User login
- All psychotropic medications cross the placenta. If psychotropic medication is used, prescribe carefully during the first trimester, giving the minimum number of drugs and the lowest dosages needed to restore or maintain well-being.
- No psychotropic agents are FDA-approved for use in pregnancy.
- Teratogenicity notwithstanding, psychotropic intervention is the most effective treatment for women with bipolar disorder.
- We recommend that women who continue to take valproate or carbamazepine during pregnancy receive folate, 3 to 4 mg/d, as a precaution.
Managing gravidas with bipolar disorder requires obstetricians to balance the potential for neonatal malformations against the high risk of relapse when patients discontinue medications.1 This article offers an evidence-based approach that includes:
- analysis of the US Food and Drug Administration’s (FDA) teratogenicity categories for psychotropics
- safety profiles of drugs used in mood stabilization
- an algorithm for managing patients who are considering conception or are pregnant
When a patient is at risk for severe psychiatric problems before, during, and after pregnancy, a collaborative effort between the obstetrician and psychiatrist may be best for both the patient and her child.
Further, because low maternal folate levels are associated with neural tube defects, and some anticonvulsant agents used for mood stabilization diminish supplies of the nutrient, supplementation is essential. (See “Folate and neural tube defects”.)
Psychotropic risks to offspring
All psychotropic medications cross the placenta, exposing the fetus to some degree. Risks include teratogenicity, obstetric complications, perinatal syndromes, and long-term postnatal behavioral sequelae.
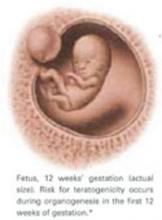
Teratogenicity. A medication is considered teratogenic when prenatal exposure significantly increases the risk of congenital deformities over the baseline, which is 2% in the United States.2 The cause of most congenital malformations is unknown. Risk for teratogenicity occurs during organogenesis in the first 12 weeks of gestation.
Obstetric complications include preterm delivery, low birth weight, and delivery complications such as low Apgar scores or behavioral effects requiring intensive care.
Perinatal syndromes include physical and behavioral symptoms noticed in the infant shortly after birth (such as jitteriness). These consequences are putatively related to drug use at or near birth and have limited duration.
Postnatal behavioral sequelae include long-term neurobehavioral abnormalities in children who were exposed to psychotropics in utero.
Balancing risks
Risks with medication. The FDA’s “use-in-pregnancy” rating system (TABLE 1) uses available data to assess the degree of teratogenic risk. These guidelines are one of many tools to use when considering drug treatment.
Most psychotropics are category “C” or “D,” which imply a chance of harm to the exposed fetus. Category “B” drugs would appear safer, but this rating could simply indicate a lack of adequate human data or that no data have shown harm in animals.
Moreover, a category “D” drug may be chosen more often during pregnancy than a category “C” drug. This may occur when more human data exist on using the category “D” drug in patients with a particular disorder (such as using lithium versus valproate or olanzapine in pregnant women).
No psychotropics are classified as “A,” meaning either some risks are associated with every psychotropic or the risk of some agents has not been adequately explored. Furthermore, no psychotropics are FDA-approved for use during pregnancy.
Risks without medication. Teratogenicity notwithstanding, psychotropic intervention is the most effective treatment for women with bipolar disorder. Patients who discontinue mood-stabilizing medication after conception significantly increase their likelihood of a relapse into depression or mania,3 either of which could lead to complications and untoward effects on the fetus.
Depression during pregnancy has been linked to low birth weight and preterm delivery.4,5 These effects may be mediated by the illness itself or by other factors that indirectly affect birth outcomes. For example, depression during pregnancy is associated with decreased appetite, substance use and abuse, and lower use of prenatal care.6
Untreated mania may also be associated with perinatal risks, as a pregnant patient in a manic state may engage in impulsive, high-risk behaviors that endanger herself and her fetus.7
TABLE 1
FDA use-in-pregnancy ratings for medications
| CATEGORY | DESCRIPTION |
|---|---|
| A | Adequate, well-controlled studies in pregnant women have not shown an increased risk of fetal abnormalities |
| B | Animal studies have revealed no evidence of harm to the fetus; however, no adequate and well-controlled studies have been conducted in pregnant women |
| OR | |
| Animal studies have shown an adverse effect, but adequate and well-controlled studies in pregnant women have failed to demonstrate a risk to the fetus | |
| C | Animal studies have shown an adverse effect and no adequate and well-controlled studies have been conducted in pregnant women |
| OR | |
| No animal studies have been conducted and no adequate and well-controlled studies have been conducted in pregnant women | |
| D | Studies—adequate and well-controlled or observational—in pregnant women have demonstrateda risk to the fetus. However, the benefits of therapy may outweigh the potential risk |
| X | Studies—adequate and well-controlled or observational—in animals or pregnant women have demonstrated positive evidence of fetal abnormalities. The use of the product is contraindicated in women who are or may become pregnant |
| Data from US Food and Drug Administration38 | |
Mood stabilizers
The 3 most commonly used mood stabilizers—lithium, valproate, and carbamazepine—are teratogenic. The least risk may occur with lithium (0.1%) versus valproate (2% to 5%) or carbamazepine (1% to 3%). These risks must be weighed against the up to 50% chance of relapse with medication discontinuation.3
The FDA designates these 3 medications as category as “D” agents (TABLE 2). This rating implies that studies have demonstrated fetal risk; still, the drug’s potential benefit may outweigh the risk.
Lithium. The International Registry of Lithium reported increased rates of cardiovascular malformations—such as Ebstein’s anomaly—in children whose mothers took lithium during pregnancy.
• Relative risk for Ebstein’s anomaly in children with fetal exposure to lithium may be 20 times higher than the risk in unexposed children, although the absolute risk with lithium exposure remains low (1 in 1,000 births).1,8
No significant neurobehavioral teratogenicity has been reported in infants exposed in utero to lithium, although few cases have been studied. One study reported that 22 lithium-exposed infants attained developmental milestones at a pace comparable to that of unexposed controls.9
•“Floppy baby” syndrome, in which infants experience hypotonicity and cyanosis, is the most recognized adverse effect in infants exposed to lithium in utero.10 Its frequency is unknown, but rare. Neonatal hypothyroidism and nephrogenic diabetes insipidus have also been documented.
Anticonvulsants. To date, no studies have examined the outcomes of children whose mothers took anticonvulsants for bipolar disorder during pregnancy, though the research concerning epileptic mothers is extensive.
• Neural tube defects. Infants exposed to anticonvulsant agents have a significantly greater risk for malformations than do neonates in the general population. Specifically, anticonvulsants may cause neural tube defects such as spina bifida, ancephaly, and encephaly in 2% to 5% of those exposed, as well as craniofacial anomalies, microcephaly, growth retardation, and heart defects.11-14
• Other minor malformations—such as rotated ears, depressed nasal bridge, short nose, elongated upper lip, and fingernail hypoplasia—have been reported in infants exposed to anticonvulsants in utero.14 These malformations disappear with age.13 Teratogenicity increases with the use of multiple anticonvulsants and possibly with higher maternal plasma levels and toxic metabolites.15
TABLE 2
FDA teratogenicity ratings of mood stabilizers, antimanic drugs, and antidepressants
| MEDICATION | PREGNANCY CATEGORY |
|---|---|
| Mood stabilizers | |
| Lithium | D |
| Carbamazepine | D |
| Valproate | D |
| Anticonvulsants | |
| Gabapentin | C |
| Lamotrigine | C |
| Topiramate | C |
| Antipsychotics | |
| Olanzapine | C |
| Risperidone | C |
| Chlorpromazine | C |
| Haloperidol | C |
| Trifluoperazine | C |
| Tricyclic antidepressants | |
| Amitriptyline | C |
| Clomipramine | C |
| Desipramine | C |
| Imipramine | C |
| Nortriptyline | D |
| Selective serotonin reuptake inhibitors | |
| Citalopram | C |
| Escitalopram | C |
| Fluoxetine | C |
| Fluvoxamine | C |
| Paroxetine | C |
| Sertraline | C |
| Other antidepressants | |
| Bupropion | B |
| Phenelzine | C |
| Tranylcypromine | C |
Antipsychotics
Psychotic illness itself may increase the risk of poor fetal outcome to a greater extent than does antipsychotic use. Prenatal exposure to low-potency phenothiazines may further increase this risk, although only slightly. The effect of prenatal exposure to atypical antipsychotics requires additional study.
