User login
CASE: Pelvic pain and a complex cyst: What now?
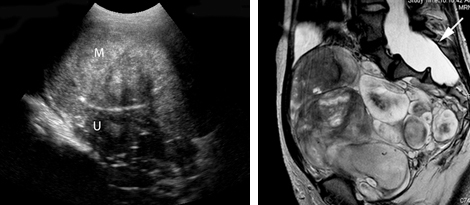
Your patient, a 41-year-old woman, has come to see you, reporting left lower quadrant pain. Physical examination is remarkable for fullness in the left adnexa. You order pelvic ultrasonography (US), which shows heterogeneous appearance to the left ovary (calipers), measuring 3.4 cm at its greatest dimension (see FIGURE). There is through transmission, but the lesion does not have the appearance of a physiologic cyst. Color Doppler shows no flow but there are areas that appear solid with septations.
With the full extent of the cyst unknown, what imaging study would be most helpful for you to order next?

Greyscale ultrasound (top left) shows heterogeneous appearance to the left ovary (calipers), measuring 3.4 cm in greatest dimension. A T1-weighted MRI (top right) shows a 10-cm lesion (arrows) with fat. The full extent of the tumor was not recognized during the ultrasound examination.When the appearance of an adnexal lesion on US is inconclusive or nonspecific, MRI becomes a very worthwhile tool. In the case presented, MRI revealed a 10-cm fatty tumor.
I want to stress at the outset: US is always the first-line imaging tool when you assess a pelvic mass. This modality is inexpensive, widely available, and involves no exposure to radiation. In the great majority of cases in which a cyst is seen on US, it can be characterized and diagnosed appropriately and the proper treatment plan—if any is needed—established.
In women of menstrual age, most cysts that are seen on US are physiologic. If a cyst is sufficiently small and its appearance characteristic, it does not require follow-up imaging.
MRI in its appropriate role does have advantages across a range of gyn abnormalities and problems, as I describe in this article, and, therefore, appropriate indications for use in clinical problem-solving. Those advantages include:
- a detailed view of anatomy, including information gleaned from characterization of tissues
- imaging in any plane.
Skilled US imaging of the adnexal mass (4-part series)
Ilan E. Timor-Tritsch, MD and Steven R. Goldstein, MD (September-December, April 2010)
MRI isn’t of much benefit to women with breast cancer-despite a rise in its use
Janelle Yates, Senior Editor (December 2011)
Fibroids and adenomyosis
MRI is helpful for assessing the size, location, number, and type of degeneration of leiomyomata in patients in whom specific information is needed to determine the choice of therapy. MRI also can be used to distinguish between fibroids and adenomyosis— an important distinction when you are selecting appropriate therapy for bleeding, pain, and bulk-related symptoms. Adenomyomata tend to be myometrial masses with an ill-defined margin, ovoid in shape; high signal-intensity glands are seen within the myometrium on T2-weighted imaging. Fibroids, on the other hand, tend to be round and well-defined.
Prep for uterine artery embolization. Consider how MRI might be used to assess leiomyomata in a patient who is considering nonsurgical uterine artery embolization (UAE). MRI can be used to appropriately triage her, based on the likelihood of success, to hysteroscopic resection of submucosal fibroids, hysterectomy, or UAE.
Because degenerated fibroids already have lost their vascular supply, they are unlikely to respond to UAE; fibroids that exhibit preprocedure hemorrhagic degeneration, therefore, represent a relative contraindication to UAE. Such hemorrhagic degeneration is demonstrated as high signal intensity on a T1-weighted MRI scan.
MRI angiography is performed as part of preprocedure UAE, providing information on the anatomy of the uterine and ovarian arteries. This information is important: If the ovarian artery supplies the fibroids, then the procedure might not yield a good or durable result.
After UAE. Postprocedure, MRI is helpful for predicting outcome; persistent perfusion of fibroids predicts treatment failure. Outcome correlates with the degree of devascularization, not the degree of shrinkage.
MRI also can be used to assess complications of UAE, such as fibroid expulsion, endometritis, and uterine abscess. Contrast-enhanced MRI can reveal viable attachment to the uterine wall, allowing for preoperative planning when UAE has not provided a satisfactory outcome.
Complex Müllerian anomalies that cannot be fully assessed sonographically
Müllerian anomalies affect approximately 1% of all women and as many as 25% of women with infertility or who have a history of multiple spontaneous miscarriages.
In most cases, US is adequate to appropriately characterize Müllerian anomalies. Three-dimensional US in particular is helpful for assessing the fundal contour; this modality has decreased the need for MRI significantly in such cases.
When is MRI useful in this setting? MRI can be used 1) in cases in which distinguishing a septate from a bicornuate uterus will affect management and 2) when the fundal contour cannot be assessed completely sonographically. A septate uterus, for example, can be treated with hysteroscopic resection, especially if the patient has a history of more than one miscarriage; a bicornuate uterus, on the other hand, is usually not treated surgically—although such a patient needs to be followed when she is pregnant because she is at increased risk of an incompetent cervix.
In rare cases, a complex Müllerian anomaly requires further assessment. Then, MRI can:
- determine the contour of the fundus
- measure any fundal indentation
- distinguish the nature of a septum (myometrial or fibrous)
- assess for an atrophic horn in a case of unicornuate uterus
- assess for complications associated with a uterine anomaly, such as endometriosis and abnormal location of pregnancy.
Cervical Ca. MRI can be used in cases of cervical cancer to:
- demonstrate the tumor
- allow accurate depiction of its size and location
- aid in treatment selection by showing direct tumor extension to the lower uterus, vagina, paracervical and parametrial tissues, as well as to adjacent bladder and rectum.
