Phenylephrine, a sympathomimetic drug, is commonly used in eye exams to dilate the pupil of the eye and to differentiate scleritis from episcleritis. Common adverse effects (AEs) of phenylephrine include subjective burning, stinging with lacrimation, rebound hyperemia, and liberation of iris pigment into the anterior chamber. Less common, systemic AEs include tachycardia and elevation of systemic blood pressure. Although instances of allergic reactions are rare, phenylephrine has been reported to cause contact dermatitis, blepharoconjunctivitis, and as in this case, keratoconjunctivitis.
Case Report
An 83-year-old white male presented for a red eye evaluation 2 days after having undergone a comprehensive eye exam with dilation at the Malcom Randall VAMC clinic in Gainesville, Florida. The patient reported onset of blurred vision, which he described as looking through a fog. He further compared the feeling to pins sticking in his eyes. The patient noted he had experienced similar symptoms on a few other occasions following eye exams. At the most recent eye exam, proparacaine and fluorescein had been used for tonometry, and phenylephrine 2.5% and tropicamide 0.5% had been used for pupillary dilation.
The patient’s best-corrected visual acuity was counting fingers at 2 feet in the right eye (OD) and left eye (OS). The best-corrected visual acuity 2 days prior had been 20/20 OD and OS. Pupils and extraocular motilities were unremarkable. Intraocular pressures were not obtained due to concern for a possible adverse reaction to proparacaine.
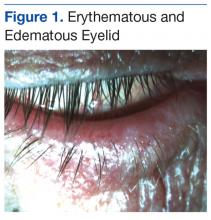
Slit-lamp evaluation revealed the lids to be lax, erythematous, and edematous in both eyes (Figure 1).
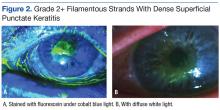
A marked papillary reaction and 3+ bulbar conjunctival injection in both eyes (OU) also was evident. The corneas had 2+ filamentous strands with dense superficial punctate keratitis bilaterally (Figures 2a & 2b). Anterior chamber angles were open, but it was difficult to assess for cells and flare through the hazy corneas. Irides were flat and clear OU. Lens exam revealed modest nuclear sclerosis OU. Due to concern for allergic reaction to tropicamide or phenylephrine, the patient was not redilated. The level of vision loss was consistent with the degree of keratitis observed OU.The initial diagnosis was acute chemical conjunctivitis most likely due to an AE to proparacaine. The plan was to start the patient on antibiotic eye drops qid OU, prednisolone qid OU, and artificial tears every hour OU. The patient was scheduled to return to clinic 4 days later for an anterior segment follow-up.
At the follow-up visit, the patient reported significant visual improvement. His best-corrected visual acuity was 20/40-2 without improvement on pinhole OD and 20/50-2 with improvement to 20/30+ on pinhole OS. Slit-lamp evaluation revealed 1+ bulbar conjunctival injection OU, intact corneal epithelium OU, and no cells or flare in the anterior chambers OU. Due to improving punctate epitheliopathy, the frequency of the antibiotic drops, the prednisolone, and the artificial tears was reduced to bid. After 3 days, he was instructed to discontinue them. The patient was scheduled to return in 2 weeks for an anterior segment follow-up.
At the next follow-up visit, the patient reported that his vision had returned to normal, and he had no further ocular AEs. His best-corrected visual acuity was 20/20-2 OD and 20/20 OS. Slit-lamp evaluation revealed mild blepharitis OU, trace bulbar conjunctival injection OU, and complete resolution of the keratitis OU. The assessment was acute allergic conjunctivitis thought to be secondary to an AE to proparacaine OU, yet the need to rule out hypersensitivity to tropicamide and/or phenylephrine remained. The plan was to educate the patient of the possibility of allergic reaction on future visits and to recommend continued use of artificial tears as needed.
Through a careful and extensive chart review of all past visits, it was suspected that phenylephrine might be to blame rather than proparacaine. At the subsequent visit, the patient agreed to undergo testing to determine the culprit via instillation of proparacaine in one eye and tropicamide in the other. The patient had no reaction to either drop (checked 45 minutes after instillation and the following day). By process of elimination, phenylephrine was determined to be the offending agent.
Discussion
Following a thorough review of the patient’s chart, it was found that on other occasions he had presented with suspected allergic reactions following routine eye examinations. The patient reported he had experienced a reaction in 2007 but could not recall what drops were instilled in his eyes at the time. In addition, there was no documentation in his medical record of the subsequent reaction following that visit. Another reaction occurred in July 2010 with instillation of tropicamide 1%, phenylephrine 2.5%, and Fluress (fluorescein sodium and benoxinate hydrochloride ophthalmic solution USP). In October 2013, when tropicamide 0.5%, proparacaine, and fluorescein strips were instilled, there was no reaction. The next reaction occurred in October 2014, when tropicamide 0.5%, phenylephrine 2.5%, proparacaine, and fluorescein strips were instilled.