User login
It could be the end of the affair with HPV!1 With this exclamation, Prof. Margaret Stanley, the noted human papillomavirus immunologist, expressed the optimism we all share, now that the possibility of conquering cervical cancer is within view. Not yet 25 years have passed since the first sequencing of a genital HPV type, and scarcely 10 years since the International Agency for Research on Cancer proclaimed that HPV causes cervical cancer. It has been 57 years since the discovery that launched an international quest to reduce the cervical cancer rate: George Papanicolaou’s test for early abnormal cell changes that, decades later, were found to be secondary to HPV. We’ve made great progress. What was the 2nd leading cancer in US women in incidence and mortality is now 11th in incidence and 13th in mortality.
But even with perfect attendance at annual screenings, women still get cervical cancer. And many still do not have screenings—they account for about half of all cervical cancers. And the Pap, as good as it is, has flaws. The test is subjective, and sensitivity varies from lab to lab and country to country.
What is new in 2006 that we may soon be able to put into practice, bringing us closer to a new world—with respect to cervical cancer prevention—different from any we’ve known?
- 1 More sensitive and objective screening
- 2 Better management of screen positives
- 3 HPV vaccine, soon to be in our offices
1 More sensitive and more objective screening
A comforting combo: Negative Pap and HPV tests
ACOG Practice Bulletin, Number 61. Human papillomavirus. Washington, DC: American College of Obstetricians and Gynecologists; April 2005.
Because HPV testing is more sensitive than cervical cytology in detecting CIN 2 and CIN 3, women with concurrent negative Pap and HPV tests can be reassured that their risk of unidentified CIN 2, CIN 3, or cervical cancer is approximately 1 in 1,000. (Level A evidence)
The American Cancer Society and the American College of Obstetricians and Gynecologists have both provided as an option the screening of women age 30 and older with the combination of the Pap and a test for high-risk HPV types.2,3 These “sophisticated new tests for the detection of HPV…hold great promise for improved screening for cervical cancer precursors and invasive cancer, and for triage of cervical cytology,” the Bulletin states.
Not all women get annual screening, however, and even if they do, the IARC estimates, the lifetime risk for cervical cancer for women who have conventional Paps annually is approximately 216 per 100,000, if the Pap sensitivity is about 70%. The prospect of reducing the risk of missing significant cervical neoplasia at each screen to 1 per 1,000 should be of comfort to women and the clinicians who watch over their health.
Dilemma: Women over 30, with normal Pap and high-risk HPV
What about the approximately 4% of women aged 30 and older with normal cytology and high-risk HPV? How should these women be managed? A panel of experts on HPV and cervical screening published “interim guidance” in 2004, recommending that until further data are available, these women should be retested in 6 to 12 months for persistence of HPV or development of abnormal cytology, and referred to colposcopy if still HPV-positive or if Pap results show low-grade squamous intraepithelial lesion (LSIL) or worse, regardless of HPV result.4
Although the April 2005 ACOG Bulletin affirmed that guideline, concern persisted that, while some women so identified might be better evaluated immediately by colposcopy, the majority would not, and there was no good way to identify HPV-positive women most at risk. Several longitudinal studies (discussed in the following section) have now made the path clearer.
REFERENCES
1. Stanley M. The end for genital human papillomavirus infections? Lancet Oncol 2005;6:256-257.
2. Sasow D, Runowicz CD, Solomon D, et al, for the American Cancer Society. American Cancer Society guideline for the early detection of cervical neoplasia and cancer. CA Cancer J Clin. 2002;52:342-362.
3. ACOG Practice Bulletin, Number 45. Cervical cytology screening. Washington, DC: American College of Obstetricians and Gynecologists; 2003.
4. Wright TC, Jr, Schiffman M, Solomon D, et al. Interim guidance for the use of human papillomavirus DNA testing as an adjunct to cervical cytology for screening. Obstet Gynecol. 2004;103:304-309.
Type-specific testing identifies highest risk
Khan MJ, Castle PE, Lorincz AT, et al. The elevated 10-year risk of cervical precancer and cancer in women with human papillomavirus (HPV) type 16 or 18 and the possible utility of type-specific HPV testing in clinical practice. J Natl Cancer Inst. 2005;97:1072–1079.
HPV screening that distinguishes types 16 and 18 from other oncogenic (high-risk) HPV types identifies women at the greatest risk of CIN 2/3+ and may permit less aggressive management of women with other high-risk HPV infections.
The solution to the dilemma of having to wait 6 to 12 months to repeat Pap and HPV tests for women with a normal cytology but a positive HPV test before determining the need for colposcopy may be solved by type-specific HPV testing. The 10-year cumulative incidence of CIN 3 and cervical cancer (CIN 3+) in 20,810 women tested once for HPV at enrollment was only 0.8% in the women who tested negative for high-risk HPV by Hybrid Capture 2. In contrast, CIN 3+ developed in 17% of the HPV-16-positive women and 14% of the HPV-18-positive women within 10 years.
Women positive for other high-risk types of HPV, but negative for HPV 16 and 18 had far less risk: only 3% developed CIN 3+.
When stratified by age to limit the analysis to women aged 30 and older, the cumulative incidence of CIN 3+ was 20% in HPV-16-positive women and 15% in HPV-18-positive women (FIGURE 1). Contrast these results to the 10-year predictive value of 11% for an LSIL Pap for the same level of cervical neoplasia. In other words, a single positive HPV 16 or 18 test is almost twice as likely to identify women at high risk for CIN 3+ as an LSIL Pap result, over time.
FIGURE 1 Positive HPV 16 or 18 linked to 14% to 17% incidence of CIN3+
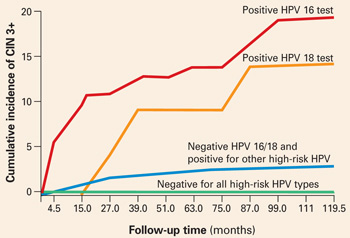
The cumulative incidence of CIN 3+ over a 10-year period, as a function of a single HPV test result at enrollment. Women positive for HPV 16 or 18 had a much greater incidence of CIN 3+, compared to women negative for HPV 16 and 18 but positive for other high-risk HPV types by Hybrid Capture 2, or negative for all high-risk HPV types. Adapted from Khan et al.
Follow-up according to risk
These findings support a follow-up strategy that would permit risk stratification of HPV-infected women for whom an optimal repeat screening interval has been unclear.
- Women positive for HPV 16 or 18 warrant referral to colposcopy, for they carry the majority of risk from a positive high-risk HPV test.
- Women positive only for other high-risk types could be reassured of the safety of a 12-month interval without colposcopy, and referred to colposcopy only if the repeat Pap shows worse than atypical squamous cells of undetermined significance (ASC-US) or the HPV test is again positive (FIGURE 2).
FIGURE 2 Type-specific testing in clinical practice
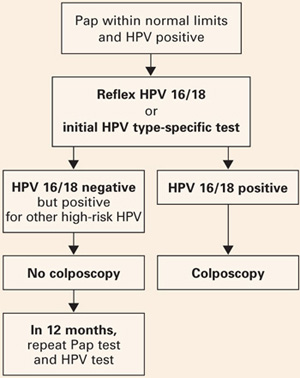
Proposed management of women aged 30 or older, who are screened concurrently with both a Pap test and an HPV test, with typing for HPV 16/18. Adapted from Khan et al.