Endometrial Ca. MRI can be used to stage endometrial cancer by showing 1) the depth of myometrial invasion and extension into the cervix, broad ligaments, and parametrium and 2) abnormal lymph nodes.
Ovarian Ca. MRI can be used to better define the imaging characteristics of an adnexal mass that is not clearly benign on US. Staging of ovarian cancer, however, is typically performed by CT; MRI is reserved for cases in which the use of iodinated contrast material is contraindicated.
Imaging of the pelvic floor
Dynamic MRI can be utilized when imaging assessment of the pelvic floor in motion is needed to determine whether surgery or other therapy for prolapse or urinary incontinence, or both, is appropriate. The pelvic floor is assessed at rest and during strain in patients with symptoms. MRI can be used to:
- quantify descent
- identify enterocele or rectocele
- assess for the position of the urethra
- assess for muscle atrophy and tears.
Problem: Endometriosis
US is the first-line modality when endometriosis needs to be assessed by imaging. Sonography depicts focal endometriomas as complex cysts with homogenous, low-level internal echoes.
Small endometrial implants, however, cannot be seen with US; contrast-enhanced MRI with fat saturation can be used to demonstrate small implants and adhesions that involve surrounding organs.
Keep in mind that, typically, laparoscopy is needed for thorough staging of endometriosis because small implants and adhesions are better seen under direct visualization.
Problem: Determining the nature of an indeterminate adnexal mass
Most adnexal lesions seen on US are self-limited physiologic cysts that have a classic appearance; they generally resolve on follow-up. Other lesions—dermoids, endometriomas, and cystadenofibromas—often have a classic appearance on US that allows for confident diagnosis.
At times, however, the diagnosis of an adnexal mass is not definitive on US, and MRI can then be very helpful in problem-solving.
Fibrous lesions. In the case of a fibrous lesion, when it is unclear if the mass is adnexal (fibroma, fibrothecoma) or uterine (an exophytic or pedunculated fibroid), MRI can be helpful in determining the organ of origin of the mass, allowing for avoidance of surgery in cases of fibroids.
Complex cysts. In the case of a complex cyst that is not clearly an endometrioma or a dermoid, MRI can be helpful in making the distinction—and can affect management if used preoperatively to 1) allow the patient to avoid surgery or 2) triage her to a less-invasive surgical procedure.
Dermoids have imaging characteristics of fat that can be brought out with specialized MRI techniques (for example, fat suppression or chemical shift artifact) that show differences between fat and water. MRI is particularly helpful in determining the size of a dermoid that might be difficult to assess sonographically because its echogenicity is similar to that of surrounding pelvic fat.
Endometriomas have blood in many stages of their evolution. The very bright signal intensity seen on T1-weighted images is characteristic of the methemoglobin seen in endometriomas.
Adnexal cysts. At times, the entire wall of an adnexal cyst cannot be assessed adequately by US because the cyst is very large (>7 cm in diameter). In such a case, MRI can help assess the entire cyst and surrounding tissue.
Hydrosalpinx. Last, the distinction between hydrosalpinx and a complex ovarian cyst or neoplasm can, at times, be difficult on US. In such a case, MRI allows for visualization of the ovary distinct from the fallopian tube, thereby providing you with a confident diagnosis of hydrosalpinx and obviating the need for further imaging assessment or surgery.
Left: Transabdominal sonogram of an 18-year-old woman reveals a large, solid mass (M) anterior to the uterus (U). The mass has heterogeneous echo-texture. It is unclear on US whether the mass arises from the uterus—although the echo-texture is similar to what would be expected of a fibroid or fibroma.
Right: A T2-weighted MRI parasagittal image shows the large, lobulated pelvic mass. Other images showed no communication with the uterus but, rather, extension of some of the mass from enlarged neural foramina. Note also the enlarged thecal sac (arrow), which is compatible with dural ectasia. Taken together, these findings are compatible with plexiform neurofibroma. This woman has neurofibromatosis, previously undiagnosed.
Problem solving in pregnancy
To begin, note that, although MRI at 1.5 Tesla* is safe for use in pregnancy, studies on pregnant women should be performed only on patients in whom the diagnostic benefit is considered to outweigh the theoretical risk of the scan.
Malignancy is found in 2% to 5% of adnexal masses that are removed during pregnancy. Knowledge of the type of lesion is important to judge whether surgery can wait until after delivery or, if a malignancy is a concern, whether it is safest for the patient to have surgery during her pregnancy.
*Tesla is the unit of measurement of the strength of the magnetic field in an MRI scanner that determines the degree and quality of the visualization of anatomic detail.
Presentation: Pain. MRI is very helpful in pregnancy for assessing a patient who has right-lower-quadrant pain when US already has been utilized and the cause of the pain is unclear. MRI can be used in pregnancy to diagnose:
- appendicitis
- Crohn’s disease
- unusual cases of ectopic pregnancy
- ovarian torsion
- ureteral obstruction.
Placenta accreta. Typically, US is utilized to diagnosis placenta accreta. Sonographic findings of accreta include:
- loss of the hypoechoic retroplacental myometrial zone
- thinning or disruption of the hyperechoic uterine serosa or bladder interface
- focal exophytic masses
- lacunar flow.
Typically, a combination of transabdominal and transvaginal US scanning, with assessment of flow using color or power Doppler, or both, is sufficient in the postcesarean-delivery patient who has an anterior placenta previa. In a case in which a patient has had a myomectomy and has scars in the uterus in various locations, MRI can be helpful
In a case of suspected uterine dehiscence, MRI can be used to assess the entire uterine contour—a study that can be difficult with US.
