(3):256-261.
11. Huerta S, Dredar S, Hayden E, et al. Preoperative weight loss decreases the operative time of gastric bypass at a Veterans Administration hospital. Obes Surg. 2008;18(5):508-512.
12. Still CD, Benotti P, Wood GC, et al. Outcomes of preoperative weight loss in high-risk patients undergoing gastric bypass surgery. Arch Surg. 2007;142(10):994-998; discussion 999.
13. Pekkarinen, T, Mustajoki P. Use of a very low- calorie diet in preoperative weight loss: Efficacy and safety. Obes Res. 1997;5(6):595-602.
14. Van Nieuwenhove Y, Dambrauskas Z, Campillo-Soto A, et al. Preoperative very low-calorie diet and operative outcome after laparoscopic gastric bypass: A randomized multicenter study. Arch Surg. 2011;146(11):1300-1305.
15. Hauser DL, Titchner RL, Wilson MA, Eid GM. Long-term outcomes of laparoscopic Roux-en-Y gastric bypass in US veterans. Obes Surg. 2010;20(3):283-289.
16. Livingston EH, Arterburn D, Schifftner TL, Henderson WG, DePalma RG. National Surgical Quality Improvement Program analysis of bariatric operations: Modifiable risk factors contribute to bariatric surgical adverse outcomes. J Am Coll Surg. 2006;203(5):625-633.
17. National Joint Registry. 11th Annual Report 2014: National Joint Registry for England and Wales and Northern Ireland. Hemel Hempsted, England: National Joint Registry; 2014.
18. Pritchett JW, Bortel DT. Knee replacement in morbidly obese women. Surg Gynecol Obstet. 1991;173(2):119-122.
19. Winiarsky R, Barth P, Lotke P. Total knee arthroplasty in morbidly obese patients. J Bone Joint Surg Am. 1998;80(12):1770-1774.
20. Krushell RJ, Fingeroth RJ. Primary total knee arthroplasty in morbidly obese patients: A 5- to 14-year follow-up study. J Arthroplasty. 2007;22 (6 suppl 2):77-80.
21. Sridhar MS, Jarrett CD, Xerogeanes JW, Labib SA. Obesity and symptomatic osteoarthritis of the knee. J Bone Joint Surg Br. 2012;94(4):433-440.
22. Amin AK, Clayton RA, Patton JT, Gaston M, Cook RE, Brenkel IJ. Total knee replacement in morbidly obese patients: Results of a prospective, matched study. J Bone Joint Surg Br. 2006;88(10):1321-1326.
23. Bamgbade OA, Rutter TW, Nafiu OO, Dorje P. Postoperative complications in obese and nonobese patients. World J Surg. 2007;31(3):556-560; discussion 561.
24. U.S. Department of Agriculture, U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2010. 7th ed. Washington, DC: U.S. Government Printing Office; 2010.
25. Kamrath RO, Plummer LJ, Sadur CN, et al. Cholelithiasis in patients treated with a very-low-calorie diet. Am J Clin Nutr. 1992;56(suppl 1):255S-257S.
26. Cram P, Lu X, Kates SL, Singh JA, Li Y, Wolf BR. Total knee arthroplasty volume, utilization, and outcomes among Medicare beneficiaries, 1991-2010. JAMA. 2012;308(12):1227-1236.
27. Lavernia C, Lee DJ, Hernandez VH. The increasing financial burden of knee revision surgery in the United States. Clin Orthop Relat Res. 2006;446: 221-226.
28. Cremieux PY, Buchwald H, Shikora SA, Ghosh A, Yang HE, Buessing M. A study on the economic impact of bariatric surgery. Am J Manag Care. 2008;14(9):589-596.
The prevalence of overweight and obesity has continued to increase over the past several decades.1,2 Data specific to the veteran population indicates prevalence rates are considerably higher than that of the general population, with overweight or obese veteran women and men at 68.4% and 73%, respectively.3-6
Traditional weight-loss programs (> 1,200 calories per day) fail to produce the degree of weight loss required to reduce surgical risk to a safe level for individuals with a body mass index (BMI) > 35. In contrast, intensive weight-loss programs using very low calorie diets (< 800 calories per day) combined with lifestyle modifications have been effective in generating considerable weight loss. These intensive weight-loss programs have also improved comorbid conditions such as insulin resistance, diabetes, hypertension, hyperlipidemia, and hypertriglyceridemia.7-10 Additionally, these programs have reduced surgical risks by decreasing operative time and reducing hospital length of stay.11,12 Weight loss not only improves surgical risk, but also impacts health care resource allocation.
Related: A Call to Action: Intensive Lifestyle Intervention Against Diabesity
Very low calorie diets have proven to be safe for preoperative weight loss. One prospective study evaluated the safety of a weight-reduction program with 30 patients with morbid obesity and whose elective surgery had been postponed due to patient’s weight status.13 Study participants lost ≥ 15% of their body weight. Subsequently, only 15 patients underwent surgery. Surgery was no longer indicated for 4 participants, 9 did not have surgery for reasons that were unreported, and 2 discontinued the diet. The authors suggested a very low calorie diet program is suitable for preoperative weight reduction in morbid obesity without significant complications.