Antipsychotics are often used to treat mania because of their rapid effects and sedative properties. Most antipsychotics—specifically, haloperidol, olanzapine, and risperidone—are designated as pregnancy category “C,” specifying that fetal risk cannot be ruled out.
Chlorpromazine and haloperidol have been most studied during pregnancy, but in relation to treating hyperemesis gravidarum and psychosis, not bipolar disorder. Results regarding antipsychotics’ teratogenic and behavioral risks are mixed,16-21 probably because the various compounds have different effects on the fetus.
The underlying illness—rather than the mother’s medications—may increase the rate of anomalies seen with exposure to antipsychotics:
• Rieder et al22 reported an increased rate of perinatal death in infants of schizophrenic mothers but no significant association between the mothers’ use of antipsychotics and perinatal death.
• Sobel23 compared psychotic women with and without histories of chlorpromazine exposure during pregnancy. Rates of fetal damage were similar and approximately twice that of the general population.
A meta-analysis of 74,337 live births revealed that first-trimester exposure to lowpotency antipsychotics increases the relative risk of fetal anomalies in nonpsychotic women. Phenothiazines may increase the 2% baseline incidence of malformations to 2.4%.1 No specific organ malformation following fetal exposure to phenothiazines has been consistently identified.
Olanzapine was recently approved for treating mania. Very little data exist regarding its impact on fetal development when used during pregnancy, though studies on small numbers of women have not revealed teratogenicity.24,25
Benzodiazepines
Benzodiazepines are rarely a primary treatment for mania or depression. Thus, a comprehensive review of their effect on fetal outcome is beyond the scope of this review. A meta-analysis of exposure during the first trimester suggests a very small but significant increase in risk for cleft palate.1 The absolute risk with exposure appears to be less than 1 in 1,000 cases.
Antidepressants
Acute mania is an emergency requiring immediate treatment. The other possibility in women with bipolar disorder is relapse into depression. An antidepressant should not be used without a mood stabilizer when treating bipolar I disorder. Similarly, a mood stabilizer alone may be inadequate to treat depression. The use of tricyclics or selective serotonin reuptake inhibitors (SSRIs) during pregnancy has not to date been associated with teratogenicity (TABLE 3),26 but larger numbers of women need to be studied. Perinatal effects in some cases have been reported.1
Tricyclics. In case-control studies of more than 300,000 live births, researchers followed 414 incidences of first-trimester exposure to tricyclics. They discovered no significant association between fetal exposure to tricyclics and increased rates of congenital malformations.1
The few studies that have been performed suggest that no long-term effects stem from exposure in utero.26 Although these results suggest that prenatal exposure to tricyclics is relatively safe, more research is needed.
SSRIs. No significant teratogenic effects of SSRIs have been identified.
The manufacturer’s register for fluoxetine contains approximately 2,000 cases of treated patients, with no excess cases of congenital anomalies or malformations following prenatal exposure. Citalopram has the next largest database of in-utero exposure (n = 365), again with no increased risk for teratogenicity. Several smaller systematic reports are available on in-utero exposure to sertraline, paroxetine, or escitalopram.26
Most studies of pregnant women taking fluoxetine during the first trimester found no increased risk of obstetric complications—including spontaneous pregnancy loss, preterm labor, or low birth weight—compared with women not taking fluoxetine. Third-trimester use of fluoxetine may increase the risk for perinatal complications,27 but this effect has been reported inconsistently and requires further study.26 Effects of other SSRIs during the third trimester have not been systematically explored.
Case reports and 1 controlled study have addressed possible perinatal symptoms from in-utero exposure to SSRIs.28,29 Preliminary data show no adverse neurobehavioral function in exposed neonates.26
Electroconvulsive therapy (ECT) has been proven effective for acute mania and depression, demonstrating few deleterious effects on neonates. ECT has few side effects and may be safer than drug therapy in this population. Two reviews support the efficacy and relative safety of ECT during pregnancy, although more evidence is needed.30,31
Recommendations
Discuss pregnancy and medication risks with all women with bipolar disorder, regardless of plans for pregnancy (FIGURE). If psychotropic medication is used, prescribe carefully during the first trimester, using the minimum number of drugs and the lowest dosages needed to restore or maintain well-being.32
Pros and cons of switching. Some clinicians may encourage a patient to taper a medication during the first trimester because of its unknown or high teratogenicity. Depending on the patient’s illness severity, this might not be the optimal decision. A more conservative option would be to switch to a lower-risk drug during pregnancy.
Lithium has both antidepressant and antimanic properties and is less teratogenic than anticonvulsants in the first trimester. However, if lithium has not been successful for the woman’s mania prophylaxis in the past and she has experienced antimanic response to an anticonvulsant, switching to lithium or another anticonvulsant is not recommended.
Folate and neural tube defects. First-trimester exposure to carbamazepine or valproate increases the risk for neural tube defects; using the lowest available dosage may decrease the risk for spina bifida—at least with valproate.
Low maternal folate levels are often associated with neural tube defects from any cause.33 Valproate lowers folate levels by inhibiting 1 of the enzymes necessary for its formation, which may be a mechanism for the increased risk of spina bifida.34
• Precautionary folate supplementation. No study has demonstrated that folate supplementation reduces the risk of neural tube defects in women taking anticonvulsants during pregnancy.35 Nonetheless, we recommend that women who continue to take valproate or carbamazepine during pregnancy receive folate, 3 to 4 mg/d, as a precaution.
• Treating manic relapse. Patients who stop taking lithium, particularly those who halt therapy abruptly, incur a high rate of relapse.3 Counsel women taking lithium to plan their pregnancies to allow enough time to taper off the medication before conception, if they want to try this. Lithium should be decreased slowly—by approximately 50% every 2 weeks—to avoid relapse. Treat aggressively if relapse occurs during pregnancy. Consider:
- psychiatric hospitalization in case of psychosis or suicidal ideation
- reinstituting drug therapy with a less teratogenic agent
- ECT for a manic or depressive episode
As the pregnancy advances and the mother’s volume of distribution increases, dosage increases may be needed to maintain therapeutic drug levels.
Treating depressive relapse. Should depression occur during pregnancy, SSRIs or tricyclics added to mood stabilizer therapy have been shown to be effective, with few teratogenic effects.
Cognitive-behavioral and interpersonal psychotherapies also have shown efficacy in pregnant women with major depressive disorder36 and may be effective for gravidas with bipolar disorder. Cognitive psychotherapies, when used with medication, have been reported effective in preventing relapse in nongravid bipolar patients.36,37
Dr. Altshuler reports that she is a scientific adviser and consultant for Abbott, Lilly, Forest, and AstraZeneca. Dr. Yonkers and Ms. Richards report no financial relationship with any companies whose products are mentioned in this article.
1. Altshuler L, Cohen L, Szuba MP, et al. Pharmacologic management of psychiatric illness during pregnancy: dilemmas and guidelines. Am J Psychiatry. 1996;153:592-606.
2. Nelson K, Holmes LB. Malformations due to presumed spontaneous mutations in newborn infants. N Engl J Med. 1989;320:19-23.
3. Viguera AC, Nonacs R, Cohen LS, et al. Risk of recurrence of bipolar disorder in pregnant and nonpregnant women after discontinuing lithium maintenance. Am J Psychiatry. 2000;157:179-184.
4. Steer RA, Scholl TO, Hediger ML, Fischer RL. Self-reported depression and negative pregnancy outcomes. J Clin Epidemiol. 1992;45:1093-1099.
5. Orr ST, Miller CA. Maternal depressive symptoms and the risk of poor pregnancy outcome. Review of the literature and preliminary findings. Epidemiol Rev. 1995;17:165-171.
6. Zuckerman B, Amaro H, Bauchner H, Cabral H. Depressive symptoms during pregnancy: relationship to poor health behaviors. Am J Obstet Gynecol. 1989;160:1107-1111.