2 type-specific tests in the pipeline
Currently, the only FDA-approved test for combined screening of women aged 30 and older is the Hybrid Capture 2 High-risk HPV test, which tests for a panel of the 13 most common HPV types known to cause cervical cancer, but does not report on individual types.
But 2 type-specific HPV tests may become available in 2006, which would enable clinicians to follow this strategy.
Digene is nearly ready to launch a 16, 18, 45 type-specific “reflex” test (to a positive Hybrid Capture 2 HPV panel), and Roche is preparing to get its type-specific Linear Array HPV test approved.
2 Better management of screen positives
New practice bulletin on managing abnormal tests
ACOG Practice Bulletin, Number 66. Management of abnormal cervical cytology and histology. Washington, DC: American College of Obstetricians and Gynecologists; September 2005.
The new Practice Bulletin published last September in most respects mirrors the most recent American Society for Colposcopy and Cervical Pathology (ASCCP) Consensus guidelines.1
Key points
- ASC-US may be managed by referral to immediate colposcopy, by repeat Pap, or by HPV testing. However, “reflex HPV testing” when ASC-US is derived from liquid-based cytology has advantages. (It is estimated that a large majority of ASC-US is now managed by HPV testing.)
- Initial management of all other Pap abnormalities is by immediate referral to colposcopy, ie, the finding of atypical squamous cells cannot rule out high-grade (ASC-H), atypical glandular cells (AGC), LSIL, and high-grade intraepithelial lesions (HSIL).
- Management of ASC-US and LSIL in adolescence and postmenopause: ACOG provides an alternative strategy for adolescents with either ASC-US or LSIL cytology, who may have either repeat cytology at 6 and 12 months or a single HPV test at 12 months. ACOG did not differentiate postmenopausal women with either ASC-US or LSIL as “special situations” with additional management strategies.
- CIN 2/3 should usually be treated, both guidelines say. The only exception is the adolescent with CIN 2, who may be followed with repeat cytology and colposcopy at 4 to 6 months if she is deemed reliable for follow-up, the colposcopy is adequate, and the endocervical sampling is negative.
- HPV-positive ASC-US, ASC-H, or LSIL and either CIN 1 or normal colposcopy findings should be followed by repeat Pap at 6 and 12 months, or a single HPV test at 12 months, with referral to colposcopy if either the Pap results show ASC-US or more advanced abnormality or the HPV test is positive.
- In contrast, an excisional procedure is required for normal findings, or an unsatisfactory colposcopy in nonpregnant women referred for atypical glandular cells “favor neoplasia” (AGC-H), or adenocarcinoma in situ (AIS), or repeat atypical glandular cells “not otherwise specified” (AGC-NOS), or HSIL. The only exception is an adolescent with HSIL cytology and a satisfactory and normal colposcopy and biopsy, who may be followed closely.
- Women treated for CIN 2/3 can be monitored after treatment by cytology screening at 6-month intervals 3 or 4 times or by a single HPV test at 6 months, before returning to annual screening. Any repeat abnormal Pap at the threshold of ASC-US or more advanced abnormality or a positive HPV test requires colposcopic evaluation.
REFERENCES
1. Wright TC, Jr, Cox JT, Massad LS, Carlson J, Twiggs LB, Wilkinson EJ. American Society for Colposcopy and Cervical Pathology. 2001 consensus guidelines for the management of women with cervical intraepithelial neoplasia. Am J Obstet Gynecol. 2003;189:295-304.
3 HPV vaccine, soon to be in our offices
Vaccines will stop CIN 2/3 and cancer
Mao C, Koutsky LA, Ault KA, et al. Efficacy of human papillomavirus-16 vaccine to prevent cervical intraepithelial neoplasia: a randomized controlled trial. Obstet Gynecol. 2006;107:18–27.
The HPV 16 vaccine provided 100% protection against development of HPV-16-related CIN 2/3 during an average of 3.5 years of follow-up.
This preliminary study to the Quadrivalent HPV 6, 11, 16, 18 trial on an HPV 16 virus-like particle (VLP) vaccine reached an average of 3.5 years of follow-up. CIN 2/3 developed in 12 of the 750 women receiving placebo, in contrast to none of the 755 vaccine recipients. Persistent HPV 16 infections were defined as testing positive for type-specific HPV 16 on 2 or more visits, with the caveat that women testing positive on the last visit were considered persistent because they would have no further follow-up to determine that status. As a result, some women with a positive test only on the last visit were included as “persisters,” perhaps explaining why the efficacy in preventing persistent HPV 16 in the vaccine recipients was only 94%. Single-test positives can be transient infections, vaginal contamination with infected cells from a partner during recent intercourse, or early persistent infections. Although antibody titers to HPV 16 in vaccine recipients waned over time, they still exceeded titers in placebo recipients who already had natural immunity to HPV 16.
Benefit of the HPV 16 vaccine was also seen for women already HPV-16 positive at enrollment, but only if they were seronegative for HPV 16. It is possible that, if an immune response has not yet been mounted, the vaccine may still have a positive effect for women already HPV-16 infected.
Who will be vaccinated?
Although the primary target group for the HPV vaccine will be children before natural exposure can occur after the onset of sexual activity, many women already sexually active will likely want to be vaccinated.
It is this “catch-up” group that will challenge the OBGyn to become familiar with and to provide the HPV vaccine when it becomes available, likely later this year.
Quadrivalent vaccine 100% effective
Skjeldstad FE, Koutsky LA, for the Merck Phase 3 HPV Vaccine Steering Committee (Future II). Phase II trial of prophylactic quadrivalent HPV 6, 11, 16, 18 L1 virus-like particle (VLP) vaccine; prevention of cervical intraepithelial neoplasia (CIN) 2/3 including adeno- and squamous cell carcinoma in situ (CIS). Presented at: Infectious Diseases Society of America, Late Breaker Session 66, LB–8A; October 7, 2005; San Francisco, Calif.
The Quadrivalent HPV 6, 11, 16, 18 vaccine provided 100% protection against persistent HPV 16/18 and HPV 16/18-related CIN 2/3.
This trial became center stage in the world media in early October 2005, with headlines such as “First anti-cancer vaccine 100% effective.” The results are truly astounding, as there were no CIN 2/3 cases in the Per Protocol group, among the 5,301 women vaccinated, in contrast to 21 cases in the 5,258 women who received the placebo (TABLE 1).
In the group most likely to mirror a typical vaccinated population, only 1 case of HPV-16/18-positive CIN 2/3 occurred in the 5,736 vaccinated women, some of whom were already positive for 1 or more HPV types, or had serologic evidence of prior type-specific infection, or received fewer than the 3 recommended doses. In contrast, 36 HPV-16/18 CIN 2/3 occurred in the 5,766 women who received placebo.
TABLE 1
Efficacy of quadrivalent HPV 6, 11, 16, 18 vaccine in preventing CIN 2/3
| VACCINE GROUPS | PLACEBO GROUPS | EFFICACY | |||||
|---|---|---|---|---|---|---|---|
| NUMBER OF WOMEN | NUMBER OF CIN 2/3 CASES | RATE | NUMBER OF WOMEN | NUMBER OF CIN 2/3 CASES | RATE | % | |
| Per protocol | 5,301 | 0 | 0.0 | 5,258 | 21 | 0.3 | 100 |
| Modified intention to treat | 5,301 | 1 | <0.1 | 5,766 | 36 | 0.3 | 97 |
| Source: Skjeldstad et al | |||||||
No warts, either
Subsequent analysis revealed similar protection from HPV 6 or 11 genital warts.