Most investigations of preoperative very low calorie diets included only those patients awaiting bariatric surgery. These studies confirmed bariatric preoperative weight loss correlates with reduced postoperative complications.11,14,15 Additionally, the National Surgery Quality Improvement Program analysis of bariatric outcomes identified superobesity (defined as > 350 pounds) as a preoperative risk factor associated with postoperative complications.16
Related: Minimally Invasive Surgical Treatments for Obstructive Sleep Apnea
Obesity-related intra- and postoperative complications during elective surgeries are concerning because of the increasing number of obese surgical patients. With a growing aging population and rising rates of obesity, the number of total knee arthroplasties (TKAs) are increasing and now surpass total hip arthoplasties.17 The risk of intra-operative surgical complications is higher in patients with an elevated BMI than in those without, including higher blood transfusion requirements as a result of operative blood loss, difficulty in identifying anatomy leading to iatrogenic damage, or malalignment of the prosthesis.18-20
The risk of postoperative complications in obese patients is reported with rates as high as 32% and is primarily caused by superficial and deep surgical site infections and postoperative venous thromboembolic complications.18,19,21,22 One retrospective study evaluated prevalence, pattern, and severity of 7,721 postoperative complications in obese and nonobese surgical patients occurring within 30 days of surgery.23 Obese patients had significantly higher rates of postoperative myocardial infarction, wound infection, nerve injury, and urinary tract infections. The evidence suggests a higher risk of intra- and postoperative complications of TKA in obese patients, but there remains continued controversy in this area. Furthermore, there is a paucity of data regarding actual postponement or cancellation rate in elective procedures related to obesity. There is a lack of literature evaluating the impact of significant preoperative weight loss by nonsurgical interventions on outcomes of subsequent elective surgery.
The primary aim of this study was to determine whether a medically supervised, very low calorie weight loss program (Optifast, Nestlé Health Science) could safely and effectively produce the weight loss necessary to achieve surgical clearance at the Phoenix VA Health Care System (PVAHCS). The secondary aim was to determine whether a decrease in medication utilization during the diet intervention would offset the cost of the nutrition intervention.
Methods
This was a prospective, theory-based pilot study exploring weight status in response to a very low calorie diet, utilizing a quasi-experimental design. The PVAHCS Institutional Review Board approved the study.
Subjects participated in a medically supervised weight-loss program, including a liquid-meal replacement and weekly education administered by a registered dietitian. Twenty male and female veterans with obesity who had been denied medically indicated nonbariatric elective surgery due to obesity/morbid obesity and who met the study’s inclusion criteria were recruited.
Inclusion criteria included veterans aged 18 to 70 years, BMI > 30, and a nutritional consult for weight loss prior to elective (nonbariatric) surgery. The exclusion criteria included active medical conditions for which weight loss would be contraindicated, active alcohol or substance abuse, and psychological issues that could prevent compliance.
Screening Measures
A complete metabolic panel and prealbumin levels were assessed at baseline and used as indicators of overall electrolyte, hydration, and nutritional status. A complete blood count and thyroid stimulating test were used to rule out anemia, infections, and thyroid disorders. Because rapid weight loss may precipitate serious ventricular arrhythmias, an electrocardiogram was performed at baseline and after each 50 pounds of weight loss.
Intervention
Subjects consumed 5 Optifast packets per day (each mixed with 6-10 ounces of water), providing 800 calories per day (34% protein, 49% carbohydrate, and 17% fat; with 100% of the Dietary Reference Intake for vitamins and minerals). Participants were enrolled in the program for a minimum of 6 weeks and a maximum of 16 weeks.
The research dietitian provided participants with weekly modules focused on lifestyle and education plans developed by Nestlé (eTable 1). Concentrating initially on behavior modification techniques and later introducing concepts dealing with food minimized distracting stimuli for participants. Subjects were required to consume an additional 2 quarts of noncaloric liquid to maintain hydration and were educated not to consume any liquids or solids containing calories. Subjects were required to maintain a diary on timing of Optifast and fluid consumption. Caffeine intake was limited (< 200 mg per day) because of its effects on fluid loss, cardiac stimulation, and irritation to the gastric mucosa. Participants served as their own controls.
Three weeks prior to completing the liquid diet, patients were instructed on a 3-week dietary transition plan, incorporating solid foods into their meal plan. Transition guidelines used the plate method, based on recommendations from the Dietary Guidelines for Americans to assist individuals in making healthy food choices, as patients were transitioned from the liquid to solid food.24 During transition week 1, subjects consumed 4 shakes per day and 1 meal (885 kcal per day); the second transition week consisted of 3 shakes and 2 meals (1,030 kcal per day); and the final transition week included 1 shake and 3 meals (1,080 kcal per day).
Outcome Measurements
Subjects were weighed weekly. To assess dietary compliance, participants were given a log to record daily intake of the liquid diet, additional liquids consumed, and physical activity. Bioelectrical impedance analysis was used pre- and postintervention to determine body composition, including body fat percentile.
Biochemical outcome measures affected by very low calorie diets (lipids, hemoglobin A1c, fasting glucose) were measured at baseline and every 4 weeks, and clinical outcomes were measured weekly. A BodyGem hand-held indirect calorimeter measured resting energy expenditure (REE) to monitor caloric needs during weight loss and to guide the transition to solid food. Medication use related to obesity was recorded weekly, and the total medication costs were calculated pre- and postintervention.
Medication Management
Blood pressure was monitored weekly. If a patient was prescribed warfarin, the primary care provider and pharmacist were alerted, because it was anticipated that dosages would change with weight loss. Patients on insulin had a 50% reduction on week 1, and subsequent adjustments were made at the discretion of the provider based on glucose monitoring. Oral hypoglycemic agent adjustments were also made based on glucose monitoring.