7. Miller LJ. Psychotic denial of pregnancy: phenomenology and clinical management. Hosp Community Psychiatry. 1990;41:1233-1237.
8. Cohen LS, Friedman JM, Jefferson JW, et al. A reevaluation of risk of in utero exposure to lithium. JAMA. 1994;271:146-150;correction JAMA. 1994;271:1485.
9. Schou M. What happened later to the lithium babies? A follow-up study of children born without malformations. Acta Psychiatr Scand. 1976;54:193-197.
10. Woody JN, London WL, Wilbanks GD. Lithium toxicity in a newborn. Pediatrics. 1971;47:94-96.
11. Jones K, Lacro R, Johnson K, Adams J. Patterns of malformations in the children of women treated with carbamazepine during pregnancy. N Engl J Med. 1989;320:1661-1666.
12. Rosa F. Spina bifida in infants of women treated with carbamazepine during pregnancy. N Engl J Med. 1991;324:674-677.
13. Koch S, Losche G, Jager-Roman E, et al. Major and minor birth malformations and antiepileptic drugs. Neurology. 1992;42:83-88.
14. Jager-Roman E, Deichl A, Jakob S, et al. Fetal growth, major malformations, and minor anomalies in infants born to women receiving valproic acid. J Pediatr. 1986;108:997-1004.
15. Nakane Y, Okuma T, Takahashi R, et al. Multi-institutional study on the teratogenicity and fetal toxicity of antiepileptic drugs: a report of a collaborative study group in Japan. Epilepsia. 1980;21:663-680.
16. Edlund MJ, Craig TJ. Antipsychotic drug use and birth defects: an epidemiologic reassessment. Compr Psychiatry. 1984;25:32-38.
17. Kris EB. Children of mothers maintained on pharmacotherapy during pregnancy and postpartum. Curr Ther Res. 1965;7:785-789.
18. Clark CVH, Gorman D, Vernadakis A. Effects of prenatal administration of psychotropic drugs on behavior of developing rats. Dev Psychobiol. 1970;3:225-235.
19. Golub M, Kornetsky C. Seizure susceptibility and avoidance conditioning in adult rats treated prenatally with chlorpromazine. Dev Psychobiol. 1974;7:79-88.
20. Spear LP, Shalaby IA, Brick J. Chronic administration of haloperidol during development: behavioral and psychopharmacological effects. Psychopharmacology. (Berl) 1980;70:47-58.
21. Cagiano R, Barfield RJ, White NR, et al. Subtle behavioral changes produced in rat pups exposed in utero to haloperidol. Eur J Pharmacol. 1988;157:45-50.
22. Rieder RO, Rosenthal D, Wender P, Blumenthal H. The offspring of schizophrenics: fetal and neonatal deaths. Arch Gen Psychiatry. 1975;32:200-211.
23. Sobel DE. Fetal damage due to ECT, insulin coma, chlorpromazine, or reserpine. Arch Gen Psychiatry. 1960;2:606-611.
24. Dickson R. Olanzapine and pregnancy. Can J Psychiatry. 1998;43:196-197.
25. Goldstein DJ, Corbin LA, Fung MC. Olanzapine-exposed pregnancies and lactation; early experience. J Clin Psychopharmacol. 2000;24:399-403.
26. Altshuler LL, Cohen LS, Moline ML, et al. The expert consensus guideline series: treatment of depression in women. Postgrad Med. 2001;Mar(Spec No):1-22.
27. Chambers CD, Johnson KA, Dick LM, et al. Birth outcomes in pregnant women taking fluoxetine. N Engl J Med. 1996;335:1010-1015.
28. Spencer MJ. Fluoxetine hydrochloride (Prozac) toxicity in the neonate. Pediatrics. 1993;92:721-722.
29. Cabrera FM, Battaglia G. Delayed decreases in brain 5-HT 2a and 2c receptor density and function in male rat progeny following prenatal fluoxetine. J Pharmacol Exp Ther. 1994;269:637-645.
30. Miller LJ. Use of electroconvulsive therapy during pregnancy. Hosp Community Psychiatry. 1994;45:444-450.
31. Ferrill MJ, Kehoe WA, Jacisin JJ. ECT during pregnancy: physiologic and pharmacologic considerations. Convuls Ther. 1992;8:186-200.
32. Yonkers K, Wisner K, Cohen L, et al. Management of bipolar disorder during pregnancy and the postpartum period. Bipolar Consensus Statement. Submitted for publication.
33. Dansky L, Rosenblatt D, Andermann E. Mechanisms of teratogenesis: folic acid and antiepileptic therapy. Neurology. 1992;42(suppl 5):32-42.
34. Wegner C, Nau H. Alteration of embryonic folate metabolism by valproic acid during organogenesis: implications for mechanism of teratogenesis. Neurology. 1992;42(suppl 5):17-24.
35. MRC Vitamin Study Research Group. Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. Lancet. 1991;338:131-137.
36. Spinelli MG, Endicott J. Controlled clinical trial of interpersonal psychotherapy versus parenting education program for depressed pregnant women. Am J Psychiatry. 2003;160:555-562.
37. Lam DH, Watkins ER, Hayward P, et al. A randomized controlled study of cognitive therapy for relapse prevention for bipolar affective disorder: outcome in the first year. Arch Gen Psychiatry. 2003;60:145-152.
38. US Food and Drug Administration. Current categories for drug use in pregnancy. Available at: http://www.fda.gov/fdac/features/2001/301_preg.html#categories. Accessed November 19, 2003.
- All psychotropic medications cross the placenta. If psychotropic medication is used, prescribe carefully during the first trimester, giving the minimum number of drugs and the lowest dosages needed to restore or maintain well-being.
- No psychotropic agents are FDA-approved for use in pregnancy.
- Teratogenicity notwithstanding, psychotropic intervention is the most effective treatment for women with bipolar disorder.
- We recommend that women who continue to take valproate or carbamazepine during pregnancy receive folate, 3 to 4 mg/d, as a precaution.
Managing gravidas with bipolar disorder requires obstetricians to balance the potential for neonatal malformations against the high risk of relapse when patients discontinue medications.1 This article offers an evidence-based approach that includes:
- analysis of the US Food and Drug Administration’s (FDA) teratogenicity categories for psychotropics
- safety profiles of drugs used in mood stabilization
- an algorithm for managing patients who are considering conception or are pregnant
When a patient is at risk for severe psychiatric problems before, during, and after pregnancy, a collaborative effort between the obstetrician and psychiatrist may be best for both the patient and her child.
Further, because low maternal folate levels are associated with neural tube defects, and some anticonvulsant agents used for mood stabilization diminish supplies of the nutrient, supplementation is essential. (See “Folate and neural tube defects”.)
Psychotropic risks to offspring
All psychotropic medications cross the placenta, exposing the fetus to some degree. Risks include teratogenicity, obstetric complications, perinatal syndromes, and long-term postnatal behavioral sequelae.
Teratogenicity. A medication is considered teratogenic when prenatal exposure significantly increases the risk of congenital deformities over the baseline, which is 2% in the United States.2 The cause of most congenital malformations is unknown. Risk for teratogenicity occurs during organogenesis in the first 12 weeks of gestation.
Obstetric complications include preterm delivery, low birth weight, and delivery complications such as low Apgar scores or behavioral effects requiring intensive care.
Perinatal syndromes include physical and behavioral symptoms noticed in the infant shortly after birth (such as jitteriness). These consequences are putatively related to drug use at or near birth and have limited duration.
Postnatal behavioral sequelae include long-term neurobehavioral abnormalities in children who were exposed to psychotropics in utero.
Balancing risks
Risks with medication. The FDA’s “use-in-pregnancy” rating system (TABLE 1) uses available data to assess the degree of teratogenic risk. These guidelines are one of many tools to use when considering drug treatment.
Most psychotropics are category “C” or “D,” which imply a chance of harm to the exposed fetus. Category “B” drugs would appear safer, but this rating could simply indicate a lack of adequate human data or that no data have shown harm in animals.