No serious adverse events were recorded in the entire trial.
Because HPV 16 and 18 together cause approximately 70% of all cervical cancers, and HPV 6 and 11 cause 90% of genital warts, these results are surely something about which to rejoice!
Gardasil and Cervarix vaccines
Now the challenge will be in getting the population vaccinated. Merck is expected to have its Gardasil Quadrivalent vaccine on the market mid- to late 2006. GlaxoSmithKline expects to put Cervarix Bivalent HPV 16, 18 vaccine on the market sometime in 2007.
How HPV vaccine will—and won’t—change practice
Franco EL, Harper DM. Vaccination against human papillomavirus infection: a new paradigm in cervical cancer control. Vaccine. 2005;23:2388–2394.
Cervical screening will continue, but will be more accurate and more efficient.
Yes, we are on the verge of the possibility of reducing the risk of cervical cancer to close to zero, but it will take decades. Vaccinating young girls will not significantly reduce cervical cancer rates until these girls reach the median ages of microinvasive (early 40s) and invasive (late 40s) cervical cancer.
Even then, cervical cancer rates will depend on these factors:
- the extent of vaccination coverage
- the number of high-risk HPV types in the vaccine
- whether vaccination provides multidecade protection or falls off with time
- whether the medical community and the public continue to diligently follow recommended screening guidelines
If immune protection falls with time, booster HPV vaccine shots should provide ongoing protection, but population protection will depend on the percent of the population obtaining the booster. If the population becomes complacent about cervical screening as risk for cervical cancer decreases, then cancers will develop that would have otherwise been prevented.
Why screening will continue
Virus-like particle (VLP) vaccines for all of the important oncogenic HPV types could, theoretically, be produced. But until long after multivalent HPV vaccines that include all the important oncogenic types are available, women will require screening to prevent the 30% of cancers that occur from other high-risk HPV types not in the present vaccine. And, we will need screening to protect women who are not vaccinated, and those already infected.
As Franco and Harper stressed, “Although the future seems bright on the vaccine front, policy makers are strongly cautioned to avoid scaling back cervical cancer screening. Any premature relaxation of cervical cancer control measures already in place will bring a resurgence of the disease to the unacceptable levels of the not too distant past.”
In other words, cervical screening will continue for the foreseeable future.
A peek at a “new world”
Fewer abnormal Pap tests. The vaccine will likely steadily decrease the rate of abnormal Paps that are important, as an increasing proportion of women are vaccinated against the 2 most common types in high-grade CIN.
Colposcopies and cervical treatments will decline in number coincident with the proportion of the population vaccinated.
A training challenge? This change will decrease the number of significant lesions that a colposcopist may see, increasing the challenge of training and maintaining expertise in identification and treatment of these lesions. As significant Pap abnormalities decrease, maintaining expertise in cytologic interpretation, and even in maintaining attention to detail, may become more difficult.
Specific testing. Finding women with significant abnormalities may more and more be accomplished with the accuracy afforded by testing for specific HPV types known to be most at-risk for CIN 3+.
With respect to cervical cancer prevention, the years to come will surely be a new world, different from what we all have known.
Piyathilake CJ, Henao OL, Macaluso M, et al. Folate is associated with the natural history of high-risk human papillomaviruses. Cancer Res. 2004;64:8788–8793.
Improving folate status in women at risk of getting infected or already infected with high-risk HPV may help prevent cervical cancer. It is reasonable to advise women with HPV that folate supplements may be helpful.
Recommending oral folate supplements is one of the few things we can offer that can empower our patients with something positive that they can do for themselves.
A subset of women in the ASCUS LSIL Triage (ALT) study were evaluated prospectively to determine whether systemic levels of folic acid are associated with the occurrence and duration of HPV infections after controlling for other micronutrients (vitamins B12, A, E, C, and total carotene) and for known risk factors for high-risk HPV infections and cervical cancer. Hybrid Capture 2 and serum levels of these micronutrients were obtained at 6-month intervals throughout the trial’s 2-year follow-up.
Women with higher folate status were significantly less likely to be repeatedly HPV positive, more likely to become testnegative during the 2-year study, and 73% less likely to become newly HPV positive.
These associations held after controlling for other micronutrients and known risk factors for HPV. The authors reviewed a possible role of folate in preventing integration of HPV, thereby improving clearance of HPV infections, and documented that increased folate levels were also protective against the development of CIN 2/3.
Food fortification with folate became mandatory in the United States in 1998. The median folate level in women in this study mirrored the median post-fortification level for women in the United States—indicating that folate levels in food are not adequate to affect HPV status.
Therefore, it appears reasonable to advise women with HPV that taking folic acid supplementation in the levels usually advised for pregnant women may be helpful.
Dr. Cox served as a member of the American Cancer Society Cervical Guidelines Committee, the 2001 Bethesda Workshop, and was one of the primary authors of the 2001 ASCCP Consensus Guidelines for the management of women with abnormal cervical cytology and cervical cancer precursors. He is President-elect of the American Society for Colposcopy and Cervical Pathology and presently is on the ACS HPV Vaccine Advisory Committee, and the Data and Safety Monitoring Board of the HPV 6, 11, 16, 18 Quadrivalent Vaccine Trial. Dr. Cox is a consultant to Digene, GlaxoSmithKline, and the Merck Data and Safety Monitoring Board, and a speaker for Digene.
It could be the end of the affair with HPV!1 With this exclamation, Prof. Margaret Stanley, the noted human papillomavirus immunologist, expressed the optimism we all share, now that the possibility of conquering cervical cancer is within view. Not yet 25 years have passed since the first sequencing of a genital HPV type, and scarcely 10 years since the International Agency for Research on Cancer proclaimed that HPV causes cervical cancer. It has been 57 years since the discovery that launched an international quest to reduce the cervical cancer rate: George Papanicolaou’s test for early abnormal cell changes that, decades later, were found to be secondary to HPV. We’ve made great progress. What was the 2nd leading cancer in US women in incidence and mortality is now 11th in incidence and 13th in mortality.
But even with perfect attendance at annual screenings, women still get cervical cancer. And many still do not have screenings—they account for about half of all cervical cancers. And the Pap, as good as it is, has flaws. The test is subjective, and sensitivity varies from lab to lab and country to country.
What is new in 2006 that we may soon be able to put into practice, bringing us closer to a new world—with respect to cervical cancer prevention—different from any we’ve known?
- 1 More sensitive and objective screening
- 2 Better management of screen positives
- 3 HPV vaccine, soon to be in our offices
1 More sensitive and more objective screening
A comforting combo: Negative Pap and HPV tests
ACOG Practice Bulletin, Number 61. Human papillomavirus. Washington, DC: American College of Obstetricians and Gynecologists; April 2005.
Because HPV testing is more sensitive than cervical cytology in detecting CIN 2 and CIN 3, women with concurrent negative Pap and HPV tests can be reassured that their risk of unidentified CIN 2, CIN 3, or cervical cancer is approximately 1 in 1,000. (Level A evidence)
The American Cancer Society and the American College of Obstetricians and Gynecologists have both provided as an option the screening of women age 30 and older with the combination of the Pap and a test for high-risk HPV types.2,3 These “sophisticated new tests for the detection of HPV…hold great promise for improved screening for cervical cancer precursors and invasive cancer, and for triage of cervical cytology,” the Bulletin states.
