User login
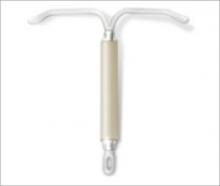
Since the levonorgestrel-releasing intrauterine system (LNG-IUS) (Mirena, Bayer Health Care Pharmaceuticals) ( FIGURE 1 ) was introduced in the United States in 2001, more than 2 million devices have been used by women here. This use has contributed to a cumulative experience of 14 million women (36 million woman-years of experience) in 120 countries over the last 18 years.
Recent Food and Drug Administration (FDA)-approved labeling changes for the LNG-IUS1 expand the pool of women who are candidates for this convenient, reversible method of contraception. This article summarizes those labeling changes and answers questions that are often asked by clinicians who, more and more, insert the LNG-IUS for their patients.
FIGURE 1 LNG-IUS, arms open
The LNG-IUS device is readily visible on radiographs. Visualization by ultrasonography is more challenging.
SOURCE: BAYER HEALTH CARE PHARMACEUTICALS. USED WITH PERMISSION.
What changes have been made to labeling?
Patient profile. The so-called recommended patient profile has been streamlined. The label now indicates only that the LNG-IUS is recommended for women who have given birth to at least one child. A more detailed description of women who were included in the phase-II US clinical trial is included in that section of the labeling. Clinicians should recognize that neither nulliparity nor nulligravity is listed in labeling as a contraindication to the LNG-IUS.
Depth of cavity. Another important change in the new labeling is that the LNG-IUS can be used in women whose uterine cavity sounds to a depth of 6 to 10 cm (no longer only 6 to 9 cm). This will permit more multiparous women, who may have a larger cavity, to use the LNG-IUS.
Pregnancy risk and consequences. The risk of pregnancy is rare when the LNG-IUS is used; pregnancy with the device in situ does not appear to be associated with an increased risk of birth defects. As of September 2006, there had been only 390 live births among an estimated cumulative 9.9 million LNG-IUS users worldwide. Of those births, congenital anomalies have been infrequent; no clear trend was seen toward an increased risk of any specific anomaly after exposure to the LNG-IUS.
Adverse events. As worldwide experience with the LNG-IUS has expanded since the last labeling, the number of reported cases of relatively rare adverse events has also been updated:
- Only nine cases of infection with group A Streptococcus have been reported in 9.9 million users, constituting a risk of approximately one infection for every 1 million users
- Based on postmarketing experience, new wording has been added about the possibility of 1) device breakage (before insertion) and 2) angioedema—a rare allergic reaction that is not specific to levonorgestrel (or the IUS)
- Wording has been added to the labeling that provides reassurance, based on observational studies, that there is no evidence of an increase in the risk of breast cancer risk with use of the LNG-IUS
- Similarly, the new labeling declares that, in general, no adverse eff ects have been found with use of the LNG-IUS on breast-feeding performance in regard to the health, growth, or development of an infant—even though isolated cases of a decrease in milk production have been reported.
Contraindications. The roster of contraindications ( TABLE ) has been significantly modified to reflect scientific evidence. Removed from that list are 1) a history of ectopic pregnancy and 2) risk factors for ectopic pregnancy.
TABLE A new label lists 12 contraindications to the LNG-IUS
| The LNG-IUS is contraindicated in the presence of one or more of the following: |
| Pregnancy or suspicion of pregnancy |
| Congenital or acquired uterine anomaly, including fibroids if they distort the uterine cavity |
| Acute pelvic inflammatory disease or a history of pelvic inflammatory disease, unless there has been a subsequent intrauterine pregnancy |
| Postpartum endometritis or infected abortion in the past 3 months |
| Known or suspected uterine or cervical neoplasia or unresolved, abnormal Pap smear |
| Genital bleeding of unknown cause |
| Untreated acute cervicitis or vaginitis, including bacterial vaginosis or other lower genital tract infection, until infection is controlled |
| Acute liver disease or liver tumor (benign or malignant |
| Conditions associated with an increased susceptibility to pelvic infection |
| Previously inserted intrauterine device that has not been removed |
| Hypersensitivity to any device component |
| Known or suspected carcinoma of the breast |
When should I place the LNG-IUS?
Labeling recommends that, in a cycling woman, the LNG-IUS be placed sometime during the first 7 days of menses. A woman who has it placed at any other time in the cycle needs to be screened for pregnancy and needs to use back-up contraception for 7 days after the LNG-IUS is placed.