Moreover, a category “D” drug may be chosen more often during pregnancy than a category “C” drug. This may occur when more human data exist on using the category “D” drug in patients with a particular disorder (such as using lithium versus valproate or olanzapine in pregnant women).
No psychotropics are classified as “A,” meaning either some risks are associated with every psychotropic or the risk of some agents has not been adequately explored. Furthermore, no psychotropics are FDA-approved for use during pregnancy.
Risks without medication. Teratogenicity notwithstanding, psychotropic intervention is the most effective treatment for women with bipolar disorder. Patients who discontinue mood-stabilizing medication after conception significantly increase their likelihood of a relapse into depression or mania,3 either of which could lead to complications and untoward effects on the fetus.
Depression during pregnancy has been linked to low birth weight and preterm delivery.4,5 These effects may be mediated by the illness itself or by other factors that indirectly affect birth outcomes. For example, depression during pregnancy is associated with decreased appetite, substance use and abuse, and lower use of prenatal care.6
Untreated mania may also be associated with perinatal risks, as a pregnant patient in a manic state may engage in impulsive, high-risk behaviors that endanger herself and her fetus.7
TABLE 1
FDA use-in-pregnancy ratings for medications
| CATEGORY | DESCRIPTION |
|---|---|
| A | Adequate, well-controlled studies in pregnant women have not shown an increased risk of fetal abnormalities |
| B | Animal studies have revealed no evidence of harm to the fetus; however, no adequate and well-controlled studies have been conducted in pregnant women |
| OR | |
| Animal studies have shown an adverse effect, but adequate and well-controlled studies in pregnant women have failed to demonstrate a risk to the fetus | |
| C | Animal studies have shown an adverse effect and no adequate and well-controlled studies have been conducted in pregnant women |
| OR | |
| No animal studies have been conducted and no adequate and well-controlled studies have been conducted in pregnant women | |
| D | Studies—adequate and well-controlled or observational—in pregnant women have demonstrateda risk to the fetus. However, the benefits of therapy may outweigh the potential risk |
| X | Studies—adequate and well-controlled or observational—in animals or pregnant women have demonstrated positive evidence of fetal abnormalities. The use of the product is contraindicated in women who are or may become pregnant |
| Data from US Food and Drug Administration38 | |
Mood stabilizers
The 3 most commonly used mood stabilizers—lithium, valproate, and carbamazepine—are teratogenic. The least risk may occur with lithium (0.1%) versus valproate (2% to 5%) or carbamazepine (1% to 3%). These risks must be weighed against the up to 50% chance of relapse with medication discontinuation.3
The FDA designates these 3 medications as category as “D” agents (TABLE 2). This rating implies that studies have demonstrated fetal risk; still, the drug’s potential benefit may outweigh the risk.
Lithium. The International Registry of Lithium reported increased rates of cardiovascular malformations—such as Ebstein’s anomaly—in children whose mothers took lithium during pregnancy.
• Relative risk for Ebstein’s anomaly in children with fetal exposure to lithium may be 20 times higher than the risk in unexposed children, although the absolute risk with lithium exposure remains low (1 in 1,000 births).1,8
No significant neurobehavioral teratogenicity has been reported in infants exposed in utero to lithium, although few cases have been studied. One study reported that 22 lithium-exposed infants attained developmental milestones at a pace comparable to that of unexposed controls.9
•“Floppy baby” syndrome, in which infants experience hypotonicity and cyanosis, is the most recognized adverse effect in infants exposed to lithium in utero.10 Its frequency is unknown, but rare. Neonatal hypothyroidism and nephrogenic diabetes insipidus have also been documented.
Anticonvulsants. To date, no studies have examined the outcomes of children whose mothers took anticonvulsants for bipolar disorder during pregnancy, though the research concerning epileptic mothers is extensive.
• Neural tube defects. Infants exposed to anticonvulsant agents have a significantly greater risk for malformations than do neonates in the general population. Specifically, anticonvulsants may cause neural tube defects such as spina bifida, ancephaly, and encephaly in 2% to 5% of those exposed, as well as craniofacial anomalies, microcephaly, growth retardation, and heart defects.11-14
• Other minor malformations—such as rotated ears, depressed nasal bridge, short nose, elongated upper lip, and fingernail hypoplasia—have been reported in infants exposed to anticonvulsants in utero.14 These malformations disappear with age.13 Teratogenicity increases with the use of multiple anticonvulsants and possibly with higher maternal plasma levels and toxic metabolites.15
TABLE 2
FDA teratogenicity ratings of mood stabilizers, antimanic drugs, and antidepressants
| MEDICATION | PREGNANCY CATEGORY |
|---|---|
| Mood stabilizers | |
| Lithium | D |
| Carbamazepine | D |
| Valproate | D |
| Anticonvulsants | |
| Gabapentin | C |
| Lamotrigine | C |
| Topiramate | C |
| Antipsychotics | |
| Olanzapine | C |
| Risperidone | C |
| Chlorpromazine | C |
| Haloperidol | C |
| Trifluoperazine | C |
| Tricyclic antidepressants | |
| Amitriptyline | C |
| Clomipramine | C |
| Desipramine | C |
| Imipramine | C |
| Nortriptyline | D |
| Selective serotonin reuptake inhibitors | |
| Citalopram | C |
| Escitalopram | C |
| Fluoxetine | C |
| Fluvoxamine | C |
| Paroxetine | C |
| Sertraline | C |
| Other antidepressants | |
| Bupropion | B |
| Phenelzine | C |
| Tranylcypromine | C |
Antipsychotics
Psychotic illness itself may increase the risk of poor fetal outcome to a greater extent than does antipsychotic use. Prenatal exposure to low-potency phenothiazines may further increase this risk, although only slightly. The effect of prenatal exposure to atypical antipsychotics requires additional study.
Antipsychotics are often used to treat mania because of their rapid effects and sedative properties. Most antipsychotics—specifically, haloperidol, olanzapine, and risperidone—are designated as pregnancy category “C,” specifying that fetal risk cannot be ruled out.
Chlorpromazine and haloperidol have been most studied during pregnancy, but in relation to treating hyperemesis gravidarum and psychosis, not bipolar disorder. Results regarding antipsychotics’ teratogenic and behavioral risks are mixed,16-21 probably because the various compounds have different effects on the fetus.
The underlying illness—rather than the mother’s medications—may increase the rate of anomalies seen with exposure to antipsychotics:
• Rieder et al22 reported an increased rate of perinatal death in infants of schizophrenic mothers but no significant association between the mothers’ use of antipsychotics and perinatal death.
• Sobel23 compared psychotic women with and without histories of chlorpromazine exposure during pregnancy. Rates of fetal damage were similar and approximately twice that of the general population.
A meta-analysis of 74,337 live births revealed that first-trimester exposure to lowpotency antipsychotics increases the relative risk of fetal anomalies in nonpsychotic women. Phenothiazines may increase the 2% baseline incidence of malformations to 2.4%.1 No specific organ malformation following fetal exposure to phenothiazines has been consistently identified.
Olanzapine was recently approved for treating mania. Very little data exist regarding its impact on fetal development when used during pregnancy, though studies on small numbers of women have not revealed teratogenicity.24,25
Benzodiazepines
Benzodiazepines are rarely a primary treatment for mania or depression. Thus, a comprehensive review of their effect on fetal outcome is beyond the scope of this review. A meta-analysis of exposure during the first trimester suggests a very small but significant increase in risk for cleft palate.1 The absolute risk with exposure appears to be less than 1 in 1,000 cases.
Antidepressants
Acute mania is an emergency requiring immediate treatment. The other possibility in women with bipolar disorder is relapse into depression. An antidepressant should not be used without a mood stabilizer when treating bipolar I disorder. Similarly, a mood stabilizer alone may be inadequate to treat depression. The use of tricyclics or selective serotonin reuptake inhibitors (SSRIs) during pregnancy has not to date been associated with teratogenicity (TABLE 3),26 but larger numbers of women need to be studied. Perinatal effects in some cases have been reported.1
Tricyclics. In case-control studies of more than 300,000 live births, researchers followed 414 incidences of first-trimester exposure to tricyclics. They discovered no significant association between fetal exposure to tricyclics and increased rates of congenital malformations.1
The few studies that have been performed suggest that no long-term effects stem from exposure in utero.26 Although these results suggest that prenatal exposure to tricyclics is relatively safe, more research is needed.