All patients were prescribed ursodeoxycholic acid 300 mg twice a day to reduce the risk of gallstone formation.25 Psyllium was provided to prevent constipation, a commonly reported adverse event (AE) of Optifast. Over-the-counter lactase additives were recommended for patients with known lactose intolerance. As recommended by the Optifast program, patients were instructed to avoid nonsteroidal anti-inflammatory drugs, aspirin and laxatives, amphetamines/stimulants, pseudoephedrine, and sugar-containing medications. Medications were adjusted according to clinical practices.
Statistical Analysis
Distributions of continuous measurements at the beginning (baseline) and end (follow-up) of the study and changes in these measurements (follow-up minus baseline) were tested for normality using the Shapiro-Wilk test. Where both baseline and follow-up values of a given measurement were distributed normally, both baseline and follow-up values are shown as mean ± SD (Table 1). If ≥ 1 baseline and follow-up measurements were not normally distributed, both baseline and follow-up measurements are shown as median with interquartile range. Changes in measurements are either shown as mean ± SD or median and interquartile range as appropriate. Significance of the former changes was evaluated with a paired t test; whereas the latter changes were evaluated with a Wilcoxon signed rank test.
Results
A total of 65 veterans were referred to the program. Eighteen male and 2 female veterans ranging from ages 43 to 68 years, with a mean age of 55 years (SD ± 7.9) consented to participate; 16 (80%) completed the study. Four subjects dropped out; 1 due to lactose intolerance uncontrolled by lactase, 1 due to exacerbation of obsessive compulsive disorder, 1 moved out of state, and 1 opted out before beginning the dietary intervention. Comorbidities included psychiatric diagnoses (80%), hypertension (80%), diabetes (60%), and hyperlipidemia (60%). Baseline characteristics were not different between those who withdrew and those who completed the study (Table 1). Study outcomes based on intent-to-treat analysis are presented in Table 2.
BMI decreased linearly during the intervention (Figure 1). In 10 subjects, the change in BMI postintervention was both statistically (-16 ± 8%, P < .0001) and clinically significant and sufficient for surgical clearance. Eight (40%) had surgery and 2 (10%) no longer needed surgery due to self-reported improved quality of life and decreased pain. Despite the clinically and statistically significant weight loss, 14.5% of the weight lost was fat-free mass; decrease in body fat was 9% ± 4% (P < .0001).
All study subjects consumed 5 Optifast packets per day for at least 10 weeks and no longer than 16 weeks. Of the participants who completed the intervention, the majority elected to continue the intervention time to 16 weeks; however 1 participant went to week 10 and 2 participants completed through week 13. Nonadherence in this protocol was defined as > 2 weeks of weight gain. Two participants gained weight for 6 and 7 weeks, respectively.
Mean systolic blood pressure, plasma triglyceride and fasting glucose levels, A1c, and REE levels decreased significantly postintervention. Additionally, patients experienced either dose reduction or discontinuation of diabetes or hypertension medication use postintervention (Figure 2). Discontinued diabetes medications included rosiglitazone (n = 1), glyburide (n = 1), and metformin (n = 2). Discontinued or reduced antihypertensives included furosemide (n = 1), thiazides (n = 3), beta blockers (n = 1), angiotensin-converting enzyme inhibitors (n = 4), calcium channel blockers (n = 2), and angiotensin II receptor antagonists (n = 2).
Discussion
To the authors’ knowledge, this was the first study using a low calorie liquid diet to achieve weight loss to qualify for nonbariatric elective surgery. This diet provides an alternative intervention for individuals who would otherwise be denied elective surgery due to extreme obesity. Eighty percent of participants completed 10 to 16 weeks of the 800 calorie liquid diet plan with significant weight loss of 16 BMI ± 8%. The intervention was well tolerated without significant AEs.
It is difficult to compare these results to prior studies, as the target populations differ. Previous studies utilizing calorie levels < 800 calories per day included mostly women and consequently, their preintervention weights were lower than in the current study population.10 This study population was predominately older males with a high prevalence of comorbid medical and psychiatric conditions. Despite these demographic and clinical differences, improvements in biochemistries were similar to those demonstrated previously.8-10 The observations for beneficial changes in cardiovascular and glycemic risk factors and reduced medication use related to weight loss and calorie control are consistent with previous results.8-10
Related: Moral Questions Surrounding Bariatric Surgery
To the authors’ knowledge, REE has not been reported in earlier investigations of very low calorie diet interventions. This study found significant decreases in REE, which was measured pre- and postintervention. Participants were given postintervention REE value and individualized meal plans were developed from this number. An interesting and unexpected finding was that this number seemed to provide useful reinforcement for patients as they transitioned to solid food. This may have helped improve adherence to meal plans. Despite concerns regarding possible weight gain, the weight loss continued at a similar rate during the transition, demonstrating that continued weight loss can occur with a combination of food and liquid diet.
The need for elective surgery may have increased motivation to adhere to this weight-loss program. The dropout rate was 20%; lower than previous studies using very low calorie diets and substantially better than traditional weight-loss programs.8,9
An unexpected finding was that 10% of participants who qualified for knee replacement surgery chose to postpone surgery due to decreased pain and improved quality of life. Over the past 20 years, the estimated cost of 1 TKA was $15,000 with an estimated $9 billion spent annually for this procedure in the U.S.26 Importantly, obesity increases the risk of TKA revision surgeries, which are both expensive (average cost of Medicare-covered TKA revision surgeries is $73,696) and projected to increase 66% over the next 25 years.27 Weight loss prior to surgery not only may decrease risk for revisions of TKA, but in some cases also may delay or eliminate the need for surgery.