Common indications for using MRI as a problem-solving tool in gynecology
| Distinguishing fibroids from adenomyosis |
|---|
|
| Assessing an indeterminate adnexal mass |
|
| Evaluation of pregnancy |
|
Summing up
MRI is an exceptionally helpful modality in cases of gynecologic abnormalities that have not been, and cannot be, fully characterized by US. Keep in mind, however, that MRI should be used for problem solving—not for initial imaging!
Although the expense of pelvic MRI is much greater than the expense of US, MRI can provide a precise diagnosis—allowing you to establish the appropriate treatment plan. If that plan alters the need for, or invasiveness of, surgical management, then you have improved the quality of your care; possibly made follow-up imaging unnecessary; and, perhaps, reduced the cost of care over the longer term.
We want to hear from you! Tell us what you think.
1. Ascher SM, Arnold LL, Patt RH, et al. Adenomyosis: prospective comparison of MR imaging and transvaginal sonography. Radiology. 1994;190(3):803-806.
2. Byun JY, Kim SE, Choi BG, Ko GY, Jung SE, Choi KH. Diffuse and focal adenomyosis: MR imaging findings. Radiographics. 1999;19 (supple 1):S161-S170.
3. Carrington BM, Hricak H, Nuruddin RN, Secaf E, Laros RK, Jr, Hill EC. Müllerian duct anomalies: MR imaging evaluation. Radiology. 1990;176(3):715-720.
4. Fielding JR, Griffiths DJ, Versi E, Mulkern RV, Lee ML, Jolesz FA. MR imaging of pelvic floor continence mechanisms in the supine and sitting positions. AJR. 1998;171(6):1607-1610.
5. Finberg HJ, Williams JW. Placenta accreta: prospective sonographic diagnosis in patients with placenta previa and prior cesarean section. J Ultrasound Med. 1992;11(7):333-343.
6. Guy GP, Peisner DB, Timor-Tritsch IE. Ultrasonographic evaluation of uteroplacental blood flow patterns of abnormally located and adherent placentas. Am J Obstet Gynecol. 1990;163(3):723-727.
7. Hess LW, Peaceman A, O’Brien WF, Winkel CA, Cruikshank DP, Morrison JC. Adnexal mass occurring with intrauterine pregnancy: report of fifty-four patients requiring laparotomy for definitive management. Am J Obstet Gynecol. 1988;158(5):1029-1034.
8. Kier R, McCarthy SM, Scoutt LM, Viscarello RR, Schwartz PE. Pelvic masses in pregnancy: MR imaging. Radiology. 1990;176(3):709-713.
9. Levine D. Obstetric MRI. J Magn Reson Imaging. 2006;24(1):1-15.
10. Levine D, Brown DL, Andreotti RF, et al. Society of Radiologists in Ultrasound. Management of asymptomatic ovarian and other adnexal cysts imaged at US: Society of Radiologists in Ultrasound Consensus Conference Statement. Radiology. 2010;256(3):943-954.
11. Levine D, Hulka CA, Ludmir J, Li W, Edelman RR. Placenta accreta: evaluation with color Doppler US power Doppler US, and MR imaging. Radiology. 1997;205(3):773-776.
12. Olson MC, Posniak HV, Tempany CM, Dudiak CM. MR imaging of the female pelvic region. Radiographics. 1992;12(3):445-465.
13. Omary RA, Vasireddy S, Chrisman HB, et al. The effect of pelvic MR imaging on the diagnosis and treatment of women with presumed symptomatic uterine fibroids. J Vasc Interv Radiol. 2002;13(11):1149-1153.
14. Pellerito JS, McCarthy SM, Doyle MB, Glickman MG, DeCherney AH. Diagnosis of uterine anomalies: relative accuracy of MR imaging endovaginal sonography, and hysterosalpingography. Radiology. 1992;183(3):795-800.
15. Schwartz LB, Panageas E, Lange R, Rizzo J, Comite F, McCarthy S. Female pelvis: impact of MR imaging on treatment decisions and net cost analysis. Radiology. 1994;192(1):55-60.
CASE: Pelvic pain and a complex cyst: What now?
Your patient, a 41-year-old woman, has come to see you, reporting left lower quadrant pain. Physical examination is remarkable for fullness in the left adnexa. You order pelvic ultrasonography (US), which shows heterogeneous appearance to the left ovary (calipers), measuring 3.4 cm at its greatest dimension (see FIGURE). There is through transmission, but the lesion does not have the appearance of a physiologic cyst. Color Doppler shows no flow but there are areas that appear solid with septations.
With the full extent of the cyst unknown, what imaging study would be most helpful for you to order next?

Greyscale ultrasound (top left) shows heterogeneous appearance to the left ovary (calipers), measuring 3.4 cm in greatest dimension. A T1-weighted MRI (top right) shows a 10-cm lesion (arrows) with fat. The full extent of the tumor was not recognized during the ultrasound examination.When the appearance of an adnexal lesion on US is inconclusive or nonspecific, MRI becomes a very worthwhile tool. In the case presented, MRI revealed a 10-cm fatty tumor.
I want to stress at the outset: US is always the first-line imaging tool when you assess a pelvic mass. This modality is inexpensive, widely available, and involves no exposure to radiation. In the great majority of cases in which a cyst is seen on US, it can be characterized and diagnosed appropriately and the proper treatment plan—if any is needed—established.
In women of menstrual age, most cysts that are seen on US are physiologic. If a cyst is sufficiently small and its appearance characteristic, it does not require follow-up imaging.
MRI in its appropriate role does have advantages across a range of gyn abnormalities and problems, as I describe in this article, and, therefore, appropriate indications for use in clinical problem-solving. Those advantages include:
- a detailed view of anatomy, including information gleaned from characterization of tissues
- imaging in any plane.