How should I place the device?
Complete instructions on insertion are provided in the package insert for the LNG-IUS. A few points about placement highlighted in the new labeling should be noted:
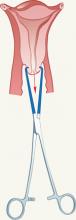
- The need to use a tenaculum to stabilize the cervix and to straighten the uterine axis has been reinforced in the new labeling ( FIGURE 2 )
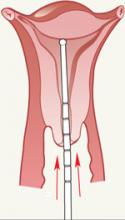
- Careful uterine sounding ( FIGURE 3 ) is needed to evaluate the uterine cavity to rule out any significant distortion of the cavity and to ensure appropriate uterine size before the LNG-IUS package is opened
- Uterine perforation is rare with the LNG-IUS, but to achieve that low risk you must wait at least 10 seconds for the arms of the device to open within the uterine cavity before it is advanced to the fundus
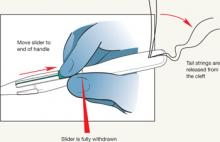
- The procedure-related expulsion rate can be reduced if the clinician moves the slider to the end of the handle (Position 3) carefully and waits for the tail strings to be released from the cleft ( FIGURE 4 ) before trying to withdraw the insertion device.
FIGURE 2 Tenaculum use is key
FIGURE 3 Sound the uterus
FIGURE 4 Release tail strings before withdrawing insertion device
What should I do if the LNG-IUS isn’t at the fundus?
Studies have shown there can be significant migration of the LNG-IUS within the uterine cavity. Fundal placement insures that the tail strings will be long enough to remove the device regardless of where it settles. A device that settles within the lower uterine segment is still effective. Removal of the device is necessary only if 1) a portion of it protrudes from the cervix or 2) the woman has excessive cramping with a low-lying device.
How can I visualize an LNG-IUS?
The LNG-IUS is visible on a radiograph. It is more challenging to image the device by ultrasonography; visualization of the shadowing beneath the device can help localize the unit. It is important to know that the tail strings are more echogenic than the device. Failure to recognize this allows the false impression that the IUD is lower than it actually is.
A woman can safely have a magnetic resonance imaging study without disrupting the position of the LNG-IUS device. It will not set off alarms in security systems—such as those used at an airport
I place only a few LNG-IUS devices each year. What should I do to remain competent and confident at performing this procedure?
Many teaching aids are available to refresh your skills. Options include instructional CD-ROMs and manuals and hands-on practice with a representative of the manufacturer of the device. As noted, the LNG-IUS package insert offers step-by-step instructions on the procedure for placing the device.
What about concerns over cost that some patients express to me?
Women can charge the cost of the device to a credit card if their health insurance does not cover it, or its insertion. Payment plans are also available from the manufacturer.
If you attempt to assist your patients by finding less expensive LNG-IUS units (that is, from a source other than the manufacturer), be aware that the device must be stored under controlled conditions in transit, similar to the way other delicate devices are (e.g., NuvaRing, Implanon). LNG-IUS devices that are shipped under less-than-optimal controlled conditions may not maintain their stability or provide the appropriate rate of release of the contraceptive hormone.
1. Mirena (levonorgestrel-releasing intrauterine system) [package insert]. Wayne, NJ: Bayer Health Care Pharmaceuticals; 2008.
Since the levonorgestrel-releasing intrauterine system (LNG-IUS) (Mirena, Bayer Health Care Pharmaceuticals) ( FIGURE 1 ) was introduced in the United States in 2001, more than 2 million devices have been used by women here. This use has contributed to a cumulative experience of 14 million women (36 million woman-years of experience) in 120 countries over the last 18 years.
Recent Food and Drug Administration (FDA)-approved labeling changes for the LNG-IUS1 expand the pool of women who are candidates for this convenient, reversible method of contraception. This article summarizes those labeling changes and answers questions that are often asked by clinicians who, more and more, insert the LNG-IUS for their patients.
FIGURE 1 LNG-IUS, arms open
The LNG-IUS device is readily visible on radiographs. Visualization by ultrasonography is more challenging.
SOURCE: BAYER HEALTH CARE PHARMACEUTICALS. USED WITH PERMISSION.
What changes have been made to labeling?
Patient profile. The so-called recommended patient profile has been streamlined. The label now indicates only that the LNG-IUS is recommended for women who have given birth to at least one child. A more detailed description of women who were included in the phase-II US clinical trial is included in that section of the labeling. Clinicians should recognize that neither nulliparity nor nulligravity is listed in labeling as a contraindication to the LNG-IUS.
