Program Expansion
The Ebola clinical research program expanded over time from the initial PREVAIL vaccine study to include studies of therapeutic agents, natural history in Ebola survivors, and an additional vaccine study. The PHS officers have been integral in conducting these studies. The initial study implemented in Liberia, known as PREVAIL I, involved the evaluation of 2 vaccine strategies vs placebo.12,14 In addition to the NIH-based Corps officers supporting the study, the Readiness and Deployment Operations Group (RedDOG) initiated deployments for an additional 2 pharmacy and 7 laboratory officers to support this study. During the deployment, the pharmacists were asked to extend their reach to Sierra Leone and later to Guinea to help establish PREVAIL II, an evaluation of ZMapp in the treatment of Ebola.13 A total of 9 Corps pharmacists, 2 nurses, and 3 physicians deployed to Sierra Leone or Guinea to assist in the PREVAIL II study.
As the epidemic came to an end in Liberia in May 2015, the need for a long-term assessment of Ebola survivors was recognized, resulting in PREVAIL III.15 Noteworthy in the survivor study was an ophthalmic substudy led by a Corps officer assigned to the National Eye Institute.16,17 The survivor study also identified that the persistence of the Ebola virus was longer than previously known and that sexual transmission via semen from infected males remained a potential mode of transmission.18 To address the lingering viral load, a study of an antiviral drug was initiated in Liberia in the summer of 2016, PREVAIL IV.19
Four Corps pharmacists helped train Liberian pharmacists to establish and sustain this randomized, double-blind, placebo-controlled study. Most recently, Corps pharmacists were deployed to support the initiation of the Partnership for Research on Ebola Vaccines (PREVAC), a collaborative partnership with researchers from Liberia, Guinea, and Sierra Leone with cosponsors from the NIH, Institut national de la santé et de la recherche médicale (Inserm) in France, and the London School of Hygiene and Tropical Medicine in the United Kingdom.20
Deployment Procedures
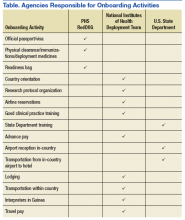
As previously mentioned, 108 staff (civil service, assigned PHS, and contractors) from within the NIH volunteered for deployment to assist in the clinical research Ebola response. The typical rotation for those volunteers was limited to 3 weeks to minimize the disruption of their normal work assignments. Volunteers were organized into small teams within the Division of Clinical Research were composed of the right mix of physicians, nurses, medical technologists, and pharmacists. The team ensured that staff obtained official government passports, scheduled airline reservations, and received an orientation to the deployment setting as well as to the research studies (Table). Additionally, the team coordinated the voucher submission process for reimbursement of expenses on return from the country. An additional team member was stationed in Liberia to coordinate the housing and transportation arrangements with a local hotel near the U.S. Embassy.
Within a week of the February 2015 initial meeting in Liberia to establish the NIH/PHS collaboration, the NIH deployment team met by phone with the Corps’ RedDOG to discuss initial requirements (eg, number of officers needed, disciplines, time lines, and documentation needed for deployment). These initial discussions resulted in the establishment of more formal processes that evolved over time as the 2 organizations gained experience. Based on the identification of the numbers and types of officers needed, RedDOG used procedures similar to the process for staffing the MMU. A communication went out to the Corps seeking interested officers.
Deployment slots were filled based on the personal availability of the officer and coordination with their immediate supervisor and agency. Officers needed to meet medical clearance requirements and provide current health care provider licensure information. Additional training requirements needed to be completed (eg, U.S. State Department training and good clinical practice [GCP] if not already current). Corps officers also took part in the NIH orientation program for deploying personnel to familiarize them to the situation on the ground in West Africa and the specific clinical research protocols that they would encounter. Given that most of the Corps officers were coming from outside the NIH, the onboarding activities required significant attention to detail as procedures for arranging travel (eg, passport, visa, and airline reservations) and processes for reimbursement of travel/per diem pay differed from more traditional deployments directed through the Corps headquarters.
Commissioned Corps Roles in the Research Response
Whereas the establishment of the research program in Liberia was based primarily on relationships forged over a 2-month period by the NIAID deputy director for clinical research and staff, the extension of the research program into Sierra Leone (March 2015) and Guinea (June 2015) was on a substantially shorter time line. As a result, Corps officers were thrust into roles that immediately employed their leadership and diplomacy skills.