SSRIs. No significant teratogenic effects of SSRIs have been identified.
The manufacturer’s register for fluoxetine contains approximately 2,000 cases of treated patients, with no excess cases of congenital anomalies or malformations following prenatal exposure. Citalopram has the next largest database of in-utero exposure (n = 365), again with no increased risk for teratogenicity. Several smaller systematic reports are available on in-utero exposure to sertraline, paroxetine, or escitalopram.26
Most studies of pregnant women taking fluoxetine during the first trimester found no increased risk of obstetric complications—including spontaneous pregnancy loss, preterm labor, or low birth weight—compared with women not taking fluoxetine. Third-trimester use of fluoxetine may increase the risk for perinatal complications,27 but this effect has been reported inconsistently and requires further study.26 Effects of other SSRIs during the third trimester have not been systematically explored.
Case reports and 1 controlled study have addressed possible perinatal symptoms from in-utero exposure to SSRIs.28,29 Preliminary data show no adverse neurobehavioral function in exposed neonates.26
Electroconvulsive therapy (ECT) has been proven effective for acute mania and depression, demonstrating few deleterious effects on neonates. ECT has few side effects and may be safer than drug therapy in this population. Two reviews support the efficacy and relative safety of ECT during pregnancy, although more evidence is needed.30,31
Recommendations
Discuss pregnancy and medication risks with all women with bipolar disorder, regardless of plans for pregnancy (FIGURE). If psychotropic medication is used, prescribe carefully during the first trimester, using the minimum number of drugs and the lowest dosages needed to restore or maintain well-being.32
Pros and cons of switching. Some clinicians may encourage a patient to taper a medication during the first trimester because of its unknown or high teratogenicity. Depending on the patient’s illness severity, this might not be the optimal decision. A more conservative option would be to switch to a lower-risk drug during pregnancy.
Lithium has both antidepressant and antimanic properties and is less teratogenic than anticonvulsants in the first trimester. However, if lithium has not been successful for the woman’s mania prophylaxis in the past and she has experienced antimanic response to an anticonvulsant, switching to lithium or another anticonvulsant is not recommended.
Folate and neural tube defects. First-trimester exposure to carbamazepine or valproate increases the risk for neural tube defects; using the lowest available dosage may decrease the risk for spina bifida—at least with valproate.
Low maternal folate levels are often associated with neural tube defects from any cause.33 Valproate lowers folate levels by inhibiting 1 of the enzymes necessary for its formation, which may be a mechanism for the increased risk of spina bifida.34
• Precautionary folate supplementation. No study has demonstrated that folate supplementation reduces the risk of neural tube defects in women taking anticonvulsants during pregnancy.35 Nonetheless, we recommend that women who continue to take valproate or carbamazepine during pregnancy receive folate, 3 to 4 mg/d, as a precaution.
• Treating manic relapse. Patients who stop taking lithium, particularly those who halt therapy abruptly, incur a high rate of relapse.3 Counsel women taking lithium to plan their pregnancies to allow enough time to taper off the medication before conception, if they want to try this. Lithium should be decreased slowly—by approximately 50% every 2 weeks—to avoid relapse. Treat aggressively if relapse occurs during pregnancy. Consider:
- psychiatric hospitalization in case of psychosis or suicidal ideation
- reinstituting drug therapy with a less teratogenic agent
- ECT for a manic or depressive episode
As the pregnancy advances and the mother’s volume of distribution increases, dosage increases may be needed to maintain therapeutic drug levels.
Treating depressive relapse. Should depression occur during pregnancy, SSRIs or tricyclics added to mood stabilizer therapy have been shown to be effective, with few teratogenic effects.
Cognitive-behavioral and interpersonal psychotherapies also have shown efficacy in pregnant women with major depressive disorder36 and may be effective for gravidas with bipolar disorder. Cognitive psychotherapies, when used with medication, have been reported effective in preventing relapse in nongravid bipolar patients.36,37
Dr. Altshuler reports that she is a scientific adviser and consultant for Abbott, Lilly, Forest, and AstraZeneca. Dr. Yonkers and Ms. Richards report no financial relationship with any companies whose products are mentioned in this article.
- All psychotropic medications cross the placenta. If psychotropic medication is used, prescribe carefully during the first trimester, giving the minimum number of drugs and the lowest dosages needed to restore or maintain well-being.
- No psychotropic agents are FDA-approved for use in pregnancy.
- Teratogenicity notwithstanding, psychotropic intervention is the most effective treatment for women with bipolar disorder.
- We recommend that women who continue to take valproate or carbamazepine during pregnancy receive folate, 3 to 4 mg/d, as a precaution.
Managing gravidas with bipolar disorder requires obstetricians to balance the potential for neonatal malformations against the high risk of relapse when patients discontinue medications.1 This article offers an evidence-based approach that includes:
- analysis of the US Food and Drug Administration’s (FDA) teratogenicity categories for psychotropics
- safety profiles of drugs used in mood stabilization
- an algorithm for managing patients who are considering conception or are pregnant
When a patient is at risk for severe psychiatric problems before, during, and after pregnancy, a collaborative effort between the obstetrician and psychiatrist may be best for both the patient and her child.
Further, because low maternal folate levels are associated with neural tube defects, and some anticonvulsant agents used for mood stabilization diminish supplies of the nutrient, supplementation is essential. (See “Folate and neural tube defects”.)
Psychotropic risks to offspring
All psychotropic medications cross the placenta, exposing the fetus to some degree. Risks include teratogenicity, obstetric complications, perinatal syndromes, and long-term postnatal behavioral sequelae.
Teratogenicity. A medication is considered teratogenic when prenatal exposure significantly increases the risk of congenital deformities over the baseline, which is 2% in the United States.2 The cause of most congenital malformations is unknown. Risk for teratogenicity occurs during organogenesis in the first 12 weeks of gestation.
Obstetric complications include preterm delivery, low birth weight, and delivery complications such as low Apgar scores or behavioral effects requiring intensive care.
Perinatal syndromes include physical and behavioral symptoms noticed in the infant shortly after birth (such as jitteriness). These consequences are putatively related to drug use at or near birth and have limited duration.
Postnatal behavioral sequelae include long-term neurobehavioral abnormalities in children who were exposed to psychotropics in utero.
Balancing risks
Risks with medication. The FDA’s “use-in-pregnancy” rating system (TABLE 1) uses available data to assess the degree of teratogenic risk. These guidelines are one of many tools to use when considering drug treatment.
Most psychotropics are category “C” or “D,” which imply a chance of harm to the exposed fetus. Category “B” drugs would appear safer, but this rating could simply indicate a lack of adequate human data or that no data have shown harm in animals.
Moreover, a category “D” drug may be chosen more often during pregnancy than a category “C” drug. This may occur when more human data exist on using the category “D” drug in patients with a particular disorder (such as using lithium versus valproate or olanzapine in pregnant women).
No psychotropics are classified as “A,” meaning either some risks are associated with every psychotropic or the risk of some agents has not been adequately explored. Furthermore, no psychotropics are FDA-approved for use during pregnancy.
Risks without medication. Teratogenicity notwithstanding, psychotropic intervention is the most effective treatment for women with bipolar disorder. Patients who discontinue mood-stabilizing medication after conception significantly increase their likelihood of a relapse into depression or mania,3 either of which could lead to complications and untoward effects on the fetus.