Not all women get annual screening, however, and even if they do, the IARC estimates, the lifetime risk for cervical cancer for women who have conventional Paps annually is approximately 216 per 100,000, if the Pap sensitivity is about 70%. The prospect of reducing the risk of missing significant cervical neoplasia at each screen to 1 per 1,000 should be of comfort to women and the clinicians who watch over their health.
Dilemma: Women over 30, with normal Pap and high-risk HPV
What about the approximately 4% of women aged 30 and older with normal cytology and high-risk HPV? How should these women be managed? A panel of experts on HPV and cervical screening published “interim guidance” in 2004, recommending that until further data are available, these women should be retested in 6 to 12 months for persistence of HPV or development of abnormal cytology, and referred to colposcopy if still HPV-positive or if Pap results show low-grade squamous intraepithelial lesion (LSIL) or worse, regardless of HPV result.4
Although the April 2005 ACOG Bulletin affirmed that guideline, concern persisted that, while some women so identified might be better evaluated immediately by colposcopy, the majority would not, and there was no good way to identify HPV-positive women most at risk. Several longitudinal studies (discussed in the following section) have now made the path clearer.
REFERENCES
1. Stanley M. The end for genital human papillomavirus infections? Lancet Oncol 2005;6:256-257.
2. Sasow D, Runowicz CD, Solomon D, et al, for the American Cancer Society. American Cancer Society guideline for the early detection of cervical neoplasia and cancer. CA Cancer J Clin. 2002;52:342-362.
3. ACOG Practice Bulletin, Number 45. Cervical cytology screening. Washington, DC: American College of Obstetricians and Gynecologists; 2003.
4. Wright TC, Jr, Schiffman M, Solomon D, et al. Interim guidance for the use of human papillomavirus DNA testing as an adjunct to cervical cytology for screening. Obstet Gynecol. 2004;103:304-309.
Type-specific testing identifies highest risk
Khan MJ, Castle PE, Lorincz AT, et al. The elevated 10-year risk of cervical precancer and cancer in women with human papillomavirus (HPV) type 16 or 18 and the possible utility of type-specific HPV testing in clinical practice. J Natl Cancer Inst. 2005;97:1072–1079.
HPV screening that distinguishes types 16 and 18 from other oncogenic (high-risk) HPV types identifies women at the greatest risk of CIN 2/3+ and may permit less aggressive management of women with other high-risk HPV infections.
The solution to the dilemma of having to wait 6 to 12 months to repeat Pap and HPV tests for women with a normal cytology but a positive HPV test before determining the need for colposcopy may be solved by type-specific HPV testing. The 10-year cumulative incidence of CIN 3 and cervical cancer (CIN 3+) in 20,810 women tested once for HPV at enrollment was only 0.8% in the women who tested negative for high-risk HPV by Hybrid Capture 2. In contrast, CIN 3+ developed in 17% of the HPV-16-positive women and 14% of the HPV-18-positive women within 10 years.
Women positive for other high-risk types of HPV, but negative for HPV 16 and 18 had far less risk: only 3% developed CIN 3+.
When stratified by age to limit the analysis to women aged 30 and older, the cumulative incidence of CIN 3+ was 20% in HPV-16-positive women and 15% in HPV-18-positive women (FIGURE 1). Contrast these results to the 10-year predictive value of 11% for an LSIL Pap for the same level of cervical neoplasia. In other words, a single positive HPV 16 or 18 test is almost twice as likely to identify women at high risk for CIN 3+ as an LSIL Pap result, over time.
FIGURE 1 Positive HPV 16 or 18 linked to 14% to 17% incidence of CIN3+

The cumulative incidence of CIN 3+ over a 10-year period, as a function of a single HPV test result at enrollment. Women positive for HPV 16 or 18 had a much greater incidence of CIN 3+, compared to women negative for HPV 16 and 18 but positive for other high-risk HPV types by Hybrid Capture 2, or negative for all high-risk HPV types. Adapted from Khan et al.
Follow-up according to risk
These findings support a follow-up strategy that would permit risk stratification of HPV-infected women for whom an optimal repeat screening interval has been unclear.
- Women positive for HPV 16 or 18 warrant referral to colposcopy, for they carry the majority of risk from a positive high-risk HPV test.
- Women positive only for other high-risk types could be reassured of the safety of a 12-month interval without colposcopy, and referred to colposcopy only if the repeat Pap shows worse than atypical squamous cells of undetermined significance (ASC-US) or the HPV test is again positive (FIGURE 2).
FIGURE 2 Type-specific testing in clinical practice

Proposed management of women aged 30 or older, who are screened concurrently with both a Pap test and an HPV test, with typing for HPV 16/18. Adapted from Khan et al.
2 type-specific tests in the pipeline
Currently, the only FDA-approved test for combined screening of women aged 30 and older is the Hybrid Capture 2 High-risk HPV test, which tests for a panel of the 13 most common HPV types known to cause cervical cancer, but does not report on individual types.
But 2 type-specific HPV tests may become available in 2006, which would enable clinicians to follow this strategy.
Digene is nearly ready to launch a 16, 18, 45 type-specific “reflex” test (to a positive Hybrid Capture 2 HPV panel), and Roche is preparing to get its type-specific Linear Array HPV test approved.
2 Better management of screen positives
New practice bulletin on managing abnormal tests
ACOG Practice Bulletin, Number 66. Management of abnormal cervical cytology and histology. Washington, DC: American College of Obstetricians and Gynecologists; September 2005.
The new Practice Bulletin published last September in most respects mirrors the most recent American Society for Colposcopy and Cervical Pathology (ASCCP) Consensus guidelines.1
Key points
- ASC-US may be managed by referral to immediate colposcopy, by repeat Pap, or by HPV testing. However, “reflex HPV testing” when ASC-US is derived from liquid-based cytology has advantages. (It is estimated that a large majority of ASC-US is now managed by HPV testing.)
- Initial management of all other Pap abnormalities is by immediate referral to colposcopy, ie, the finding of atypical squamous cells cannot rule out high-grade (ASC-H), atypical glandular cells (AGC), LSIL, and high-grade intraepithelial lesions (HSIL).
- Management of ASC-US and LSIL in adolescence and postmenopause: ACOG provides an alternative strategy for adolescents with either ASC-US or LSIL cytology, who may have either repeat cytology at 6 and 12 months or a single HPV test at 12 months. ACOG did not differentiate postmenopausal women with either ASC-US or LSIL as “special situations” with additional management strategies.
- CIN 2/3 should usually be treated, both guidelines say. The only exception is the adolescent with CIN 2, who may be followed with repeat cytology and colposcopy at 4 to 6 months if she is deemed reliable for follow-up, the colposcopy is adequate, and the endocervical sampling is negative.
- HPV-positive ASC-US, ASC-H, or LSIL and either CIN 1 or normal colposcopy findings should be followed by repeat Pap at 6 and 12 months, or a single HPV test at 12 months, with referral to colposcopy if either the Pap results show ASC-US or more advanced abnormality or the HPV test is positive.
- In contrast, an excisional procedure is required for normal findings, or an unsatisfactory colposcopy in nonpregnant women referred for atypical glandular cells “favor neoplasia” (AGC-H), or adenocarcinoma in situ (AIS), or repeat atypical glandular cells “not otherwise specified” (AGC-NOS), or HSIL. The only exception is an adolescent with HSIL cytology and a satisfactory and normal colposcopy and biopsy, who may be followed closely.