Skilled US imaging of the adnexal mass (4-part series)
Ilan E. Timor-Tritsch, MD and Steven R. Goldstein, MD (September-December, April 2010)
MRI isn’t of much benefit to women with breast cancer-despite a rise in its use
Janelle Yates, Senior Editor (December 2011)
Fibroids and adenomyosis
MRI is helpful for assessing the size, location, number, and type of degeneration of leiomyomata in patients in whom specific information is needed to determine the choice of therapy. MRI also can be used to distinguish between fibroids and adenomyosis— an important distinction when you are selecting appropriate therapy for bleeding, pain, and bulk-related symptoms. Adenomyomata tend to be myometrial masses with an ill-defined margin, ovoid in shape; high signal-intensity glands are seen within the myometrium on T2-weighted imaging. Fibroids, on the other hand, tend to be round and well-defined.
Prep for uterine artery embolization. Consider how MRI might be used to assess leiomyomata in a patient who is considering nonsurgical uterine artery embolization (UAE). MRI can be used to appropriately triage her, based on the likelihood of success, to hysteroscopic resection of submucosal fibroids, hysterectomy, or UAE.
Because degenerated fibroids already have lost their vascular supply, they are unlikely to respond to UAE; fibroids that exhibit preprocedure hemorrhagic degeneration, therefore, represent a relative contraindication to UAE. Such hemorrhagic degeneration is demonstrated as high signal intensity on a T1-weighted MRI scan.
MRI angiography is performed as part of preprocedure UAE, providing information on the anatomy of the uterine and ovarian arteries. This information is important: If the ovarian artery supplies the fibroids, then the procedure might not yield a good or durable result.
After UAE. Postprocedure, MRI is helpful for predicting outcome; persistent perfusion of fibroids predicts treatment failure. Outcome correlates with the degree of devascularization, not the degree of shrinkage.
MRI also can be used to assess complications of UAE, such as fibroid expulsion, endometritis, and uterine abscess. Contrast-enhanced MRI can reveal viable attachment to the uterine wall, allowing for preoperative planning when UAE has not provided a satisfactory outcome.
Complex Müllerian anomalies that cannot be fully assessed sonographically
Müllerian anomalies affect approximately 1% of all women and as many as 25% of women with infertility or who have a history of multiple spontaneous miscarriages.
In most cases, US is adequate to appropriately characterize Müllerian anomalies. Three-dimensional US in particular is helpful for assessing the fundal contour; this modality has decreased the need for MRI significantly in such cases.
When is MRI useful in this setting? MRI can be used 1) in cases in which distinguishing a septate from a bicornuate uterus will affect management and 2) when the fundal contour cannot be assessed completely sonographically. A septate uterus, for example, can be treated with hysteroscopic resection, especially if the patient has a history of more than one miscarriage; a bicornuate uterus, on the other hand, is usually not treated surgically—although such a patient needs to be followed when she is pregnant because she is at increased risk of an incompetent cervix.
In rare cases, a complex Müllerian anomaly requires further assessment. Then, MRI can:
- determine the contour of the fundus
- measure any fundal indentation
- distinguish the nature of a septum (myometrial or fibrous)
- assess for an atrophic horn in a case of unicornuate uterus
- assess for complications associated with a uterine anomaly, such as endometriosis and abnormal location of pregnancy.
Cervical Ca. MRI can be used in cases of cervical cancer to:
- demonstrate the tumor
- allow accurate depiction of its size and location
- aid in treatment selection by showing direct tumor extension to the lower uterus, vagina, paracervical and parametrial tissues, as well as to adjacent bladder and rectum.
Endometrial Ca. MRI can be used to stage endometrial cancer by showing 1) the depth of myometrial invasion and extension into the cervix, broad ligaments, and parametrium and 2) abnormal lymph nodes.
Ovarian Ca. MRI can be used to better define the imaging characteristics of an adnexal mass that is not clearly benign on US. Staging of ovarian cancer, however, is typically performed by CT; MRI is reserved for cases in which the use of iodinated contrast material is contraindicated.
Imaging of the pelvic floor
Dynamic MRI can be utilized when imaging assessment of the pelvic floor in motion is needed to determine whether surgery or other therapy for prolapse or urinary incontinence, or both, is appropriate. The pelvic floor is assessed at rest and during strain in patients with symptoms. MRI can be used to:
- quantify descent
- identify enterocele or rectocele
- assess for the position of the urethra
- assess for muscle atrophy and tears.
Problem: Endometriosis
US is the first-line modality when endometriosis needs to be assessed by imaging. Sonography depicts focal endometriomas as complex cysts with homogenous, low-level internal echoes.
Small endometrial implants, however, cannot be seen with US; contrast-enhanced MRI with fat saturation can be used to demonstrate small implants and adhesions that involve surrounding organs.
Keep in mind that, typically, laparoscopy is needed for thorough staging of endometriosis because small implants and adhesions are better seen under direct visualization.
Problem: Determining the nature of an indeterminate adnexal mass
Most adnexal lesions seen on US are self-limited physiologic cysts that have a classic appearance; they generally resolve on follow-up. Other lesions—dermoids, endometriomas, and cystadenofibromas—often have a classic appearance on US that allows for confident diagnosis.
At times, however, the diagnosis of an adnexal mass is not definitive on US, and MRI can then be very helpful in problem-solving.
Fibrous lesions. In the case of a fibrous lesion, when it is unclear if the mass is adnexal (fibroma, fibrothecoma) or uterine (an exophytic or pedunculated fibroid), MRI can be helpful in determining the organ of origin of the mass, allowing for avoidance of surgery in cases of fibroids.
