Methods
This QI project ascertained the feasibility of the IPPCS by evaluating all consults obtained during the pilot period from November 2, 2015 through May 6, 2016. The IPPCS was accessible Monday through Friday during normal business hours. Goal turnaround time for consult completion was 24 to 72 hours, given the lack of coverage on holidays and weekends. The target population included veterans hospitalized at the WPBVAMC inpatient ward or nursing home with uncontrolled pain on IV and/or oral analgesic medications. All IPPCS consults submitted during the pilot period were included in the sample.
The WPBVAMC Scientific Advisory Committee (SAC) approved this QI program prior to initiation. Following supervisory support from the OCPC, PMR, and Clinical Pharmacy departments, hospital technologists assisted in creating a consult link in the Computerized Patient Record System (CPRS), which allowed providers to submit IPPCS requests for specific patients efficiently. Consults were categorized as postoperative pain, acute or chronic pain, malignant pain, or end-of-life pain. Inpatient providers could enter requests for assistance with 1 or more of the following: opioid dose conversions, opioid taper/titration schedules, general opioid treatment recommendations, or nonopioid/adjuvant recommendations.
The Medical Records Committee approved a customized CPRS subjective-objective-assessment-recommendations (SOAR) note template, which helped standardize the pain CPS documentation. To promote consult requests and interdisciplinary collaboration, inpatient clinicians received education about the IPPCS at respective meetings (eg, General Medicine staff and Clinical Pharmacy meetings).
All CPSs involved in this project were residency-trained in direct patient care, including pain and palliative care, and maintained national board certification as pharmacotherapy specialists. Their role included reviewing patients’ electronic medical records, conducting face-to-face pain assessments, completing opioid risk assessments, evaluating analgesic regimen appropriateness, reviewing medication adherence, completing pain medication reconciliation, querying the Florida Prescription Drug Monitoring Program (PDMP), interpreting urine drug testing (UDT), and delivering provider/patient/caregiver education. Parameters used to determine the appropriateness of analgesic regimens included, but were not limited to:
- Use of oral instead of IV medications if oral dosing was feasible/possible/appropriate;
- Dose and adjustments per renal/hepatic function;
- Adequate treatment duration and titration;
- No therapeutic drug class duplications;
- Medication tolerability (eg, allergies, AEs, drug interactions); and
- Opioid risk assessment per Opioid Risk Tool (ORT) score and medical history.
Consulting providers clarified patients’ pain diagnoses prior to pharmacy consultations.
After face-to-face patient interviews, the inpatient pain CPS prepared pain management recommendations, including nonopioid/adjuvant pain medications and/or opioid dose adjustments. The IPPCS also collaborated with pain physicians for intervention procedures, nonpharmacologic recommendations, and for more complex patients who may have required additional imaging or detailed physical evaluations. Pain CPSs documented CPRS notes with the SOAR template and discussed all recommendations with appropriate inpatient teams.
Respective providers received questionnaires hosted on SurveyMonkey.com (San Mateo, CA) to gauge their satisfaction with the IPPCS at the end of the pilot period, which helped determine program utility. Data collected for the pilot included patient demographics; patient admission diagnosis; consulting inpatient service; type of pain and reason for IPPCS request; total morphine equivalent daily dose (MEDD); pertinent past medical history (ie, sleep apnea, psychiatric comorbidities, or substance use disorder [SUD]); ORT score; patients’ reported average pain severity on the 10-point Numeric Pain Rating Scale (NPRS); number of requests submitted; medications discontinued, initiated, or dose increased/decreased; and number of pharmacist recommendations, including number accepted by providers. The ORT is a 5-item questionnaire used to determine risk of opioid-related aberrant behaviors in adults to help discriminate between low-risk and high-risk individuals (Table 1).17
Descriptive statistics were used to evaluate the results, and a Likert scale was used to evaluate responses the from provider satisfaction questionnaires. The IPPCS collected and organized the data using Microsoft Excel (Redmond, WA).
Results
By the end of the pilot period in May 2016, the IPPCS had received 100 consult requests and completed 81% (Figure). The remaining consults included 11% forwarded to other disciplines. The service discontinued 8% of the requests, given patients’ hospital discharge prior to IPPCS review.
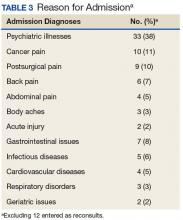
Baseline patient data are outlined in Tables 2, 3, 4, and 5. For each of the 100 consults, providers could select more than 1 reason for the request. The nonopioid/adjuvant treatment recommendations were the most common at 49% (62/128). Patients could have more than 1 pertinent medical comorbidity, with psychiatric illnesses the most prevalent at 68% (133/197). A mean ORT score of 8.1 indicated a high risk for opioid-related aberrant behavior. Overall, half the patients (37/73) were high risk, 25% (18/73) were medium risk (ORT 4-7), and 25% (18/73) were low risk (ORT 0-3). Patients’ reported average pain was often severe (NPRS 7-10) at 54% (40/74) or moderate (NPRS 4-6) at 39% (29/74).
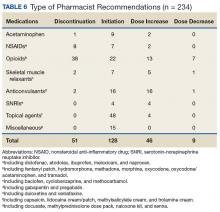
The IPPCS recommended various medications for initiation, discontinuation, or dosage changes (Table 6). For example, the IPPCS recommended initiation of topical agents in 38% (48/128) of cases. The inpatient pain CPS offered opioid initiation in 17% (22/128) of cases, with immediate-release oral morphine as the most predominant. Notably, opioids remained the most common medications suggested for discontinuation at 74% (38/51), including 47% (18/38) for IV hydromorphone. Dose titration recommendations mainly included anticonvulsants at 33% (16/48), and most dose reductions involved opioids at 78% (7/9), namely, oxycodone/acetaminophen and IV hydromorphone.
Providers accepted 76% (179/234) of IPPCS pharmacist medication recommendations. The most common included initiation/optimization of adjuvant therapy (eg, anticonvulsants, serotonin-norepinephrine reuptake inhibitors [SNRIs], and topical agents) at 46% (83/179), followed by opioid discontinuation (eg, IV hydromorphone) at 22% (40/179). Although this project primarily tracked medication interventions, examples of accepted nonpharmacologic recommendations included UDT and referrals to other programs (eg, pain psychology, substance abuse, mental health, acupuncture, chiropractor, PT/OT/PMR, and interventional pain management), which received support from each respective discipline. Declined pharmacologic recommendations mostly included topicals (eg, lidocaine, trolamine, and capsaicin cream) at 35% (19/55). However, findings also show that providers implemented 100% of medication recommendations in whole for 58% (47/81) of consults.
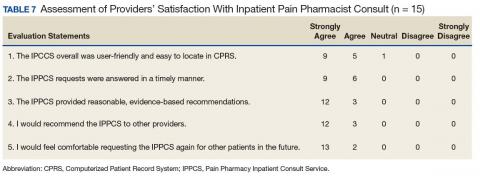
Likert scale satisfaction questionnaires offered insight into providers’ perception of the IPPCS (Table 7). One provider felt “neutral” about the consult submission process, given the time needed to complete the CPRS requests, but all other providers “agreed” or “strongly agreed” that the IPPCS was user-friendly. More importantly, 100% (15/15) “agreed” or “strongly agreed” that the inpatient pain CPS answered consults promptly with reasonable, evidence-based recommendations. All respondents declared that they would recommend the IPPCS to other practitioners and felt comfortable entering requests for future patients.