- Women treated for CIN 2/3 can be monitored after treatment by cytology screening at 6-month intervals 3 or 4 times or by a single HPV test at 6 months, before returning to annual screening. Any repeat abnormal Pap at the threshold of ASC-US or more advanced abnormality or a positive HPV test requires colposcopic evaluation.
REFERENCES
1. Wright TC, Jr, Cox JT, Massad LS, Carlson J, Twiggs LB, Wilkinson EJ. American Society for Colposcopy and Cervical Pathology. 2001 consensus guidelines for the management of women with cervical intraepithelial neoplasia. Am J Obstet Gynecol. 2003;189:295-304.
3 HPV vaccine, soon to be in our offices
Vaccines will stop CIN 2/3 and cancer
Mao C, Koutsky LA, Ault KA, et al. Efficacy of human papillomavirus-16 vaccine to prevent cervical intraepithelial neoplasia: a randomized controlled trial. Obstet Gynecol. 2006;107:18–27.
The HPV 16 vaccine provided 100% protection against development of HPV-16-related CIN 2/3 during an average of 3.5 years of follow-up.

This preliminary study to the Quadrivalent HPV 6, 11, 16, 18 trial on an HPV 16 virus-like particle (VLP) vaccine reached an average of 3.5 years of follow-up. CIN 2/3 developed in 12 of the 750 women receiving placebo, in contrast to none of the 755 vaccine recipients. Persistent HPV 16 infections were defined as testing positive for type-specific HPV 16 on 2 or more visits, with the caveat that women testing positive on the last visit were considered persistent because they would have no further follow-up to determine that status. As a result, some women with a positive test only on the last visit were included as “persisters,” perhaps explaining why the efficacy in preventing persistent HPV 16 in the vaccine recipients was only 94%. Single-test positives can be transient infections, vaginal contamination with infected cells from a partner during recent intercourse, or early persistent infections. Although antibody titers to HPV 16 in vaccine recipients waned over time, they still exceeded titers in placebo recipients who already had natural immunity to HPV 16.

Benefit of the HPV 16 vaccine was also seen for women already HPV-16 positive at enrollment, but only if they were seronegative for HPV 16. It is possible that, if an immune response has not yet been mounted, the vaccine may still have a positive effect for women already HPV-16 infected.
Who will be vaccinated?
Although the primary target group for the HPV vaccine will be children before natural exposure can occur after the onset of sexual activity, many women already sexually active will likely want to be vaccinated.
It is this “catch-up” group that will challenge the OBGyn to become familiar with and to provide the HPV vaccine when it becomes available, likely later this year.
Quadrivalent vaccine 100% effective
Skjeldstad FE, Koutsky LA, for the Merck Phase 3 HPV Vaccine Steering Committee (Future II). Phase II trial of prophylactic quadrivalent HPV 6, 11, 16, 18 L1 virus-like particle (VLP) vaccine; prevention of cervical intraepithelial neoplasia (CIN) 2/3 including adeno- and squamous cell carcinoma in situ (CIS). Presented at: Infectious Diseases Society of America, Late Breaker Session 66, LB–8A; October 7, 2005; San Francisco, Calif.
The Quadrivalent HPV 6, 11, 16, 18 vaccine provided 100% protection against persistent HPV 16/18 and HPV 16/18-related CIN 2/3.
This trial became center stage in the world media in early October 2005, with headlines such as “First anti-cancer vaccine 100% effective.” The results are truly astounding, as there were no CIN 2/3 cases in the Per Protocol group, among the 5,301 women vaccinated, in contrast to 21 cases in the 5,258 women who received the placebo (TABLE 1).
In the group most likely to mirror a typical vaccinated population, only 1 case of HPV-16/18-positive CIN 2/3 occurred in the 5,736 vaccinated women, some of whom were already positive for 1 or more HPV types, or had serologic evidence of prior type-specific infection, or received fewer than the 3 recommended doses. In contrast, 36 HPV-16/18 CIN 2/3 occurred in the 5,766 women who received placebo.
TABLE 1
Efficacy of quadrivalent HPV 6, 11, 16, 18 vaccine in preventing CIN 2/3
| VACCINE GROUPS | PLACEBO GROUPS | EFFICACY | |||||
|---|---|---|---|---|---|---|---|
| NUMBER OF WOMEN | NUMBER OF CIN 2/3 CASES | RATE | NUMBER OF WOMEN | NUMBER OF CIN 2/3 CASES | RATE | % | |
| Per protocol | 5,301 | 0 | 0.0 | 5,258 | 21 | 0.3 | 100 |
| Modified intention to treat | 5,301 | 1 | <0.1 | 5,766 | 36 | 0.3 | 97 |
| Source: Skjeldstad et al | |||||||
No warts, either
Subsequent analysis revealed similar protection from HPV 6 or 11 genital warts.
No serious adverse events were recorded in the entire trial.
Because HPV 16 and 18 together cause approximately 70% of all cervical cancers, and HPV 6 and 11 cause 90% of genital warts, these results are surely something about which to rejoice!
Gardasil and Cervarix vaccines
Now the challenge will be in getting the population vaccinated. Merck is expected to have its Gardasil Quadrivalent vaccine on the market mid- to late 2006. GlaxoSmithKline expects to put Cervarix Bivalent HPV 16, 18 vaccine on the market sometime in 2007.
How HPV vaccine will—and won’t—change practice
Franco EL, Harper DM. Vaccination against human papillomavirus infection: a new paradigm in cervical cancer control. Vaccine. 2005;23:2388–2394.
Cervical screening will continue, but will be more accurate and more efficient.
Yes, we are on the verge of the possibility of reducing the risk of cervical cancer to close to zero, but it will take decades. Vaccinating young girls will not significantly reduce cervical cancer rates until these girls reach the median ages of microinvasive (early 40s) and invasive (late 40s) cervical cancer.
Even then, cervical cancer rates will depend on these factors:
- the extent of vaccination coverage
- the number of high-risk HPV types in the vaccine
- whether vaccination provides multidecade protection or falls off with time
- whether the medical community and the public continue to diligently follow recommended screening guidelines
If immune protection falls with time, booster HPV vaccine shots should provide ongoing protection, but population protection will depend on the percent of the population obtaining the booster. If the population becomes complacent about cervical screening as risk for cervical cancer decreases, then cancers will develop that would have otherwise been prevented.
Why screening will continue
Virus-like particle (VLP) vaccines for all of the important oncogenic HPV types could, theoretically, be produced. But until long after multivalent HPV vaccines that include all the important oncogenic types are available, women will require screening to prevent the 30% of cancers that occur from other high-risk HPV types not in the present vaccine. And, we will need screening to protect women who are not vaccinated, and those already infected.
As Franco and Harper stressed, “Although the future seems bright on the vaccine front, policy makers are strongly cautioned to avoid scaling back cervical cancer screening. Any premature relaxation of cervical cancer control measures already in place will bring a resurgence of the disease to the unacceptable levels of the not too distant past.”
In other words, cervical screening will continue for the foreseeable future.
A peek at a “new world”
Fewer abnormal Pap tests. The vaccine will likely steadily decrease the rate of abnormal Paps that are important, as an increasing proportion of women are vaccinated against the 2 most common types in high-grade CIN.
Colposcopies and cervical treatments will decline in number coincident with the proportion of the population vaccinated.