Depth of cavity. Another important change in the new labeling is that the LNG-IUS can be used in women whose uterine cavity sounds to a depth of 6 to 10 cm (no longer only 6 to 9 cm). This will permit more multiparous women, who may have a larger cavity, to use the LNG-IUS.
Pregnancy risk and consequences. The risk of pregnancy is rare when the LNG-IUS is used; pregnancy with the device in situ does not appear to be associated with an increased risk of birth defects. As of September 2006, there had been only 390 live births among an estimated cumulative 9.9 million LNG-IUS users worldwide. Of those births, congenital anomalies have been infrequent; no clear trend was seen toward an increased risk of any specific anomaly after exposure to the LNG-IUS.
Adverse events. As worldwide experience with the LNG-IUS has expanded since the last labeling, the number of reported cases of relatively rare adverse events has also been updated:
- Only nine cases of infection with group A Streptococcus have been reported in 9.9 million users, constituting a risk of approximately one infection for every 1 million users
- Based on postmarketing experience, new wording has been added about the possibility of 1) device breakage (before insertion) and 2) angioedema—a rare allergic reaction that is not specific to levonorgestrel (or the IUS)
- Wording has been added to the labeling that provides reassurance, based on observational studies, that there is no evidence of an increase in the risk of breast cancer risk with use of the LNG-IUS
- Similarly, the new labeling declares that, in general, no adverse eff ects have been found with use of the LNG-IUS on breast-feeding performance in regard to the health, growth, or development of an infant—even though isolated cases of a decrease in milk production have been reported.
Contraindications. The roster of contraindications ( TABLE ) has been significantly modified to reflect scientific evidence. Removed from that list are 1) a history of ectopic pregnancy and 2) risk factors for ectopic pregnancy.
TABLE A new label lists 12 contraindications to the LNG-IUS
| The LNG-IUS is contraindicated in the presence of one or more of the following: |
| Pregnancy or suspicion of pregnancy |
| Congenital or acquired uterine anomaly, including fibroids if they distort the uterine cavity |
| Acute pelvic inflammatory disease or a history of pelvic inflammatory disease, unless there has been a subsequent intrauterine pregnancy |
| Postpartum endometritis or infected abortion in the past 3 months |
| Known or suspected uterine or cervical neoplasia or unresolved, abnormal Pap smear |
| Genital bleeding of unknown cause |
| Untreated acute cervicitis or vaginitis, including bacterial vaginosis or other lower genital tract infection, until infection is controlled |
| Acute liver disease or liver tumor (benign or malignant |
| Conditions associated with an increased susceptibility to pelvic infection |
| Previously inserted intrauterine device that has not been removed |
| Hypersensitivity to any device component |
| Known or suspected carcinoma of the breast |
When should I place the LNG-IUS?
Labeling recommends that, in a cycling woman, the LNG-IUS be placed sometime during the first 7 days of menses. A woman who has it placed at any other time in the cycle needs to be screened for pregnancy and needs to use back-up contraception for 7 days after the LNG-IUS is placed.
How should I place the device?
Complete instructions on insertion are provided in the package insert for the LNG-IUS. A few points about placement highlighted in the new labeling should be noted:
- The need to use a tenaculum to stabilize the cervix and to straighten the uterine axis has been reinforced in the new labeling ( FIGURE 2 )
- Careful uterine sounding ( FIGURE 3 ) is needed to evaluate the uterine cavity to rule out any significant distortion of the cavity and to ensure appropriate uterine size before the LNG-IUS package is opened
- Uterine perforation is rare with the LNG-IUS, but to achieve that low risk you must wait at least 10 seconds for the arms of the device to open within the uterine cavity before it is advanced to the fundus
- The procedure-related expulsion rate can be reduced if the clinician moves the slider to the end of the handle (Position 3) carefully and waits for the tail strings to be released from the cleft ( FIGURE 4 ) before trying to withdraw the insertion device.
FIGURE 2 Tenaculum use is key
FIGURE 3 Sound the uterus
FIGURE 4 Release tail strings before withdrawing insertion device
What should I do if the LNG-IUS isn’t at the fundus?
Studies have shown there can be significant migration of the LNG-IUS within the uterine cavity. Fundal placement insures that the tail strings will be long enough to remove the device regardless of where it settles. A device that settles within the lower uterine segment is still effective. Removal of the device is necessary only if 1) a portion of it protrudes from the cervix or 2) the woman has excessive cramping with a low-lying device.