Complex cysts. In the case of a complex cyst that is not clearly an endometrioma or a dermoid, MRI can be helpful in making the distinction—and can affect management if used preoperatively to 1) allow the patient to avoid surgery or 2) triage her to a less-invasive surgical procedure.
Dermoids have imaging characteristics of fat that can be brought out with specialized MRI techniques (for example, fat suppression or chemical shift artifact) that show differences between fat and water. MRI is particularly helpful in determining the size of a dermoid that might be difficult to assess sonographically because its echogenicity is similar to that of surrounding pelvic fat.
Endometriomas have blood in many stages of their evolution. The very bright signal intensity seen on T1-weighted images is characteristic of the methemoglobin seen in endometriomas.
Adnexal cysts. At times, the entire wall of an adnexal cyst cannot be assessed adequately by US because the cyst is very large (>7 cm in diameter). In such a case, MRI can help assess the entire cyst and surrounding tissue.
Hydrosalpinx. Last, the distinction between hydrosalpinx and a complex ovarian cyst or neoplasm can, at times, be difficult on US. In such a case, MRI allows for visualization of the ovary distinct from the fallopian tube, thereby providing you with a confident diagnosis of hydrosalpinx and obviating the need for further imaging assessment or surgery.

Left: Transabdominal sonogram of an 18-year-old woman reveals a large, solid mass (M) anterior to the uterus (U). The mass has heterogeneous echo-texture. It is unclear on US whether the mass arises from the uterus—although the echo-texture is similar to what would be expected of a fibroid or fibroma.
Right: A T2-weighted MRI parasagittal image shows the large, lobulated pelvic mass. Other images showed no communication with the uterus but, rather, extension of some of the mass from enlarged neural foramina. Note also the enlarged thecal sac (arrow), which is compatible with dural ectasia. Taken together, these findings are compatible with plexiform neurofibroma. This woman has neurofibromatosis, previously undiagnosed.
Problem solving in pregnancy
To begin, note that, although MRI at 1.5 Tesla* is safe for use in pregnancy, studies on pregnant women should be performed only on patients in whom the diagnostic benefit is considered to outweigh the theoretical risk of the scan.
Malignancy is found in 2% to 5% of adnexal masses that are removed during pregnancy. Knowledge of the type of lesion is important to judge whether surgery can wait until after delivery or, if a malignancy is a concern, whether it is safest for the patient to have surgery during her pregnancy.
*Tesla is the unit of measurement of the strength of the magnetic field in an MRI scanner that determines the degree and quality of the visualization of anatomic detail.
Presentation: Pain. MRI is very helpful in pregnancy for assessing a patient who has right-lower-quadrant pain when US already has been utilized and the cause of the pain is unclear. MRI can be used in pregnancy to diagnose:
- appendicitis
- Crohn’s disease
- unusual cases of ectopic pregnancy
- ovarian torsion
- ureteral obstruction.
Placenta accreta. Typically, US is utilized to diagnosis placenta accreta. Sonographic findings of accreta include:
- loss of the hypoechoic retroplacental myometrial zone
- thinning or disruption of the hyperechoic uterine serosa or bladder interface
- focal exophytic masses
- lacunar flow.
Typically, a combination of transabdominal and transvaginal US scanning, with assessment of flow using color or power Doppler, or both, is sufficient in the postcesarean-delivery patient who has an anterior placenta previa. In a case in which a patient has had a myomectomy and has scars in the uterus in various locations, MRI can be helpful
In a case of suspected uterine dehiscence, MRI can be used to assess the entire uterine contour—a study that can be difficult with US.
Common indications for using MRI as a problem-solving tool in gynecology
| Distinguishing fibroids from adenomyosis |
|---|
|
| Assessing an indeterminate adnexal mass |
|
| Evaluation of pregnancy |
|
Summing up
MRI is an exceptionally helpful modality in cases of gynecologic abnormalities that have not been, and cannot be, fully characterized by US. Keep in mind, however, that MRI should be used for problem solving—not for initial imaging!
Although the expense of pelvic MRI is much greater than the expense of US, MRI can provide a precise diagnosis—allowing you to establish the appropriate treatment plan. If that plan alters the need for, or invasiveness of, surgical management, then you have improved the quality of your care; possibly made follow-up imaging unnecessary; and, perhaps, reduced the cost of care over the longer term.
We want to hear from you! Tell us what you think.
CASE: Pelvic pain and a complex cyst: What now?
Your patient, a 41-year-old woman, has come to see you, reporting left lower quadrant pain. Physical examination is remarkable for fullness in the left adnexa. You order pelvic ultrasonography (US), which shows heterogeneous appearance to the left ovary (calipers), measuring 3.4 cm at its greatest dimension (see FIGURE). There is through transmission, but the lesion does not have the appearance of a physiologic cyst. Color Doppler shows no flow but there are areas that appear solid with septations.
With the full extent of the cyst unknown, what imaging study would be most helpful for you to order next?

Greyscale ultrasound (top left) shows heterogeneous appearance to the left ovary (calipers), measuring 3.4 cm in greatest dimension. A T1-weighted MRI (top right) shows a 10-cm lesion (arrows) with fat. The full extent of the tumor was not recognized during the ultrasound examination.When the appearance of an adnexal lesion on US is inconclusive or nonspecific, MRI becomes a very worthwhile tool. In the case presented, MRI revealed a 10-cm fatty tumor.
I want to stress at the outset: US is always the first-line imaging tool when you assess a pelvic mass. This modality is inexpensive, widely available, and involves no exposure to radiation. In the great majority of cases in which a cyst is seen on US, it can be characterized and diagnosed appropriately and the proper treatment plan—if any is needed—established.