A training challenge? This change will decrease the number of significant lesions that a colposcopist may see, increasing the challenge of training and maintaining expertise in identification and treatment of these lesions. As significant Pap abnormalities decrease, maintaining expertise in cytologic interpretation, and even in maintaining attention to detail, may become more difficult.
Specific testing. Finding women with significant abnormalities may more and more be accomplished with the accuracy afforded by testing for specific HPV types known to be most at-risk for CIN 3+.
With respect to cervical cancer prevention, the years to come will surely be a new world, different from what we all have known.
Piyathilake CJ, Henao OL, Macaluso M, et al. Folate is associated with the natural history of high-risk human papillomaviruses. Cancer Res. 2004;64:8788–8793.
Improving folate status in women at risk of getting infected or already infected with high-risk HPV may help prevent cervical cancer. It is reasonable to advise women with HPV that folate supplements may be helpful.
Recommending oral folate supplements is one of the few things we can offer that can empower our patients with something positive that they can do for themselves.
A subset of women in the ASCUS LSIL Triage (ALT) study were evaluated prospectively to determine whether systemic levels of folic acid are associated with the occurrence and duration of HPV infections after controlling for other micronutrients (vitamins B12, A, E, C, and total carotene) and for known risk factors for high-risk HPV infections and cervical cancer. Hybrid Capture 2 and serum levels of these micronutrients were obtained at 6-month intervals throughout the trial’s 2-year follow-up.
Women with higher folate status were significantly less likely to be repeatedly HPV positive, more likely to become testnegative during the 2-year study, and 73% less likely to become newly HPV positive.
These associations held after controlling for other micronutrients and known risk factors for HPV. The authors reviewed a possible role of folate in preventing integration of HPV, thereby improving clearance of HPV infections, and documented that increased folate levels were also protective against the development of CIN 2/3.
Food fortification with folate became mandatory in the United States in 1998. The median folate level in women in this study mirrored the median post-fortification level for women in the United States—indicating that folate levels in food are not adequate to affect HPV status.
Therefore, it appears reasonable to advise women with HPV that taking folic acid supplementation in the levels usually advised for pregnant women may be helpful.
It could be the end of the affair with HPV!1 With this exclamation, Prof. Margaret Stanley, the noted human papillomavirus immunologist, expressed the optimism we all share, now that the possibility of conquering cervical cancer is within view. Not yet 25 years have passed since the first sequencing of a genital HPV type, and scarcely 10 years since the International Agency for Research on Cancer proclaimed that HPV causes cervical cancer. It has been 57 years since the discovery that launched an international quest to reduce the cervical cancer rate: George Papanicolaou’s test for early abnormal cell changes that, decades later, were found to be secondary to HPV. We’ve made great progress. What was the 2nd leading cancer in US women in incidence and mortality is now 11th in incidence and 13th in mortality.
But even with perfect attendance at annual screenings, women still get cervical cancer. And many still do not have screenings—they account for about half of all cervical cancers. And the Pap, as good as it is, has flaws. The test is subjective, and sensitivity varies from lab to lab and country to country.
What is new in 2006 that we may soon be able to put into practice, bringing us closer to a new world—with respect to cervical cancer prevention—different from any we’ve known?
- 1 More sensitive and objective screening
- 2 Better management of screen positives
- 3 HPV vaccine, soon to be in our offices
1 More sensitive and more objective screening
A comforting combo: Negative Pap and HPV tests
ACOG Practice Bulletin, Number 61. Human papillomavirus. Washington, DC: American College of Obstetricians and Gynecologists; April 2005.
Because HPV testing is more sensitive than cervical cytology in detecting CIN 2 and CIN 3, women with concurrent negative Pap and HPV tests can be reassured that their risk of unidentified CIN 2, CIN 3, or cervical cancer is approximately 1 in 1,000. (Level A evidence)
The American Cancer Society and the American College of Obstetricians and Gynecologists have both provided as an option the screening of women age 30 and older with the combination of the Pap and a test for high-risk HPV types.2,3 These “sophisticated new tests for the detection of HPV…hold great promise for improved screening for cervical cancer precursors and invasive cancer, and for triage of cervical cytology,” the Bulletin states.
Not all women get annual screening, however, and even if they do, the IARC estimates, the lifetime risk for cervical cancer for women who have conventional Paps annually is approximately 216 per 100,000, if the Pap sensitivity is about 70%. The prospect of reducing the risk of missing significant cervical neoplasia at each screen to 1 per 1,000 should be of comfort to women and the clinicians who watch over their health.
Dilemma: Women over 30, with normal Pap and high-risk HPV
What about the approximately 4% of women aged 30 and older with normal cytology and high-risk HPV? How should these women be managed? A panel of experts on HPV and cervical screening published “interim guidance” in 2004, recommending that until further data are available, these women should be retested in 6 to 12 months for persistence of HPV or development of abnormal cytology, and referred to colposcopy if still HPV-positive or if Pap results show low-grade squamous intraepithelial lesion (LSIL) or worse, regardless of HPV result.4
Although the April 2005 ACOG Bulletin affirmed that guideline, concern persisted that, while some women so identified might be better evaluated immediately by colposcopy, the majority would not, and there was no good way to identify HPV-positive women most at risk. Several longitudinal studies (discussed in the following section) have now made the path clearer.
REFERENCES
1. Stanley M. The end for genital human papillomavirus infections? Lancet Oncol 2005;6:256-257.
2. Sasow D, Runowicz CD, Solomon D, et al, for the American Cancer Society. American Cancer Society guideline for the early detection of cervical neoplasia and cancer. CA Cancer J Clin. 2002;52:342-362.
3. ACOG Practice Bulletin, Number 45. Cervical cytology screening. Washington, DC: American College of Obstetricians and Gynecologists; 2003.
4. Wright TC, Jr, Schiffman M, Solomon D, et al. Interim guidance for the use of human papillomavirus DNA testing as an adjunct to cervical cytology for screening. Obstet Gynecol. 2004;103:304-309.
Type-specific testing identifies highest risk
Khan MJ, Castle PE, Lorincz AT, et al. The elevated 10-year risk of cervical precancer and cancer in women with human papillomavirus (HPV) type 16 or 18 and the possible utility of type-specific HPV testing in clinical practice. J Natl Cancer Inst. 2005;97:1072–1079.
HPV screening that distinguishes types 16 and 18 from other oncogenic (high-risk) HPV types identifies women at the greatest risk of CIN 2/3+ and may permit less aggressive management of women with other high-risk HPV infections.
The solution to the dilemma of having to wait 6 to 12 months to repeat Pap and HPV tests for women with a normal cytology but a positive HPV test before determining the need for colposcopy may be solved by type-specific HPV testing. The 10-year cumulative incidence of CIN 3 and cervical cancer (CIN 3+) in 20,810 women tested once for HPV at enrollment was only 0.8% in the women who tested negative for high-risk HPV by Hybrid Capture 2. In contrast, CIN 3+ developed in 17% of the HPV-16-positive women and 14% of the HPV-18-positive women within 10 years.
Women positive for other high-risk types of HPV, but negative for HPV 16 and 18 had far less risk: only 3% developed CIN 3+.
When stratified by age to limit the analysis to women aged 30 and older, the cumulative incidence of CIN 3+ was 20% in HPV-16-positive women and 15% in HPV-18-positive women (FIGURE 1). Contrast these results to the 10-year predictive value of 11% for an LSIL Pap for the same level of cervical neoplasia. In other words, a single positive HPV 16 or 18 test is almost twice as likely to identify women at high risk for CIN 3+ as an LSIL Pap result, over time.