Depression during pregnancy has been linked to low birth weight and preterm delivery.4,5 These effects may be mediated by the illness itself or by other factors that indirectly affect birth outcomes. For example, depression during pregnancy is associated with decreased appetite, substance use and abuse, and lower use of prenatal care.6
Untreated mania may also be associated with perinatal risks, as a pregnant patient in a manic state may engage in impulsive, high-risk behaviors that endanger herself and her fetus.7
TABLE 1
FDA use-in-pregnancy ratings for medications
| CATEGORY | DESCRIPTION |
|---|---|
| A | Adequate, well-controlled studies in pregnant women have not shown an increased risk of fetal abnormalities |
| B | Animal studies have revealed no evidence of harm to the fetus; however, no adequate and well-controlled studies have been conducted in pregnant women |
| OR | |
| Animal studies have shown an adverse effect, but adequate and well-controlled studies in pregnant women have failed to demonstrate a risk to the fetus | |
| C | Animal studies have shown an adverse effect and no adequate and well-controlled studies have been conducted in pregnant women |
| OR | |
| No animal studies have been conducted and no adequate and well-controlled studies have been conducted in pregnant women | |
| D | Studies—adequate and well-controlled or observational—in pregnant women have demonstrateda risk to the fetus. However, the benefits of therapy may outweigh the potential risk |
| X | Studies—adequate and well-controlled or observational—in animals or pregnant women have demonstrated positive evidence of fetal abnormalities. The use of the product is contraindicated in women who are or may become pregnant |
| Data from US Food and Drug Administration38 | |
Mood stabilizers
The 3 most commonly used mood stabilizers—lithium, valproate, and carbamazepine—are teratogenic. The least risk may occur with lithium (0.1%) versus valproate (2% to 5%) or carbamazepine (1% to 3%). These risks must be weighed against the up to 50% chance of relapse with medication discontinuation.3
The FDA designates these 3 medications as category as “D” agents (TABLE 2). This rating implies that studies have demonstrated fetal risk; still, the drug’s potential benefit may outweigh the risk.
Lithium. The International Registry of Lithium reported increased rates of cardiovascular malformations—such as Ebstein’s anomaly—in children whose mothers took lithium during pregnancy.
• Relative risk for Ebstein’s anomaly in children with fetal exposure to lithium may be 20 times higher than the risk in unexposed children, although the absolute risk with lithium exposure remains low (1 in 1,000 births).1,8
No significant neurobehavioral teratogenicity has been reported in infants exposed in utero to lithium, although few cases have been studied. One study reported that 22 lithium-exposed infants attained developmental milestones at a pace comparable to that of unexposed controls.9
•“Floppy baby” syndrome, in which infants experience hypotonicity and cyanosis, is the most recognized adverse effect in infants exposed to lithium in utero.10 Its frequency is unknown, but rare. Neonatal hypothyroidism and nephrogenic diabetes insipidus have also been documented.
Anticonvulsants. To date, no studies have examined the outcomes of children whose mothers took anticonvulsants for bipolar disorder during pregnancy, though the research concerning epileptic mothers is extensive.
• Neural tube defects. Infants exposed to anticonvulsant agents have a significantly greater risk for malformations than do neonates in the general population. Specifically, anticonvulsants may cause neural tube defects such as spina bifida, ancephaly, and encephaly in 2% to 5% of those exposed, as well as craniofacial anomalies, microcephaly, growth retardation, and heart defects.11-14
• Other minor malformations—such as rotated ears, depressed nasal bridge, short nose, elongated upper lip, and fingernail hypoplasia—have been reported in infants exposed to anticonvulsants in utero.14 These malformations disappear with age.13 Teratogenicity increases with the use of multiple anticonvulsants and possibly with higher maternal plasma levels and toxic metabolites.15
TABLE 2
FDA teratogenicity ratings of mood stabilizers, antimanic drugs, and antidepressants
| MEDICATION | PREGNANCY CATEGORY |
|---|---|
| Mood stabilizers | |
| Lithium | D |
| Carbamazepine | D |
| Valproate | D |
| Anticonvulsants | |
| Gabapentin | C |
| Lamotrigine | C |
| Topiramate | C |
| Antipsychotics | |
| Olanzapine | C |
| Risperidone | C |
| Chlorpromazine | C |
| Haloperidol | C |
| Trifluoperazine | C |
| Tricyclic antidepressants | |
| Amitriptyline | C |
| Clomipramine | C |
| Desipramine | C |
| Imipramine | C |
| Nortriptyline | D |
| Selective serotonin reuptake inhibitors | |
| Citalopram | C |
| Escitalopram | C |
| Fluoxetine | C |
| Fluvoxamine | C |
| Paroxetine | C |
| Sertraline | C |
| Other antidepressants | |
| Bupropion | B |
| Phenelzine | C |
| Tranylcypromine | C |
Antipsychotics
Psychotic illness itself may increase the risk of poor fetal outcome to a greater extent than does antipsychotic use. Prenatal exposure to low-potency phenothiazines may further increase this risk, although only slightly. The effect of prenatal exposure to atypical antipsychotics requires additional study.
Antipsychotics are often used to treat mania because of their rapid effects and sedative properties. Most antipsychotics—specifically, haloperidol, olanzapine, and risperidone—are designated as pregnancy category “C,” specifying that fetal risk cannot be ruled out.
Chlorpromazine and haloperidol have been most studied during pregnancy, but in relation to treating hyperemesis gravidarum and psychosis, not bipolar disorder. Results regarding antipsychotics’ teratogenic and behavioral risks are mixed,16-21 probably because the various compounds have different effects on the fetus.
The underlying illness—rather than the mother’s medications—may increase the rate of anomalies seen with exposure to antipsychotics:
• Rieder et al22 reported an increased rate of perinatal death in infants of schizophrenic mothers but no significant association between the mothers’ use of antipsychotics and perinatal death.
• Sobel23 compared psychotic women with and without histories of chlorpromazine exposure during pregnancy. Rates of fetal damage were similar and approximately twice that of the general population.
A meta-analysis of 74,337 live births revealed that first-trimester exposure to lowpotency antipsychotics increases the relative risk of fetal anomalies in nonpsychotic women. Phenothiazines may increase the 2% baseline incidence of malformations to 2.4%.1 No specific organ malformation following fetal exposure to phenothiazines has been consistently identified.
Olanzapine was recently approved for treating mania. Very little data exist regarding its impact on fetal development when used during pregnancy, though studies on small numbers of women have not revealed teratogenicity.24,25
Benzodiazepines
Benzodiazepines are rarely a primary treatment for mania or depression. Thus, a comprehensive review of their effect on fetal outcome is beyond the scope of this review. A meta-analysis of exposure during the first trimester suggests a very small but significant increase in risk for cleft palate.1 The absolute risk with exposure appears to be less than 1 in 1,000 cases.
Antidepressants
Acute mania is an emergency requiring immediate treatment. The other possibility in women with bipolar disorder is relapse into depression. An antidepressant should not be used without a mood stabilizer when treating bipolar I disorder. Similarly, a mood stabilizer alone may be inadequate to treat depression. The use of tricyclics or selective serotonin reuptake inhibitors (SSRIs) during pregnancy has not to date been associated with teratogenicity (TABLE 3),26 but larger numbers of women need to be studied. Perinatal effects in some cases have been reported.1
Tricyclics. In case-control studies of more than 300,000 live births, researchers followed 414 incidences of first-trimester exposure to tricyclics. They discovered no significant association between fetal exposure to tricyclics and increased rates of congenital malformations.1
The few studies that have been performed suggest that no long-term effects stem from exposure in utero.26 Although these results suggest that prenatal exposure to tricyclics is relatively safe, more research is needed.
SSRIs. No significant teratogenic effects of SSRIs have been identified.
The manufacturer’s register for fluoxetine contains approximately 2,000 cases of treated patients, with no excess cases of congenital anomalies or malformations following prenatal exposure. Citalopram has the next largest database of in-utero exposure (n = 365), again with no increased risk for teratogenicity. Several smaller systematic reports are available on in-utero exposure to sertraline, paroxetine, or escitalopram.26
Most studies of pregnant women taking fluoxetine during the first trimester found no increased risk of obstetric complications—including spontaneous pregnancy loss, preterm labor, or low birth weight—compared with women not taking fluoxetine. Third-trimester use of fluoxetine may increase the risk for perinatal complications,27 but this effect has been reported inconsistently and requires further study.26 Effects of other SSRIs during the third trimester have not been systematically explored.