Although there are significant costs associated with certain weight loss programs, the savings associated with reducing the need for surgery would be substantially greater than that associated with the dietary intervention. The estimated private sector cost of an 18-week weight-loss program (12-week liquid with 6-week transition) is $3,500 per participant. This study program was estimated to cost $2,400 per participant for the 16-week (13-week liquid diet and 3-week transition) program. Patients with obesity awaiting orthopedic, gastrointestinal, or neurosurgery were often referred for bariatric surgery to obtain weight loss. Bariatric surgery averages $17,000 to $26,000, which is more expensive than this diet program.28
The majority of AEs observed in this intervention were expected and similar to other studies.10 Among the 20 participants, 18 experienced a total of 60 AEs, of which 38 (63%) were considered to be study-related. Although constipation was a known AE, 25% of participants subjectively complained of decrease in frequency of bowel movements. The 2 most frequent and unanticipated AEs were increased blood urea nitrogen/ creatinine (n = 9) and reduced sodium (n = 7).
Nonadherence was often related but not limited to the following: inappropriate social cues for eating, lack of social support, sabotage by family or peers, filling an emotional void with food, and/or psychological eating related to depression and posttraumatic stress disorder. Prior to starting a similar intervention, a complete mental health assessment for individuals with known or suspected mental health diagnoses seems warranted.
Conclusion
The study limitations are its small and predominantly male sample size and lack of a randomized control. Nonetheless, this study demonstrated the feasibility of the medically supervised weight loss program to obtain the necessary weight loss in 50% of the veterans (with higher comorbidities and more advanced age). Because of the results of this investigation, the authors have initiated a randomized controlled trial utilizing this intervention. The Optifast program had a high success rate, was cost-effective, and may obviate the need for surgery.
Acknowledgements
This work was supported by pilot funding from the Department Veterans Affairs awarded on a competitive basis to Julie Kurtz. Additional support was provided by the Department of Veterans Affairs. The authors would like to acknowledge the contribution of Julie Stoneroad-Vedda, PA-C, MPAS, Northern Arizona VA Health Care System in Prescott.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
The prevalence of overweight and obesity has continued to increase over the past several decades.1,2 Data specific to the veteran population indicates prevalence rates are considerably higher than that of the general population, with overweight or obese veteran women and men at 68.4% and 73%, respectively.3-6
Traditional weight-loss programs (> 1,200 calories per day) fail to produce the degree of weight loss required to reduce surgical risk to a safe level for individuals with a body mass index (BMI) > 35. In contrast, intensive weight-loss programs using very low calorie diets (< 800 calories per day) combined with lifestyle modifications have been effective in generating considerable weight loss. These intensive weight-loss programs have also improved comorbid conditions such as insulin resistance, diabetes, hypertension, hyperlipidemia, and hypertriglyceridemia.7-10 Additionally, these programs have reduced surgical risks by decreasing operative time and reducing hospital length of stay.11,12 Weight loss not only improves surgical risk, but also impacts health care resource allocation.
Related: A Call to Action: Intensive Lifestyle Intervention Against Diabesity
Very low calorie diets have proven to be safe for preoperative weight loss. One prospective study evaluated the safety of a weight-reduction program with 30 patients with morbid obesity and whose elective surgery had been postponed due to patient’s weight status.13 Study participants lost ≥ 15% of their body weight. Subsequently, only 15 patients underwent surgery. Surgery was no longer indicated for 4 participants, 9 did not have surgery for reasons that were unreported, and 2 discontinued the diet. The authors suggested a very low calorie diet program is suitable for preoperative weight reduction in morbid obesity without significant complications.
Most investigations of preoperative very low calorie diets included only those patients awaiting bariatric surgery. These studies confirmed bariatric preoperative weight loss correlates with reduced postoperative complications.11,14,15 Additionally, the National Surgery Quality Improvement Program analysis of bariatric outcomes identified superobesity (defined as > 350 pounds) as a preoperative risk factor associated with postoperative complications.16
Related: Minimally Invasive Surgical Treatments for Obstructive Sleep Apnea
Obesity-related intra- and postoperative complications during elective surgeries are concerning because of the increasing number of obese surgical patients. With a growing aging population and rising rates of obesity, the number of total knee arthroplasties (TKAs) are increasing and now surpass total hip arthoplasties.17 The risk of intra-operative surgical complications is higher in patients with an elevated BMI than in those without, including higher blood transfusion requirements as a result of operative blood loss, difficulty in identifying anatomy leading to iatrogenic damage, or malalignment of the prosthesis.18-20
The risk of postoperative complications in obese patients is reported with rates as high as 32% and is primarily caused by superficial and deep surgical site infections and postoperative venous thromboembolic complications.18,19,21,22 One retrospective study evaluated prevalence, pattern, and severity of 7,721 postoperative complications in obese and nonobese surgical patients occurring within 30 days of surgery.23 Obese patients had significantly higher rates of postoperative myocardial infarction, wound infection, nerve injury, and urinary tract infections. The evidence suggests a higher risk of intra- and postoperative complications of TKA in obese patients, but there remains continued controversy in this area. Furthermore, there is a paucity of data regarding actual postponement or cancellation rate in elective procedures related to obesity. There is a lack of literature evaluating the impact of significant preoperative weight loss by nonsurgical interventions on outcomes of subsequent elective surgery.