How can I visualize an LNG-IUS?
The LNG-IUS is visible on a radiograph. It is more challenging to image the device by ultrasonography; visualization of the shadowing beneath the device can help localize the unit. It is important to know that the tail strings are more echogenic than the device. Failure to recognize this allows the false impression that the IUD is lower than it actually is.
A woman can safely have a magnetic resonance imaging study without disrupting the position of the LNG-IUS device. It will not set off alarms in security systems—such as those used at an airport
I place only a few LNG-IUS devices each year. What should I do to remain competent and confident at performing this procedure?
Many teaching aids are available to refresh your skills. Options include instructional CD-ROMs and manuals and hands-on practice with a representative of the manufacturer of the device. As noted, the LNG-IUS package insert offers step-by-step instructions on the procedure for placing the device.
What about concerns over cost that some patients express to me?
Women can charge the cost of the device to a credit card if their health insurance does not cover it, or its insertion. Payment plans are also available from the manufacturer.
If you attempt to assist your patients by finding less expensive LNG-IUS units (that is, from a source other than the manufacturer), be aware that the device must be stored under controlled conditions in transit, similar to the way other delicate devices are (e.g., NuvaRing, Implanon). LNG-IUS devices that are shipped under less-than-optimal controlled conditions may not maintain their stability or provide the appropriate rate of release of the contraceptive hormone.
Since the levonorgestrel-releasing intrauterine system (LNG-IUS) (Mirena, Bayer Health Care Pharmaceuticals) ( FIGURE 1 ) was introduced in the United States in 2001, more than 2 million devices have been used by women here. This use has contributed to a cumulative experience of 14 million women (36 million woman-years of experience) in 120 countries over the last 18 years.
Recent Food and Drug Administration (FDA)-approved labeling changes for the LNG-IUS1 expand the pool of women who are candidates for this convenient, reversible method of contraception. This article summarizes those labeling changes and answers questions that are often asked by clinicians who, more and more, insert the LNG-IUS for their patients.
FIGURE 1 LNG-IUS, arms open
The LNG-IUS device is readily visible on radiographs. Visualization by ultrasonography is more challenging.
SOURCE: BAYER HEALTH CARE PHARMACEUTICALS. USED WITH PERMISSION.
What changes have been made to labeling?
Patient profile. The so-called recommended patient profile has been streamlined. The label now indicates only that the LNG-IUS is recommended for women who have given birth to at least one child. A more detailed description of women who were included in the phase-II US clinical trial is included in that section of the labeling. Clinicians should recognize that neither nulliparity nor nulligravity is listed in labeling as a contraindication to the LNG-IUS.
Depth of cavity. Another important change in the new labeling is that the LNG-IUS can be used in women whose uterine cavity sounds to a depth of 6 to 10 cm (no longer only 6 to 9 cm). This will permit more multiparous women, who may have a larger cavity, to use the LNG-IUS.
Pregnancy risk and consequences. The risk of pregnancy is rare when the LNG-IUS is used; pregnancy with the device in situ does not appear to be associated with an increased risk of birth defects. As of September 2006, there had been only 390 live births among an estimated cumulative 9.9 million LNG-IUS users worldwide. Of those births, congenital anomalies have been infrequent; no clear trend was seen toward an increased risk of any specific anomaly after exposure to the LNG-IUS.
Adverse events. As worldwide experience with the LNG-IUS has expanded since the last labeling, the number of reported cases of relatively rare adverse events has also been updated:
- Only nine cases of infection with group A Streptococcus have been reported in 9.9 million users, constituting a risk of approximately one infection for every 1 million users
- Based on postmarketing experience, new wording has been added about the possibility of 1) device breakage (before insertion) and 2) angioedema—a rare allergic reaction that is not specific to levonorgestrel (or the IUS)
- Wording has been added to the labeling that provides reassurance, based on observational studies, that there is no evidence of an increase in the risk of breast cancer risk with use of the LNG-IUS
- Similarly, the new labeling declares that, in general, no adverse eff ects have been found with use of the LNG-IUS on breast-feeding performance in regard to the health, growth, or development of an infant—even though isolated cases of a decrease in milk production have been reported.
Contraindications. The roster of contraindications ( TABLE ) has been significantly modified to reflect scientific evidence. Removed from that list are 1) a history of ectopic pregnancy and 2) risk factors for ectopic pregnancy.