FIGURE 1 Positive HPV 16 or 18 linked to 14% to 17% incidence of CIN3+

The cumulative incidence of CIN 3+ over a 10-year period, as a function of a single HPV test result at enrollment. Women positive for HPV 16 or 18 had a much greater incidence of CIN 3+, compared to women negative for HPV 16 and 18 but positive for other high-risk HPV types by Hybrid Capture 2, or negative for all high-risk HPV types. Adapted from Khan et al.
Follow-up according to risk
These findings support a follow-up strategy that would permit risk stratification of HPV-infected women for whom an optimal repeat screening interval has been unclear.
- Women positive for HPV 16 or 18 warrant referral to colposcopy, for they carry the majority of risk from a positive high-risk HPV test.
- Women positive only for other high-risk types could be reassured of the safety of a 12-month interval without colposcopy, and referred to colposcopy only if the repeat Pap shows worse than atypical squamous cells of undetermined significance (ASC-US) or the HPV test is again positive (FIGURE 2).
FIGURE 2 Type-specific testing in clinical practice

Proposed management of women aged 30 or older, who are screened concurrently with both a Pap test and an HPV test, with typing for HPV 16/18. Adapted from Khan et al.
2 type-specific tests in the pipeline
Currently, the only FDA-approved test for combined screening of women aged 30 and older is the Hybrid Capture 2 High-risk HPV test, which tests for a panel of the 13 most common HPV types known to cause cervical cancer, but does not report on individual types.
But 2 type-specific HPV tests may become available in 2006, which would enable clinicians to follow this strategy.
Digene is nearly ready to launch a 16, 18, 45 type-specific “reflex” test (to a positive Hybrid Capture 2 HPV panel), and Roche is preparing to get its type-specific Linear Array HPV test approved.
2 Better management of screen positives
New practice bulletin on managing abnormal tests
ACOG Practice Bulletin, Number 66. Management of abnormal cervical cytology and histology. Washington, DC: American College of Obstetricians and Gynecologists; September 2005.
The new Practice Bulletin published last September in most respects mirrors the most recent American Society for Colposcopy and Cervical Pathology (ASCCP) Consensus guidelines.1
Key points
- ASC-US may be managed by referral to immediate colposcopy, by repeat Pap, or by HPV testing. However, “reflex HPV testing” when ASC-US is derived from liquid-based cytology has advantages. (It is estimated that a large majority of ASC-US is now managed by HPV testing.)
- Initial management of all other Pap abnormalities is by immediate referral to colposcopy, ie, the finding of atypical squamous cells cannot rule out high-grade (ASC-H), atypical glandular cells (AGC), LSIL, and high-grade intraepithelial lesions (HSIL).
- Management of ASC-US and LSIL in adolescence and postmenopause: ACOG provides an alternative strategy for adolescents with either ASC-US or LSIL cytology, who may have either repeat cytology at 6 and 12 months or a single HPV test at 12 months. ACOG did not differentiate postmenopausal women with either ASC-US or LSIL as “special situations” with additional management strategies.
- CIN 2/3 should usually be treated, both guidelines say. The only exception is the adolescent with CIN 2, who may be followed with repeat cytology and colposcopy at 4 to 6 months if she is deemed reliable for follow-up, the colposcopy is adequate, and the endocervical sampling is negative.
- HPV-positive ASC-US, ASC-H, or LSIL and either CIN 1 or normal colposcopy findings should be followed by repeat Pap at 6 and 12 months, or a single HPV test at 12 months, with referral to colposcopy if either the Pap results show ASC-US or more advanced abnormality or the HPV test is positive.
- In contrast, an excisional procedure is required for normal findings, or an unsatisfactory colposcopy in nonpregnant women referred for atypical glandular cells “favor neoplasia” (AGC-H), or adenocarcinoma in situ (AIS), or repeat atypical glandular cells “not otherwise specified” (AGC-NOS), or HSIL. The only exception is an adolescent with HSIL cytology and a satisfactory and normal colposcopy and biopsy, who may be followed closely.
- Women treated for CIN 2/3 can be monitored after treatment by cytology screening at 6-month intervals 3 or 4 times or by a single HPV test at 6 months, before returning to annual screening. Any repeat abnormal Pap at the threshold of ASC-US or more advanced abnormality or a positive HPV test requires colposcopic evaluation.
REFERENCES
1. Wright TC, Jr, Cox JT, Massad LS, Carlson J, Twiggs LB, Wilkinson EJ. American Society for Colposcopy and Cervical Pathology. 2001 consensus guidelines for the management of women with cervical intraepithelial neoplasia. Am J Obstet Gynecol. 2003;189:295-304.
3 HPV vaccine, soon to be in our offices
Vaccines will stop CIN 2/3 and cancer
Mao C, Koutsky LA, Ault KA, et al. Efficacy of human papillomavirus-16 vaccine to prevent cervical intraepithelial neoplasia: a randomized controlled trial. Obstet Gynecol. 2006;107:18–27.
The HPV 16 vaccine provided 100% protection against development of HPV-16-related CIN 2/3 during an average of 3.5 years of follow-up.

This preliminary study to the Quadrivalent HPV 6, 11, 16, 18 trial on an HPV 16 virus-like particle (VLP) vaccine reached an average of 3.5 years of follow-up. CIN 2/3 developed in 12 of the 750 women receiving placebo, in contrast to none of the 755 vaccine recipients. Persistent HPV 16 infections were defined as testing positive for type-specific HPV 16 on 2 or more visits, with the caveat that women testing positive on the last visit were considered persistent because they would have no further follow-up to determine that status. As a result, some women with a positive test only on the last visit were included as “persisters,” perhaps explaining why the efficacy in preventing persistent HPV 16 in the vaccine recipients was only 94%. Single-test positives can be transient infections, vaginal contamination with infected cells from a partner during recent intercourse, or early persistent infections. Although antibody titers to HPV 16 in vaccine recipients waned over time, they still exceeded titers in placebo recipients who already had natural immunity to HPV 16.

Benefit of the HPV 16 vaccine was also seen for women already HPV-16 positive at enrollment, but only if they were seronegative for HPV 16. It is possible that, if an immune response has not yet been mounted, the vaccine may still have a positive effect for women already HPV-16 infected.
Who will be vaccinated?
Although the primary target group for the HPV vaccine will be children before natural exposure can occur after the onset of sexual activity, many women already sexually active will likely want to be vaccinated.
It is this “catch-up” group that will challenge the OBGyn to become familiar with and to provide the HPV vaccine when it becomes available, likely later this year.
Quadrivalent vaccine 100% effective
Skjeldstad FE, Koutsky LA, for the Merck Phase 3 HPV Vaccine Steering Committee (Future II). Phase II trial of prophylactic quadrivalent HPV 6, 11, 16, 18 L1 virus-like particle (VLP) vaccine; prevention of cervical intraepithelial neoplasia (CIN) 2/3 including adeno- and squamous cell carcinoma in situ (CIS). Presented at: Infectious Diseases Society of America, Late Breaker Session 66, LB–8A; October 7, 2005; San Francisco, Calif.
The Quadrivalent HPV 6, 11, 16, 18 vaccine provided 100% protection against persistent HPV 16/18 and HPV 16/18-related CIN 2/3.