The primary aim of this study was to determine whether a medically supervised, very low calorie weight loss program (Optifast, Nestlé Health Science) could safely and effectively produce the weight loss necessary to achieve surgical clearance at the Phoenix VA Health Care System (PVAHCS). The secondary aim was to determine whether a decrease in medication utilization during the diet intervention would offset the cost of the nutrition intervention.
Methods
This was a prospective, theory-based pilot study exploring weight status in response to a very low calorie diet, utilizing a quasi-experimental design. The PVAHCS Institutional Review Board approved the study.
Subjects participated in a medically supervised weight-loss program, including a liquid-meal replacement and weekly education administered by a registered dietitian. Twenty male and female veterans with obesity who had been denied medically indicated nonbariatric elective surgery due to obesity/morbid obesity and who met the study’s inclusion criteria were recruited.
Inclusion criteria included veterans aged 18 to 70 years, BMI > 30, and a nutritional consult for weight loss prior to elective (nonbariatric) surgery. The exclusion criteria included active medical conditions for which weight loss would be contraindicated, active alcohol or substance abuse, and psychological issues that could prevent compliance.
Screening Measures
A complete metabolic panel and prealbumin levels were assessed at baseline and used as indicators of overall electrolyte, hydration, and nutritional status. A complete blood count and thyroid stimulating test were used to rule out anemia, infections, and thyroid disorders. Because rapid weight loss may precipitate serious ventricular arrhythmias, an electrocardiogram was performed at baseline and after each 50 pounds of weight loss.
Intervention
Subjects consumed 5 Optifast packets per day (each mixed with 6-10 ounces of water), providing 800 calories per day (34% protein, 49% carbohydrate, and 17% fat; with 100% of the Dietary Reference Intake for vitamins and minerals). Participants were enrolled in the program for a minimum of 6 weeks and a maximum of 16 weeks.
The research dietitian provided participants with weekly modules focused on lifestyle and education plans developed by Nestlé (eTable 1). Concentrating initially on behavior modification techniques and later introducing concepts dealing with food minimized distracting stimuli for participants. Subjects were required to consume an additional 2 quarts of noncaloric liquid to maintain hydration and were educated not to consume any liquids or solids containing calories. Subjects were required to maintain a diary on timing of Optifast and fluid consumption. Caffeine intake was limited (< 200 mg per day) because of its effects on fluid loss, cardiac stimulation, and irritation to the gastric mucosa. Participants served as their own controls.
Three weeks prior to completing the liquid diet, patients were instructed on a 3-week dietary transition plan, incorporating solid foods into their meal plan. Transition guidelines used the plate method, based on recommendations from the Dietary Guidelines for Americans to assist individuals in making healthy food choices, as patients were transitioned from the liquid to solid food.24 During transition week 1, subjects consumed 4 shakes per day and 1 meal (885 kcal per day); the second transition week consisted of 3 shakes and 2 meals (1,030 kcal per day); and the final transition week included 1 shake and 3 meals (1,080 kcal per day).
Outcome Measurements
Subjects were weighed weekly. To assess dietary compliance, participants were given a log to record daily intake of the liquid diet, additional liquids consumed, and physical activity. Bioelectrical impedance analysis was used pre- and postintervention to determine body composition, including body fat percentile.
Biochemical outcome measures affected by very low calorie diets (lipids, hemoglobin A1c, fasting glucose) were measured at baseline and every 4 weeks, and clinical outcomes were measured weekly. A BodyGem hand-held indirect calorimeter measured resting energy expenditure (REE) to monitor caloric needs during weight loss and to guide the transition to solid food. Medication use related to obesity was recorded weekly, and the total medication costs were calculated pre- and postintervention.
Medication Management
Blood pressure was monitored weekly. If a patient was prescribed warfarin, the primary care provider and pharmacist were alerted, because it was anticipated that dosages would change with weight loss. Patients on insulin had a 50% reduction on week 1, and subsequent adjustments were made at the discretion of the provider based on glucose monitoring. Oral hypoglycemic agent adjustments were also made based on glucose monitoring.
All patients were prescribed ursodeoxycholic acid 300 mg twice a day to reduce the risk of gallstone formation.25 Psyllium was provided to prevent constipation, a commonly reported adverse event (AE) of Optifast. Over-the-counter lactase additives were recommended for patients with known lactose intolerance. As recommended by the Optifast program, patients were instructed to avoid nonsteroidal anti-inflammatory drugs, aspirin and laxatives, amphetamines/stimulants, pseudoephedrine, and sugar-containing medications. Medications were adjusted according to clinical practices.
Statistical Analysis
Distributions of continuous measurements at the beginning (baseline) and end (follow-up) of the study and changes in these measurements (follow-up minus baseline) were tested for normality using the Shapiro-Wilk test. Where both baseline and follow-up values of a given measurement were distributed normally, both baseline and follow-up values are shown as mean ± SD (Table 1). If ≥ 1 baseline and follow-up measurements were not normally distributed, both baseline and follow-up measurements are shown as median with interquartile range. Changes in measurements are either shown as mean ± SD or median and interquartile range as appropriate. Significance of the former changes was evaluated with a paired t test; whereas the latter changes were evaluated with a Wilcoxon signed rank test.
Results
A total of 65 veterans were referred to the program. Eighteen male and 2 female veterans ranging from ages 43 to 68 years, with a mean age of 55 years (SD ± 7.9) consented to participate; 16 (80%) completed the study. Four subjects dropped out; 1 due to lactose intolerance uncontrolled by lactase, 1 due to exacerbation of obsessive compulsive disorder, 1 moved out of state, and 1 opted out before beginning the dietary intervention. Comorbidities included psychiatric diagnoses (80%), hypertension (80%), diabetes (60%), and hyperlipidemia (60%). Baseline characteristics were not different between those who withdrew and those who completed the study (Table 1). Study outcomes based on intent-to-treat analysis are presented in Table 2.