In women of menstrual age, most cysts that are seen on US are physiologic. If a cyst is sufficiently small and its appearance characteristic, it does not require follow-up imaging.
MRI in its appropriate role does have advantages across a range of gyn abnormalities and problems, as I describe in this article, and, therefore, appropriate indications for use in clinical problem-solving. Those advantages include:
- a detailed view of anatomy, including information gleaned from characterization of tissues
- imaging in any plane.
Skilled US imaging of the adnexal mass (4-part series)
Ilan E. Timor-Tritsch, MD and Steven R. Goldstein, MD (September-December, April 2010)
MRI isn’t of much benefit to women with breast cancer-despite a rise in its use
Janelle Yates, Senior Editor (December 2011)
Fibroids and adenomyosis
MRI is helpful for assessing the size, location, number, and type of degeneration of leiomyomata in patients in whom specific information is needed to determine the choice of therapy. MRI also can be used to distinguish between fibroids and adenomyosis— an important distinction when you are selecting appropriate therapy for bleeding, pain, and bulk-related symptoms. Adenomyomata tend to be myometrial masses with an ill-defined margin, ovoid in shape; high signal-intensity glands are seen within the myometrium on T2-weighted imaging. Fibroids, on the other hand, tend to be round and well-defined.
Prep for uterine artery embolization. Consider how MRI might be used to assess leiomyomata in a patient who is considering nonsurgical uterine artery embolization (UAE). MRI can be used to appropriately triage her, based on the likelihood of success, to hysteroscopic resection of submucosal fibroids, hysterectomy, or UAE.
Because degenerated fibroids already have lost their vascular supply, they are unlikely to respond to UAE; fibroids that exhibit preprocedure hemorrhagic degeneration, therefore, represent a relative contraindication to UAE. Such hemorrhagic degeneration is demonstrated as high signal intensity on a T1-weighted MRI scan.
MRI angiography is performed as part of preprocedure UAE, providing information on the anatomy of the uterine and ovarian arteries. This information is important: If the ovarian artery supplies the fibroids, then the procedure might not yield a good or durable result.
After UAE. Postprocedure, MRI is helpful for predicting outcome; persistent perfusion of fibroids predicts treatment failure. Outcome correlates with the degree of devascularization, not the degree of shrinkage.
MRI also can be used to assess complications of UAE, such as fibroid expulsion, endometritis, and uterine abscess. Contrast-enhanced MRI can reveal viable attachment to the uterine wall, allowing for preoperative planning when UAE has not provided a satisfactory outcome.
Complex Müllerian anomalies that cannot be fully assessed sonographically
Müllerian anomalies affect approximately 1% of all women and as many as 25% of women with infertility or who have a history of multiple spontaneous miscarriages.
In most cases, US is adequate to appropriately characterize Müllerian anomalies. Three-dimensional US in particular is helpful for assessing the fundal contour; this modality has decreased the need for MRI significantly in such cases.
When is MRI useful in this setting? MRI can be used 1) in cases in which distinguishing a septate from a bicornuate uterus will affect management and 2) when the fundal contour cannot be assessed completely sonographically. A septate uterus, for example, can be treated with hysteroscopic resection, especially if the patient has a history of more than one miscarriage; a bicornuate uterus, on the other hand, is usually not treated surgically—although such a patient needs to be followed when she is pregnant because she is at increased risk of an incompetent cervix.
In rare cases, a complex Müllerian anomaly requires further assessment. Then, MRI can:
- determine the contour of the fundus
- measure any fundal indentation
- distinguish the nature of a septum (myometrial or fibrous)
- assess for an atrophic horn in a case of unicornuate uterus
- assess for complications associated with a uterine anomaly, such as endometriosis and abnormal location of pregnancy.
Cervical Ca. MRI can be used in cases of cervical cancer to:
- demonstrate the tumor
- allow accurate depiction of its size and location
- aid in treatment selection by showing direct tumor extension to the lower uterus, vagina, paracervical and parametrial tissues, as well as to adjacent bladder and rectum.
Endometrial Ca. MRI can be used to stage endometrial cancer by showing 1) the depth of myometrial invasion and extension into the cervix, broad ligaments, and parametrium and 2) abnormal lymph nodes.
Ovarian Ca. MRI can be used to better define the imaging characteristics of an adnexal mass that is not clearly benign on US. Staging of ovarian cancer, however, is typically performed by CT; MRI is reserved for cases in which the use of iodinated contrast material is contraindicated.
Imaging of the pelvic floor
Dynamic MRI can be utilized when imaging assessment of the pelvic floor in motion is needed to determine whether surgery or other therapy for prolapse or urinary incontinence, or both, is appropriate. The pelvic floor is assessed at rest and during strain in patients with symptoms. MRI can be used to:
- quantify descent
- identify enterocele or rectocele
- assess for the position of the urethra
- assess for muscle atrophy and tears.
Problem: Endometriosis
US is the first-line modality when endometriosis needs to be assessed by imaging. Sonography depicts focal endometriomas as complex cysts with homogenous, low-level internal echoes.
Small endometrial implants, however, cannot be seen with US; contrast-enhanced MRI with fat saturation can be used to demonstrate small implants and adhesions that involve surrounding organs.
Keep in mind that, typically, laparoscopy is needed for thorough staging of endometriosis because small implants and adhesions are better seen under direct visualization.
Problem: Determining the nature of an indeterminate adnexal mass
Most adnexal lesions seen on US are self-limited physiologic cysts that have a classic appearance; they generally resolve on follow-up. Other lesions—dermoids, endometriomas, and cystadenofibromas—often have a classic appearance on US that allows for confident diagnosis.