Case reports and 1 controlled study have addressed possible perinatal symptoms from in-utero exposure to SSRIs.28,29 Preliminary data show no adverse neurobehavioral function in exposed neonates.26
Electroconvulsive therapy (ECT) has been proven effective for acute mania and depression, demonstrating few deleterious effects on neonates. ECT has few side effects and may be safer than drug therapy in this population. Two reviews support the efficacy and relative safety of ECT during pregnancy, although more evidence is needed.30,31
Recommendations
Discuss pregnancy and medication risks with all women with bipolar disorder, regardless of plans for pregnancy (FIGURE). If psychotropic medication is used, prescribe carefully during the first trimester, using the minimum number of drugs and the lowest dosages needed to restore or maintain well-being.32
Pros and cons of switching. Some clinicians may encourage a patient to taper a medication during the first trimester because of its unknown or high teratogenicity. Depending on the patient’s illness severity, this might not be the optimal decision. A more conservative option would be to switch to a lower-risk drug during pregnancy.
Lithium has both antidepressant and antimanic properties and is less teratogenic than anticonvulsants in the first trimester. However, if lithium has not been successful for the woman’s mania prophylaxis in the past and she has experienced antimanic response to an anticonvulsant, switching to lithium or another anticonvulsant is not recommended.
Folate and neural tube defects. First-trimester exposure to carbamazepine or valproate increases the risk for neural tube defects; using the lowest available dosage may decrease the risk for spina bifida—at least with valproate.
Low maternal folate levels are often associated with neural tube defects from any cause.33 Valproate lowers folate levels by inhibiting 1 of the enzymes necessary for its formation, which may be a mechanism for the increased risk of spina bifida.34
• Precautionary folate supplementation. No study has demonstrated that folate supplementation reduces the risk of neural tube defects in women taking anticonvulsants during pregnancy.35 Nonetheless, we recommend that women who continue to take valproate or carbamazepine during pregnancy receive folate, 3 to 4 mg/d, as a precaution.
• Treating manic relapse. Patients who stop taking lithium, particularly those who halt therapy abruptly, incur a high rate of relapse.3 Counsel women taking lithium to plan their pregnancies to allow enough time to taper off the medication before conception, if they want to try this. Lithium should be decreased slowly—by approximately 50% every 2 weeks—to avoid relapse. Treat aggressively if relapse occurs during pregnancy. Consider:
- psychiatric hospitalization in case of psychosis or suicidal ideation
- reinstituting drug therapy with a less teratogenic agent
- ECT for a manic or depressive episode
As the pregnancy advances and the mother’s volume of distribution increases, dosage increases may be needed to maintain therapeutic drug levels.
Treating depressive relapse. Should depression occur during pregnancy, SSRIs or tricyclics added to mood stabilizer therapy have been shown to be effective, with few teratogenic effects.
Cognitive-behavioral and interpersonal psychotherapies also have shown efficacy in pregnant women with major depressive disorder36 and may be effective for gravidas with bipolar disorder. Cognitive psychotherapies, when used with medication, have been reported effective in preventing relapse in nongravid bipolar patients.36,37
Dr. Altshuler reports that she is a scientific adviser and consultant for Abbott, Lilly, Forest, and AstraZeneca. Dr. Yonkers and Ms. Richards report no financial relationship with any companies whose products are mentioned in this article.
1. Altshuler L, Cohen L, Szuba MP, et al. Pharmacologic management of psychiatric illness during pregnancy: dilemmas and guidelines. Am J Psychiatry. 1996;153:592-606.
2. Nelson K, Holmes LB. Malformations due to presumed spontaneous mutations in newborn infants. N Engl J Med. 1989;320:19-23.
3. Viguera AC, Nonacs R, Cohen LS, et al. Risk of recurrence of bipolar disorder in pregnant and nonpregnant women after discontinuing lithium maintenance. Am J Psychiatry. 2000;157:179-184.
4. Steer RA, Scholl TO, Hediger ML, Fischer RL. Self-reported depression and negative pregnancy outcomes. J Clin Epidemiol. 1992;45:1093-1099.
5. Orr ST, Miller CA. Maternal depressive symptoms and the risk of poor pregnancy outcome. Review of the literature and preliminary findings. Epidemiol Rev. 1995;17:165-171.
6. Zuckerman B, Amaro H, Bauchner H, Cabral H. Depressive symptoms during pregnancy: relationship to poor health behaviors. Am J Obstet Gynecol. 1989;160:1107-1111.
7. Miller LJ. Psychotic denial of pregnancy: phenomenology and clinical management. Hosp Community Psychiatry. 1990;41:1233-1237.
8. Cohen LS, Friedman JM, Jefferson JW, et al. A reevaluation of risk of in utero exposure to lithium. JAMA. 1994;271:146-150;correction JAMA. 1994;271:1485.
9. Schou M. What happened later to the lithium babies? A follow-up study of children born without malformations. Acta Psychiatr Scand. 1976;54:193-197.
10. Woody JN, London WL, Wilbanks GD. Lithium toxicity in a newborn. Pediatrics. 1971;47:94-96.
11. Jones K, Lacro R, Johnson K, Adams J. Patterns of malformations in the children of women treated with carbamazepine during pregnancy. N Engl J Med. 1989;320:1661-1666.
12. Rosa F. Spina bifida in infants of women treated with carbamazepine during pregnancy. N Engl J Med. 1991;324:674-677.
13. Koch S, Losche G, Jager-Roman E, et al. Major and minor birth malformations and antiepileptic drugs. Neurology. 1992;42:83-88.
14. Jager-Roman E, Deichl A, Jakob S, et al. Fetal growth, major malformations, and minor anomalies in infants born to women receiving valproic acid. J Pediatr. 1986;108:997-1004.
15. Nakane Y, Okuma T, Takahashi R, et al. Multi-institutional study on the teratogenicity and fetal toxicity of antiepileptic drugs: a report of a collaborative study group in Japan. Epilepsia. 1980;21:663-680.
16. Edlund MJ, Craig TJ. Antipsychotic drug use and birth defects: an epidemiologic reassessment. Compr Psychiatry. 1984;25:32-38.
17. Kris EB. Children of mothers maintained on pharmacotherapy during pregnancy and postpartum. Curr Ther Res. 1965;7:785-789.
18. Clark CVH, Gorman D, Vernadakis A. Effects of prenatal administration of psychotropic drugs on behavior of developing rats. Dev Psychobiol. 1970;3:225-235.
19. Golub M, Kornetsky C. Seizure susceptibility and avoidance conditioning in adult rats treated prenatally with chlorpromazine. Dev Psychobiol. 1974;7:79-88.
20. Spear LP, Shalaby IA, Brick J. Chronic administration of haloperidol during development: behavioral and psychopharmacological effects. Psychopharmacology. (Berl) 1980;70:47-58.
21. Cagiano R, Barfield RJ, White NR, et al. Subtle behavioral changes produced in rat pups exposed in utero to haloperidol. Eur J Pharmacol. 1988;157:45-50.
22. Rieder RO, Rosenthal D, Wender P, Blumenthal H. The offspring of schizophrenics: fetal and neonatal deaths. Arch Gen Psychiatry. 1975;32:200-211.
23. Sobel DE. Fetal damage due to ECT, insulin coma, chlorpromazine, or reserpine. Arch Gen Psychiatry. 1960;2:606-611.
24. Dickson R. Olanzapine and pregnancy. Can J Psychiatry. 1998;43:196-197.
25. Goldstein DJ, Corbin LA, Fung MC. Olanzapine-exposed pregnancies and lactation; early experience. J Clin Psychopharmacol. 2000;24:399-403.
26. Altshuler LL, Cohen LS, Moline ML, et al. The expert consensus guideline series: treatment of depression in women. Postgrad Med. 2001;Mar(Spec No):1-22.
27. Chambers CD, Johnson KA, Dick LM, et al. Birth outcomes in pregnant women taking fluoxetine. N Engl J Med. 1996;335:1010-1015.
28. Spencer MJ. Fluoxetine hydrochloride (Prozac) toxicity in the neonate. Pediatrics. 1993;92:721-722.
29. Cabrera FM, Battaglia G. Delayed decreases in brain 5-HT 2a and 2c receptor density and function in male rat progeny following prenatal fluoxetine. J Pharmacol Exp Ther. 1994;269:637-645.