BMI decreased linearly during the intervention (Figure 1). In 10 subjects, the change in BMI postintervention was both statistically (-16 ± 8%, P < .0001) and clinically significant and sufficient for surgical clearance. Eight (40%) had surgery and 2 (10%) no longer needed surgery due to self-reported improved quality of life and decreased pain. Despite the clinically and statistically significant weight loss, 14.5% of the weight lost was fat-free mass; decrease in body fat was 9% ± 4% (P < .0001).
All study subjects consumed 5 Optifast packets per day for at least 10 weeks and no longer than 16 weeks. Of the participants who completed the intervention, the majority elected to continue the intervention time to 16 weeks; however 1 participant went to week 10 and 2 participants completed through week 13. Nonadherence in this protocol was defined as > 2 weeks of weight gain. Two participants gained weight for 6 and 7 weeks, respectively.
Mean systolic blood pressure, plasma triglyceride and fasting glucose levels, A1c, and REE levels decreased significantly postintervention. Additionally, patients experienced either dose reduction or discontinuation of diabetes or hypertension medication use postintervention (Figure 2). Discontinued diabetes medications included rosiglitazone (n = 1), glyburide (n = 1), and metformin (n = 2). Discontinued or reduced antihypertensives included furosemide (n = 1), thiazides (n = 3), beta blockers (n = 1), angiotensin-converting enzyme inhibitors (n = 4), calcium channel blockers (n = 2), and angiotensin II receptor antagonists (n = 2).
Discussion
To the authors’ knowledge, this was the first study using a low calorie liquid diet to achieve weight loss to qualify for nonbariatric elective surgery. This diet provides an alternative intervention for individuals who would otherwise be denied elective surgery due to extreme obesity. Eighty percent of participants completed 10 to 16 weeks of the 800 calorie liquid diet plan with significant weight loss of 16 BMI ± 8%. The intervention was well tolerated without significant AEs.
It is difficult to compare these results to prior studies, as the target populations differ. Previous studies utilizing calorie levels < 800 calories per day included mostly women and consequently, their preintervention weights were lower than in the current study population.10 This study population was predominately older males with a high prevalence of comorbid medical and psychiatric conditions. Despite these demographic and clinical differences, improvements in biochemistries were similar to those demonstrated previously.8-10 The observations for beneficial changes in cardiovascular and glycemic risk factors and reduced medication use related to weight loss and calorie control are consistent with previous results.8-10
Related: Moral Questions Surrounding Bariatric Surgery
To the authors’ knowledge, REE has not been reported in earlier investigations of very low calorie diet interventions. This study found significant decreases in REE, which was measured pre- and postintervention. Participants were given postintervention REE value and individualized meal plans were developed from this number. An interesting and unexpected finding was that this number seemed to provide useful reinforcement for patients as they transitioned to solid food. This may have helped improve adherence to meal plans. Despite concerns regarding possible weight gain, the weight loss continued at a similar rate during the transition, demonstrating that continued weight loss can occur with a combination of food and liquid diet.
The need for elective surgery may have increased motivation to adhere to this weight-loss program. The dropout rate was 20%; lower than previous studies using very low calorie diets and substantially better than traditional weight-loss programs.8,9
An unexpected finding was that 10% of participants who qualified for knee replacement surgery chose to postpone surgery due to decreased pain and improved quality of life. Over the past 20 years, the estimated cost of 1 TKA was $15,000 with an estimated $9 billion spent annually for this procedure in the U.S.26 Importantly, obesity increases the risk of TKA revision surgeries, which are both expensive (average cost of Medicare-covered TKA revision surgeries is $73,696) and projected to increase 66% over the next 25 years.27 Weight loss prior to surgery not only may decrease risk for revisions of TKA, but in some cases also may delay or eliminate the need for surgery.
Although there are significant costs associated with certain weight loss programs, the savings associated with reducing the need for surgery would be substantially greater than that associated with the dietary intervention. The estimated private sector cost of an 18-week weight-loss program (12-week liquid with 6-week transition) is $3,500 per participant. This study program was estimated to cost $2,400 per participant for the 16-week (13-week liquid diet and 3-week transition) program. Patients with obesity awaiting orthopedic, gastrointestinal, or neurosurgery were often referred for bariatric surgery to obtain weight loss. Bariatric surgery averages $17,000 to $26,000, which is more expensive than this diet program.28
The majority of AEs observed in this intervention were expected and similar to other studies.10 Among the 20 participants, 18 experienced a total of 60 AEs, of which 38 (63%) were considered to be study-related. Although constipation was a known AE, 25% of participants subjectively complained of decrease in frequency of bowel movements. The 2 most frequent and unanticipated AEs were increased blood urea nitrogen/ creatinine (n = 9) and reduced sodium (n = 7).
Nonadherence was often related but not limited to the following: inappropriate social cues for eating, lack of social support, sabotage by family or peers, filling an emotional void with food, and/or psychological eating related to depression and posttraumatic stress disorder. Prior to starting a similar intervention, a complete mental health assessment for individuals with known or suspected mental health diagnoses seems warranted.