At times, however, the diagnosis of an adnexal mass is not definitive on US, and MRI can then be very helpful in problem-solving.
Fibrous lesions. In the case of a fibrous lesion, when it is unclear if the mass is adnexal (fibroma, fibrothecoma) or uterine (an exophytic or pedunculated fibroid), MRI can be helpful in determining the organ of origin of the mass, allowing for avoidance of surgery in cases of fibroids.
Complex cysts. In the case of a complex cyst that is not clearly an endometrioma or a dermoid, MRI can be helpful in making the distinction—and can affect management if used preoperatively to 1) allow the patient to avoid surgery or 2) triage her to a less-invasive surgical procedure.
Dermoids have imaging characteristics of fat that can be brought out with specialized MRI techniques (for example, fat suppression or chemical shift artifact) that show differences between fat and water. MRI is particularly helpful in determining the size of a dermoid that might be difficult to assess sonographically because its echogenicity is similar to that of surrounding pelvic fat.
Endometriomas have blood in many stages of their evolution. The very bright signal intensity seen on T1-weighted images is characteristic of the methemoglobin seen in endometriomas.
Adnexal cysts. At times, the entire wall of an adnexal cyst cannot be assessed adequately by US because the cyst is very large (>7 cm in diameter). In such a case, MRI can help assess the entire cyst and surrounding tissue.
Hydrosalpinx. Last, the distinction between hydrosalpinx and a complex ovarian cyst or neoplasm can, at times, be difficult on US. In such a case, MRI allows for visualization of the ovary distinct from the fallopian tube, thereby providing you with a confident diagnosis of hydrosalpinx and obviating the need for further imaging assessment or surgery.

Left: Transabdominal sonogram of an 18-year-old woman reveals a large, solid mass (M) anterior to the uterus (U). The mass has heterogeneous echo-texture. It is unclear on US whether the mass arises from the uterus—although the echo-texture is similar to what would be expected of a fibroid or fibroma.
Right: A T2-weighted MRI parasagittal image shows the large, lobulated pelvic mass. Other images showed no communication with the uterus but, rather, extension of some of the mass from enlarged neural foramina. Note also the enlarged thecal sac (arrow), which is compatible with dural ectasia. Taken together, these findings are compatible with plexiform neurofibroma. This woman has neurofibromatosis, previously undiagnosed.
Problem solving in pregnancy
To begin, note that, although MRI at 1.5 Tesla* is safe for use in pregnancy, studies on pregnant women should be performed only on patients in whom the diagnostic benefit is considered to outweigh the theoretical risk of the scan.
Malignancy is found in 2% to 5% of adnexal masses that are removed during pregnancy. Knowledge of the type of lesion is important to judge whether surgery can wait until after delivery or, if a malignancy is a concern, whether it is safest for the patient to have surgery during her pregnancy.
*Tesla is the unit of measurement of the strength of the magnetic field in an MRI scanner that determines the degree and quality of the visualization of anatomic detail.
Presentation: Pain. MRI is very helpful in pregnancy for assessing a patient who has right-lower-quadrant pain when US already has been utilized and the cause of the pain is unclear. MRI can be used in pregnancy to diagnose:
- appendicitis
- Crohn’s disease
- unusual cases of ectopic pregnancy
- ovarian torsion
- ureteral obstruction.
Placenta accreta. Typically, US is utilized to diagnosis placenta accreta. Sonographic findings of accreta include:
- loss of the hypoechoic retroplacental myometrial zone
- thinning or disruption of the hyperechoic uterine serosa or bladder interface
- focal exophytic masses
- lacunar flow.
Typically, a combination of transabdominal and transvaginal US scanning, with assessment of flow using color or power Doppler, or both, is sufficient in the postcesarean-delivery patient who has an anterior placenta previa. In a case in which a patient has had a myomectomy and has scars in the uterus in various locations, MRI can be helpful
In a case of suspected uterine dehiscence, MRI can be used to assess the entire uterine contour—a study that can be difficult with US.
Common indications for using MRI as a problem-solving tool in gynecology
| Distinguishing fibroids from adenomyosis |
|---|
|
| Assessing an indeterminate adnexal mass |
|
| Evaluation of pregnancy |
|
Summing up
MRI is an exceptionally helpful modality in cases of gynecologic abnormalities that have not been, and cannot be, fully characterized by US. Keep in mind, however, that MRI should be used for problem solving—not for initial imaging!
Although the expense of pelvic MRI is much greater than the expense of US, MRI can provide a precise diagnosis—allowing you to establish the appropriate treatment plan. If that plan alters the need for, or invasiveness of, surgical management, then you have improved the quality of your care; possibly made follow-up imaging unnecessary; and, perhaps, reduced the cost of care over the longer term.
We want to hear from you! Tell us what you think.
1. Ascher SM, Arnold LL, Patt RH, et al. Adenomyosis: prospective comparison of MR imaging and transvaginal sonography. Radiology. 1994;190(3):803-806.
2. Byun JY, Kim SE, Choi BG, Ko GY, Jung SE, Choi KH. Diffuse and focal adenomyosis: MR imaging findings. Radiographics. 1999;19 (supple 1):S161-S170.
3. Carrington BM, Hricak H, Nuruddin RN, Secaf E, Laros RK, Jr, Hill EC. Müllerian duct anomalies: MR imaging evaluation. Radiology. 1990;176(3):715-720.
4. Fielding JR, Griffiths DJ, Versi E, Mulkern RV, Lee ML, Jolesz FA. MR imaging of pelvic floor continence mechanisms in the supine and sitting positions. AJR. 1998;171(6):1607-1610.