30. Miller LJ. Use of electroconvulsive therapy during pregnancy. Hosp Community Psychiatry. 1994;45:444-450.
31. Ferrill MJ, Kehoe WA, Jacisin JJ. ECT during pregnancy: physiologic and pharmacologic considerations. Convuls Ther. 1992;8:186-200.
32. Yonkers K, Wisner K, Cohen L, et al. Management of bipolar disorder during pregnancy and the postpartum period. Bipolar Consensus Statement. Submitted for publication.
33. Dansky L, Rosenblatt D, Andermann E. Mechanisms of teratogenesis: folic acid and antiepileptic therapy. Neurology. 1992;42(suppl 5):32-42.
34. Wegner C, Nau H. Alteration of embryonic folate metabolism by valproic acid during organogenesis: implications for mechanism of teratogenesis. Neurology. 1992;42(suppl 5):17-24.
35. MRC Vitamin Study Research Group. Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. Lancet. 1991;338:131-137.
36. Spinelli MG, Endicott J. Controlled clinical trial of interpersonal psychotherapy versus parenting education program for depressed pregnant women. Am J Psychiatry. 2003;160:555-562.
37. Lam DH, Watkins ER, Hayward P, et al. A randomized controlled study of cognitive therapy for relapse prevention for bipolar affective disorder: outcome in the first year. Arch Gen Psychiatry. 2003;60:145-152.
38. US Food and Drug Administration. Current categories for drug use in pregnancy. Available at: http://www.fda.gov/fdac/features/2001/301_preg.html#categories. Accessed November 19, 2003.
1. Altshuler L, Cohen L, Szuba MP, et al. Pharmacologic management of psychiatric illness during pregnancy: dilemmas and guidelines. Am J Psychiatry. 1996;153:592-606.
2. Nelson K, Holmes LB. Malformations due to presumed spontaneous mutations in newborn infants. N Engl J Med. 1989;320:19-23.
3. Viguera AC, Nonacs R, Cohen LS, et al. Risk of recurrence of bipolar disorder in pregnant and nonpregnant women after discontinuing lithium maintenance. Am J Psychiatry. 2000;157:179-184.
4. Steer RA, Scholl TO, Hediger ML, Fischer RL. Self-reported depression and negative pregnancy outcomes. J Clin Epidemiol. 1992;45:1093-1099.
5. Orr ST, Miller CA. Maternal depressive symptoms and the risk of poor pregnancy outcome. Review of the literature and preliminary findings. Epidemiol Rev. 1995;17:165-171.
6. Zuckerman B, Amaro H, Bauchner H, Cabral H. Depressive symptoms during pregnancy: relationship to poor health behaviors. Am J Obstet Gynecol. 1989;160:1107-1111.
7. Miller LJ. Psychotic denial of pregnancy: phenomenology and clinical management. Hosp Community Psychiatry. 1990;41:1233-1237.
8. Cohen LS, Friedman JM, Jefferson JW, et al. A reevaluation of risk of in utero exposure to lithium. JAMA. 1994;271:146-150;correction JAMA. 1994;271:1485.
9. Schou M. What happened later to the lithium babies? A follow-up study of children born without malformations. Acta Psychiatr Scand. 1976;54:193-197.
10. Woody JN, London WL, Wilbanks GD. Lithium toxicity in a newborn. Pediatrics. 1971;47:94-96.
11. Jones K, Lacro R, Johnson K, Adams J. Patterns of malformations in the children of women treated with carbamazepine during pregnancy. N Engl J Med. 1989;320:1661-1666.
12. Rosa F. Spina bifida in infants of women treated with carbamazepine during pregnancy. N Engl J Med. 1991;324:674-677.
13. Koch S, Losche G, Jager-Roman E, et al. Major and minor birth malformations and antiepileptic drugs. Neurology. 1992;42:83-88.
14. Jager-Roman E, Deichl A, Jakob S, et al. Fetal growth, major malformations, and minor anomalies in infants born to women receiving valproic acid. J Pediatr. 1986;108:997-1004.
15. Nakane Y, Okuma T, Takahashi R, et al. Multi-institutional study on the teratogenicity and fetal toxicity of antiepileptic drugs: a report of a collaborative study group in Japan. Epilepsia. 1980;21:663-680.
16. Edlund MJ, Craig TJ. Antipsychotic drug use and birth defects: an epidemiologic reassessment. Compr Psychiatry. 1984;25:32-38.
17. Kris EB. Children of mothers maintained on pharmacotherapy during pregnancy and postpartum. Curr Ther Res. 1965;7:785-789.
18. Clark CVH, Gorman D, Vernadakis A. Effects of prenatal administration of psychotropic drugs on behavior of developing rats. Dev Psychobiol. 1970;3:225-235.
19. Golub M, Kornetsky C. Seizure susceptibility and avoidance conditioning in adult rats treated prenatally with chlorpromazine. Dev Psychobiol. 1974;7:79-88.
20. Spear LP, Shalaby IA, Brick J. Chronic administration of haloperidol during development: behavioral and psychopharmacological effects. Psychopharmacology. (Berl) 1980;70:47-58.
21. Cagiano R, Barfield RJ, White NR, et al. Subtle behavioral changes produced in rat pups exposed in utero to haloperidol. Eur J Pharmacol. 1988;157:45-50.
22. Rieder RO, Rosenthal D, Wender P, Blumenthal H. The offspring of schizophrenics: fetal and neonatal deaths. Arch Gen Psychiatry. 1975;32:200-211.
23. Sobel DE. Fetal damage due to ECT, insulin coma, chlorpromazine, or reserpine. Arch Gen Psychiatry. 1960;2:606-611.
24. Dickson R. Olanzapine and pregnancy. Can J Psychiatry. 1998;43:196-197.
25. Goldstein DJ, Corbin LA, Fung MC. Olanzapine-exposed pregnancies and lactation; early experience. J Clin Psychopharmacol. 2000;24:399-403.
26. Altshuler LL, Cohen LS, Moline ML, et al. The expert consensus guideline series: treatment of depression in women. Postgrad Med. 2001;Mar(Spec No):1-22.
27. Chambers CD, Johnson KA, Dick LM, et al. Birth outcomes in pregnant women taking fluoxetine. N Engl J Med. 1996;335:1010-1015.
28. Spencer MJ. Fluoxetine hydrochloride (Prozac) toxicity in the neonate. Pediatrics. 1993;92:721-722.
29. Cabrera FM, Battaglia G. Delayed decreases in brain 5-HT 2a and 2c receptor density and function in male rat progeny following prenatal fluoxetine. J Pharmacol Exp Ther. 1994;269:637-645.
30. Miller LJ. Use of electroconvulsive therapy during pregnancy. Hosp Community Psychiatry. 1994;45:444-450.
31. Ferrill MJ, Kehoe WA, Jacisin JJ. ECT during pregnancy: physiologic and pharmacologic considerations. Convuls Ther. 1992;8:186-200.
32. Yonkers K, Wisner K, Cohen L, et al. Management of bipolar disorder during pregnancy and the postpartum period. Bipolar Consensus Statement. Submitted for publication.
33. Dansky L, Rosenblatt D, Andermann E. Mechanisms of teratogenesis: folic acid and antiepileptic therapy. Neurology. 1992;42(suppl 5):32-42.
34. Wegner C, Nau H. Alteration of embryonic folate metabolism by valproic acid during organogenesis: implications for mechanism of teratogenesis. Neurology. 1992;42(suppl 5):17-24.
35. MRC Vitamin Study Research Group. Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. Lancet. 1991;338:131-137.
36. Spinelli MG, Endicott J. Controlled clinical trial of interpersonal psychotherapy versus parenting education program for depressed pregnant women. Am J Psychiatry. 2003;160:555-562.
37. Lam DH, Watkins ER, Hayward P, et al. A randomized controlled study of cognitive therapy for relapse prevention for bipolar affective disorder: outcome in the first year. Arch Gen Psychiatry. 2003;60:145-152.
38. US Food and Drug Administration. Current categories for drug use in pregnancy. Available at: http://www.fda.gov/fdac/features/2001/301_preg.html#categories. Accessed November 19, 2003.