Conclusion
The study limitations are its small and predominantly male sample size and lack of a randomized control. Nonetheless, this study demonstrated the feasibility of the medically supervised weight loss program to obtain the necessary weight loss in 50% of the veterans (with higher comorbidities and more advanced age). Because of the results of this investigation, the authors have initiated a randomized controlled trial utilizing this intervention. The Optifast program had a high success rate, was cost-effective, and may obviate the need for surgery.
Acknowledgements
This work was supported by pilot funding from the Department Veterans Affairs awarded on a competitive basis to Julie Kurtz. Additional support was provided by the Department of Veterans Affairs. The authors would like to acknowledge the contribution of Julie Stoneroad-Vedda, PA-C, MPAS, Northern Arizona VA Health Care System in Prescott.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999-2000. JAMA. 2002;288(14):1723-1727.
2. Ogden CL, Flegal KM, Carroll MD, Johnson CL. Prevalence and trends in overweight among US children and adolescents, 1999-2000. JAMA. 2002;288(14):1728-1732.
3. Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. JAMA. 2010;303(3):235-241.
4. Ogden CL, Carroll MD, Curtin LR, Lamb MM, Flegal KM. Prevalence of high body mass index in US children and adolescents, 2007-2008. JAMA. 2010;303(3):242-249.
5. Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA. 2012;307(5):491-497.
6. Das SR, Kinsinger LS, Yancy WS Jr, et al. Obesity prevalence among veterans at Veterans Affairs medical facilities. Am J Prev Med. 2005;28(3):291-294.
7. Drawert S, Bedford K, Largent D. Change in glucose, blood pressure, and cholesterol with weight loss in medically obese patients. Obes Res. 1996;4(suppl 1):67S.
8. Kirschner MA, Schneider G, Ertel NH, Gorman J. An eight-year experience with very-low-calorie formula diet for control of major obesity. Int J Obes. 1988;12(1):69-80.
9. Wadden TA, Foster GD, Letizia KA, Stunkard AJ. A multicenter evaluation of a proprietary weight reduction program for the treatment of marked obesity. Arch Intern Med. 1992;152(5):961-966.
10. Anderson JW, Brinkman-Kaplan VL, Lee H, Wood CL. Relationship of weight loss to cardiovascular risk factors in morbidly obese individuals. J Am Coll Nutr. 1994;13(3):256-261.
11. Huerta S, Dredar S, Hayden E, et al. Preoperative weight loss decreases the operative time of gastric bypass at a Veterans Administration hospital. Obes Surg. 2008;18(5):508-512.
12. Still CD, Benotti P, Wood GC, et al. Outcomes of preoperative weight loss in high-risk patients undergoing gastric bypass surgery. Arch Surg. 2007;142(10):994-998; discussion 999.
13. Pekkarinen, T, Mustajoki P. Use of a very low- calorie diet in preoperative weight loss: Efficacy and safety. Obes Res. 1997;5(6):595-602.
14. Van Nieuwenhove Y, Dambrauskas Z, Campillo-Soto A, et al. Preoperative very low-calorie diet and operative outcome after laparoscopic gastric bypass: A randomized multicenter study. Arch Surg. 2011;146(11):1300-1305.
15. Hauser DL, Titchner RL, Wilson MA, Eid GM. Long-term outcomes of laparoscopic Roux-en-Y gastric bypass in US veterans. Obes Surg. 2010;20(3):283-289.
16. Livingston EH, Arterburn D, Schifftner TL, Henderson WG, DePalma RG. National Surgical Quality Improvement Program analysis of bariatric operations: Modifiable risk factors contribute to bariatric surgical adverse outcomes. J Am Coll Surg. 2006;203(5):625-633.
17. National Joint Registry. 11th Annual Report 2014: National Joint Registry for England and Wales and Northern Ireland. Hemel Hempsted, England: National Joint Registry; 2014.
18. Pritchett JW, Bortel DT. Knee replacement in morbidly obese women. Surg Gynecol Obstet. 1991;173(2):119-122.
19. Winiarsky R, Barth P, Lotke P. Total knee arthroplasty in morbidly obese patients. J Bone Joint Surg Am. 1998;80(12):1770-1774.
20. Krushell RJ, Fingeroth RJ. Primary total knee arthroplasty in morbidly obese patients: A 5- to 14-year follow-up study. J Arthroplasty. 2007;22 (6 suppl 2):77-80.
21. Sridhar MS, Jarrett CD, Xerogeanes JW, Labib SA. Obesity and symptomatic osteoarthritis of the knee. J Bone Joint Surg Br. 2012;94(4):433-440.
22. Amin AK, Clayton RA, Patton JT, Gaston M, Cook RE, Brenkel IJ. Total knee replacement in morbidly obese patients: Results of a prospective, matched study. J Bone Joint Surg Br. 2006;88(10):1321-1326.
23. Bamgbade OA, Rutter TW, Nafiu OO, Dorje P. Postoperative complications in obese and nonobese patients. World J Surg. 2007;31(3):556-560; discussion 561.
24. U.S. Department of Agriculture, U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2010. 7th ed. Washington, DC: U.S. Government Printing Office; 2010.
25. Kamrath RO, Plummer LJ, Sadur CN, et al. Cholelithiasis in patients treated with a very-low-calorie diet. Am J Clin Nutr. 1992;56(suppl 1):255S-257S.
26. Cram P, Lu X, Kates SL, Singh JA, Li Y, Wolf BR. Total knee arthroplasty volume, utilization, and outcomes among Medicare beneficiaries, 1991-2010. JAMA. 2012;308(12):1227-1236.