5. Finberg HJ, Williams JW. Placenta accreta: prospective sonographic diagnosis in patients with placenta previa and prior cesarean section. J Ultrasound Med. 1992;11(7):333-343.
6. Guy GP, Peisner DB, Timor-Tritsch IE. Ultrasonographic evaluation of uteroplacental blood flow patterns of abnormally located and adherent placentas. Am J Obstet Gynecol. 1990;163(3):723-727.
7. Hess LW, Peaceman A, O’Brien WF, Winkel CA, Cruikshank DP, Morrison JC. Adnexal mass occurring with intrauterine pregnancy: report of fifty-four patients requiring laparotomy for definitive management. Am J Obstet Gynecol. 1988;158(5):1029-1034.
8. Kier R, McCarthy SM, Scoutt LM, Viscarello RR, Schwartz PE. Pelvic masses in pregnancy: MR imaging. Radiology. 1990;176(3):709-713.
9. Levine D. Obstetric MRI. J Magn Reson Imaging. 2006;24(1):1-15.
10. Levine D, Brown DL, Andreotti RF, et al. Society of Radiologists in Ultrasound. Management of asymptomatic ovarian and other adnexal cysts imaged at US: Society of Radiologists in Ultrasound Consensus Conference Statement. Radiology. 2010;256(3):943-954.
11. Levine D, Hulka CA, Ludmir J, Li W, Edelman RR. Placenta accreta: evaluation with color Doppler US power Doppler US, and MR imaging. Radiology. 1997;205(3):773-776.
12. Olson MC, Posniak HV, Tempany CM, Dudiak CM. MR imaging of the female pelvic region. Radiographics. 1992;12(3):445-465.
13. Omary RA, Vasireddy S, Chrisman HB, et al. The effect of pelvic MR imaging on the diagnosis and treatment of women with presumed symptomatic uterine fibroids. J Vasc Interv Radiol. 2002;13(11):1149-1153.
14. Pellerito JS, McCarthy SM, Doyle MB, Glickman MG, DeCherney AH. Diagnosis of uterine anomalies: relative accuracy of MR imaging endovaginal sonography, and hysterosalpingography. Radiology. 1992;183(3):795-800.
15. Schwartz LB, Panageas E, Lange R, Rizzo J, Comite F, McCarthy S. Female pelvis: impact of MR imaging on treatment decisions and net cost analysis. Radiology. 1994;192(1):55-60.
1. Ascher SM, Arnold LL, Patt RH, et al. Adenomyosis: prospective comparison of MR imaging and transvaginal sonography. Radiology. 1994;190(3):803-806.
2. Byun JY, Kim SE, Choi BG, Ko GY, Jung SE, Choi KH. Diffuse and focal adenomyosis: MR imaging findings. Radiographics. 1999;19 (supple 1):S161-S170.
3. Carrington BM, Hricak H, Nuruddin RN, Secaf E, Laros RK, Jr, Hill EC. Müllerian duct anomalies: MR imaging evaluation. Radiology. 1990;176(3):715-720.
4. Fielding JR, Griffiths DJ, Versi E, Mulkern RV, Lee ML, Jolesz FA. MR imaging of pelvic floor continence mechanisms in the supine and sitting positions. AJR. 1998;171(6):1607-1610.
5. Finberg HJ, Williams JW. Placenta accreta: prospective sonographic diagnosis in patients with placenta previa and prior cesarean section. J Ultrasound Med. 1992;11(7):333-343.
6. Guy GP, Peisner DB, Timor-Tritsch IE. Ultrasonographic evaluation of uteroplacental blood flow patterns of abnormally located and adherent placentas. Am J Obstet Gynecol. 1990;163(3):723-727.
7. Hess LW, Peaceman A, O’Brien WF, Winkel CA, Cruikshank DP, Morrison JC. Adnexal mass occurring with intrauterine pregnancy: report of fifty-four patients requiring laparotomy for definitive management. Am J Obstet Gynecol. 1988;158(5):1029-1034.
8. Kier R, McCarthy SM, Scoutt LM, Viscarello RR, Schwartz PE. Pelvic masses in pregnancy: MR imaging. Radiology. 1990;176(3):709-713.
9. Levine D. Obstetric MRI. J Magn Reson Imaging. 2006;24(1):1-15.
10. Levine D, Brown DL, Andreotti RF, et al. Society of Radiologists in Ultrasound. Management of asymptomatic ovarian and other adnexal cysts imaged at US: Society of Radiologists in Ultrasound Consensus Conference Statement. Radiology. 2010;256(3):943-954.
11. Levine D, Hulka CA, Ludmir J, Li W, Edelman RR. Placenta accreta: evaluation with color Doppler US power Doppler US, and MR imaging. Radiology. 1997;205(3):773-776.
12. Olson MC, Posniak HV, Tempany CM, Dudiak CM. MR imaging of the female pelvic region. Radiographics. 1992;12(3):445-465.
13. Omary RA, Vasireddy S, Chrisman HB, et al. The effect of pelvic MR imaging on the diagnosis and treatment of women with presumed symptomatic uterine fibroids. J Vasc Interv Radiol. 2002;13(11):1149-1153.
14. Pellerito JS, McCarthy SM, Doyle MB, Glickman MG, DeCherney AH. Diagnosis of uterine anomalies: relative accuracy of MR imaging endovaginal sonography, and hysterosalpingography. Radiology. 1992;183(3):795-800.
15. Schwartz LB, Panageas E, Lange R, Rizzo J, Comite F, McCarthy S. Female pelvis: impact of MR imaging on treatment decisions and net cost analysis. Radiology. 1994;192(1):55-60.