This trial became center stage in the world media in early October 2005, with headlines such as “First anti-cancer vaccine 100% effective.” The results are truly astounding, as there were no CIN 2/3 cases in the Per Protocol group, among the 5,301 women vaccinated, in contrast to 21 cases in the 5,258 women who received the placebo (TABLE 1).
In the group most likely to mirror a typical vaccinated population, only 1 case of HPV-16/18-positive CIN 2/3 occurred in the 5,736 vaccinated women, some of whom were already positive for 1 or more HPV types, or had serologic evidence of prior type-specific infection, or received fewer than the 3 recommended doses. In contrast, 36 HPV-16/18 CIN 2/3 occurred in the 5,766 women who received placebo.
TABLE 1
Efficacy of quadrivalent HPV 6, 11, 16, 18 vaccine in preventing CIN 2/3
| VACCINE GROUPS | PLACEBO GROUPS | EFFICACY | |||||
|---|---|---|---|---|---|---|---|
| NUMBER OF WOMEN | NUMBER OF CIN 2/3 CASES | RATE | NUMBER OF WOMEN | NUMBER OF CIN 2/3 CASES | RATE | % | |
| Per protocol | 5,301 | 0 | 0.0 | 5,258 | 21 | 0.3 | 100 |
| Modified intention to treat | 5,301 | 1 | <0.1 | 5,766 | 36 | 0.3 | 97 |
| Source: Skjeldstad et al | |||||||
No warts, either
Subsequent analysis revealed similar protection from HPV 6 or 11 genital warts.
No serious adverse events were recorded in the entire trial.
Because HPV 16 and 18 together cause approximately 70% of all cervical cancers, and HPV 6 and 11 cause 90% of genital warts, these results are surely something about which to rejoice!
Gardasil and Cervarix vaccines
Now the challenge will be in getting the population vaccinated. Merck is expected to have its Gardasil Quadrivalent vaccine on the market mid- to late 2006. GlaxoSmithKline expects to put Cervarix Bivalent HPV 16, 18 vaccine on the market sometime in 2007.
How HPV vaccine will—and won’t—change practice
Franco EL, Harper DM. Vaccination against human papillomavirus infection: a new paradigm in cervical cancer control. Vaccine. 2005;23:2388–2394.
Cervical screening will continue, but will be more accurate and more efficient.
Yes, we are on the verge of the possibility of reducing the risk of cervical cancer to close to zero, but it will take decades. Vaccinating young girls will not significantly reduce cervical cancer rates until these girls reach the median ages of microinvasive (early 40s) and invasive (late 40s) cervical cancer.
Even then, cervical cancer rates will depend on these factors:
- the extent of vaccination coverage
- the number of high-risk HPV types in the vaccine
- whether vaccination provides multidecade protection or falls off with time
- whether the medical community and the public continue to diligently follow recommended screening guidelines
If immune protection falls with time, booster HPV vaccine shots should provide ongoing protection, but population protection will depend on the percent of the population obtaining the booster. If the population becomes complacent about cervical screening as risk for cervical cancer decreases, then cancers will develop that would have otherwise been prevented.
Why screening will continue
Virus-like particle (VLP) vaccines for all of the important oncogenic HPV types could, theoretically, be produced. But until long after multivalent HPV vaccines that include all the important oncogenic types are available, women will require screening to prevent the 30% of cancers that occur from other high-risk HPV types not in the present vaccine. And, we will need screening to protect women who are not vaccinated, and those already infected.
As Franco and Harper stressed, “Although the future seems bright on the vaccine front, policy makers are strongly cautioned to avoid scaling back cervical cancer screening. Any premature relaxation of cervical cancer control measures already in place will bring a resurgence of the disease to the unacceptable levels of the not too distant past.”
In other words, cervical screening will continue for the foreseeable future.
A peek at a “new world”
Fewer abnormal Pap tests. The vaccine will likely steadily decrease the rate of abnormal Paps that are important, as an increasing proportion of women are vaccinated against the 2 most common types in high-grade CIN.
Colposcopies and cervical treatments will decline in number coincident with the proportion of the population vaccinated.
A training challenge? This change will decrease the number of significant lesions that a colposcopist may see, increasing the challenge of training and maintaining expertise in identification and treatment of these lesions. As significant Pap abnormalities decrease, maintaining expertise in cytologic interpretation, and even in maintaining attention to detail, may become more difficult.
Specific testing. Finding women with significant abnormalities may more and more be accomplished with the accuracy afforded by testing for specific HPV types known to be most at-risk for CIN 3+.
With respect to cervical cancer prevention, the years to come will surely be a new world, different from what we all have known.
Piyathilake CJ, Henao OL, Macaluso M, et al. Folate is associated with the natural history of high-risk human papillomaviruses. Cancer Res. 2004;64:8788–8793.
Improving folate status in women at risk of getting infected or already infected with high-risk HPV may help prevent cervical cancer. It is reasonable to advise women with HPV that folate supplements may be helpful.
Recommending oral folate supplements is one of the few things we can offer that can empower our patients with something positive that they can do for themselves.
A subset of women in the ASCUS LSIL Triage (ALT) study were evaluated prospectively to determine whether systemic levels of folic acid are associated with the occurrence and duration of HPV infections after controlling for other micronutrients (vitamins B12, A, E, C, and total carotene) and for known risk factors for high-risk HPV infections and cervical cancer. Hybrid Capture 2 and serum levels of these micronutrients were obtained at 6-month intervals throughout the trial’s 2-year follow-up.
Women with higher folate status were significantly less likely to be repeatedly HPV positive, more likely to become testnegative during the 2-year study, and 73% less likely to become newly HPV positive.
These associations held after controlling for other micronutrients and known risk factors for HPV. The authors reviewed a possible role of folate in preventing integration of HPV, thereby improving clearance of HPV infections, and documented that increased folate levels were also protective against the development of CIN 2/3.
Food fortification with folate became mandatory in the United States in 1998. The median folate level in women in this study mirrored the median post-fortification level for women in the United States—indicating that folate levels in food are not adequate to affect HPV status.
Therefore, it appears reasonable to advise women with HPV that taking folic acid supplementation in the levels usually advised for pregnant women may be helpful.
Dr. Cox served as a member of the American Cancer Society Cervical Guidelines Committee, the 2001 Bethesda Workshop, and was one of the primary authors of the 2001 ASCCP Consensus Guidelines for the management of women with abnormal cervical cytology and cervical cancer precursors. He is President-elect of the American Society for Colposcopy and Cervical Pathology and presently is on the ACS HPV Vaccine Advisory Committee, and the Data and Safety Monitoring Board of the HPV 6, 11, 16, 18 Quadrivalent Vaccine Trial. Dr. Cox is a consultant to Digene, GlaxoSmithKline, and the Merck Data and Safety Monitoring Board, and a speaker for Digene.
Dr. Cox served as a member of the American Cancer Society Cervical Guidelines Committee, the 2001 Bethesda Workshop, and was one of the primary authors of the 2001 ASCCP Consensus Guidelines for the management of women with abnormal cervical cytology and cervical cancer precursors. He is President-elect of the American Society for Colposcopy and Cervical Pathology and presently is on the ACS HPV Vaccine Advisory Committee, and the Data and Safety Monitoring Board of the HPV 6, 11, 16, 18 Quadrivalent Vaccine Trial. Dr. Cox is a consultant to Digene, GlaxoSmithKline, and the Merck Data and Safety Monitoring Board, and a speaker for Digene.