27. Lavernia C, Lee DJ, Hernandez VH. The increasing financial burden of knee revision surgery in the United States. Clin Orthop Relat Res. 2006;446: 221-226.
28. Cremieux PY, Buchwald H, Shikora SA, Ghosh A, Yang HE, Buessing M. A study on the economic impact of bariatric surgery. Am J Manag Care. 2008;14(9):589-596.
1. Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999-2000. JAMA. 2002;288(14):1723-1727.
2. Ogden CL, Flegal KM, Carroll MD, Johnson CL. Prevalence and trends in overweight among US children and adolescents, 1999-2000. JAMA. 2002;288(14):1728-1732.
3. Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. JAMA. 2010;303(3):235-241.
4. Ogden CL, Carroll MD, Curtin LR, Lamb MM, Flegal KM. Prevalence of high body mass index in US children and adolescents, 2007-2008. JAMA. 2010;303(3):242-249.
5. Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA. 2012;307(5):491-497.
6. Das SR, Kinsinger LS, Yancy WS Jr, et al. Obesity prevalence among veterans at Veterans Affairs medical facilities. Am J Prev Med. 2005;28(3):291-294.
7. Drawert S, Bedford K, Largent D. Change in glucose, blood pressure, and cholesterol with weight loss in medically obese patients. Obes Res. 1996;4(suppl 1):67S.
8. Kirschner MA, Schneider G, Ertel NH, Gorman J. An eight-year experience with very-low-calorie formula diet for control of major obesity. Int J Obes. 1988;12(1):69-80.
9. Wadden TA, Foster GD, Letizia KA, Stunkard AJ. A multicenter evaluation of a proprietary weight reduction program for the treatment of marked obesity. Arch Intern Med. 1992;152(5):961-966.
10. Anderson JW, Brinkman-Kaplan VL, Lee H, Wood CL. Relationship of weight loss to cardiovascular risk factors in morbidly obese individuals. J Am Coll Nutr. 1994;13(3):256-261.
11. Huerta S, Dredar S, Hayden E, et al. Preoperative weight loss decreases the operative time of gastric bypass at a Veterans Administration hospital. Obes Surg. 2008;18(5):508-512.
12. Still CD, Benotti P, Wood GC, et al. Outcomes of preoperative weight loss in high-risk patients undergoing gastric bypass surgery. Arch Surg. 2007;142(10):994-998; discussion 999.
13. Pekkarinen, T, Mustajoki P. Use of a very low- calorie diet in preoperative weight loss: Efficacy and safety. Obes Res. 1997;5(6):595-602.
14. Van Nieuwenhove Y, Dambrauskas Z, Campillo-Soto A, et al. Preoperative very low-calorie diet and operative outcome after laparoscopic gastric bypass: A randomized multicenter study. Arch Surg. 2011;146(11):1300-1305.
15. Hauser DL, Titchner RL, Wilson MA, Eid GM. Long-term outcomes of laparoscopic Roux-en-Y gastric bypass in US veterans. Obes Surg. 2010;20(3):283-289.
16. Livingston EH, Arterburn D, Schifftner TL, Henderson WG, DePalma RG. National Surgical Quality Improvement Program analysis of bariatric operations: Modifiable risk factors contribute to bariatric surgical adverse outcomes. J Am Coll Surg. 2006;203(5):625-633.
17. National Joint Registry. 11th Annual Report 2014: National Joint Registry for England and Wales and Northern Ireland. Hemel Hempsted, England: National Joint Registry; 2014.
18. Pritchett JW, Bortel DT. Knee replacement in morbidly obese women. Surg Gynecol Obstet. 1991;173(2):119-122.
19. Winiarsky R, Barth P, Lotke P. Total knee arthroplasty in morbidly obese patients. J Bone Joint Surg Am. 1998;80(12):1770-1774.
20. Krushell RJ, Fingeroth RJ. Primary total knee arthroplasty in morbidly obese patients: A 5- to 14-year follow-up study. J Arthroplasty. 2007;22 (6 suppl 2):77-80.
21. Sridhar MS, Jarrett CD, Xerogeanes JW, Labib SA. Obesity and symptomatic osteoarthritis of the knee. J Bone Joint Surg Br. 2012;94(4):433-440.
22. Amin AK, Clayton RA, Patton JT, Gaston M, Cook RE, Brenkel IJ. Total knee replacement in morbidly obese patients: Results of a prospective, matched study. J Bone Joint Surg Br. 2006;88(10):1321-1326.
23. Bamgbade OA, Rutter TW, Nafiu OO, Dorje P. Postoperative complications in obese and nonobese patients. World J Surg. 2007;31(3):556-560; discussion 561.
24. U.S. Department of Agriculture, U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2010. 7th ed. Washington, DC: U.S. Government Printing Office; 2010.
25. Kamrath RO, Plummer LJ, Sadur CN, et al. Cholelithiasis in patients treated with a very-low-calorie diet. Am J Clin Nutr. 1992;56(suppl 1):255S-257S.
26. Cram P, Lu X, Kates SL, Singh JA, Li Y, Wolf BR. Total knee arthroplasty volume, utilization, and outcomes among Medicare beneficiaries, 1991-2010. JAMA. 2012;308(12):1227-1236.
27. Lavernia C, Lee DJ, Hernandez VH. The increasing financial burden of knee revision surgery in the United States. Clin Orthop Relat Res. 2006;446: 221-226.
28. Cremieux PY, Buchwald H, Shikora SA, Ghosh A, Yang HE, Buessing M. A study on the economic impact of bariatric surgery. Am J Manag Care. 2008;14(9):589-596.