TABLE A new label lists 12 contraindications to the LNG-IUS
| The LNG-IUS is contraindicated in the presence of one or more of the following: |
| Pregnancy or suspicion of pregnancy |
| Congenital or acquired uterine anomaly, including fibroids if they distort the uterine cavity |
| Acute pelvic inflammatory disease or a history of pelvic inflammatory disease, unless there has been a subsequent intrauterine pregnancy |
| Postpartum endometritis or infected abortion in the past 3 months |
| Known or suspected uterine or cervical neoplasia or unresolved, abnormal Pap smear |
| Genital bleeding of unknown cause |
| Untreated acute cervicitis or vaginitis, including bacterial vaginosis or other lower genital tract infection, until infection is controlled |
| Acute liver disease or liver tumor (benign or malignant |
| Conditions associated with an increased susceptibility to pelvic infection |
| Previously inserted intrauterine device that has not been removed |
| Hypersensitivity to any device component |
| Known or suspected carcinoma of the breast |
When should I place the LNG-IUS?
Labeling recommends that, in a cycling woman, the LNG-IUS be placed sometime during the first 7 days of menses. A woman who has it placed at any other time in the cycle needs to be screened for pregnancy and needs to use back-up contraception for 7 days after the LNG-IUS is placed.
How should I place the device?
Complete instructions on insertion are provided in the package insert for the LNG-IUS. A few points about placement highlighted in the new labeling should be noted:
- The need to use a tenaculum to stabilize the cervix and to straighten the uterine axis has been reinforced in the new labeling ( FIGURE 2 )
- Careful uterine sounding ( FIGURE 3 ) is needed to evaluate the uterine cavity to rule out any significant distortion of the cavity and to ensure appropriate uterine size before the LNG-IUS package is opened
- Uterine perforation is rare with the LNG-IUS, but to achieve that low risk you must wait at least 10 seconds for the arms of the device to open within the uterine cavity before it is advanced to the fundus
- The procedure-related expulsion rate can be reduced if the clinician moves the slider to the end of the handle (Position 3) carefully and waits for the tail strings to be released from the cleft ( FIGURE 4 ) before trying to withdraw the insertion device.
FIGURE 2 Tenaculum use is key
FIGURE 3 Sound the uterus
FIGURE 4 Release tail strings before withdrawing insertion device
What should I do if the LNG-IUS isn’t at the fundus?
Studies have shown there can be significant migration of the LNG-IUS within the uterine cavity. Fundal placement insures that the tail strings will be long enough to remove the device regardless of where it settles. A device that settles within the lower uterine segment is still effective. Removal of the device is necessary only if 1) a portion of it protrudes from the cervix or 2) the woman has excessive cramping with a low-lying device.
How can I visualize an LNG-IUS?
The LNG-IUS is visible on a radiograph. It is more challenging to image the device by ultrasonography; visualization of the shadowing beneath the device can help localize the unit. It is important to know that the tail strings are more echogenic than the device. Failure to recognize this allows the false impression that the IUD is lower than it actually is.
A woman can safely have a magnetic resonance imaging study without disrupting the position of the LNG-IUS device. It will not set off alarms in security systems—such as those used at an airport
I place only a few LNG-IUS devices each year. What should I do to remain competent and confident at performing this procedure?
Many teaching aids are available to refresh your skills. Options include instructional CD-ROMs and manuals and hands-on practice with a representative of the manufacturer of the device. As noted, the LNG-IUS package insert offers step-by-step instructions on the procedure for placing the device.
What about concerns over cost that some patients express to me?
Women can charge the cost of the device to a credit card if their health insurance does not cover it, or its insertion. Payment plans are also available from the manufacturer.
If you attempt to assist your patients by finding less expensive LNG-IUS units (that is, from a source other than the manufacturer), be aware that the device must be stored under controlled conditions in transit, similar to the way other delicate devices are (e.g., NuvaRing, Implanon). LNG-IUS devices that are shipped under less-than-optimal controlled conditions may not maintain their stability or provide the appropriate rate of release of the contraceptive hormone.
1. Mirena (levonorgestrel-releasing intrauterine system) [package insert]. Wayne, NJ: Bayer Health Care Pharmaceuticals; 2008.
1. Mirena (levonorgestrel-releasing intrauterine system) [package insert]. Wayne, NJ: Bayer Health Care Pharmaceuticals; 2008.