The COPD CARE service improved patient access to follow-up with no COPD readmissions in the intervention group. The COPD CARE service also validated the use of a coordinated medical home consisting of clinical pharmacists and nurses who provided the initial COPD disease monitoring and plan development. This intervention also resulted in patients receiving greater access to their PACT teams within 30 days of discharge and a higher rate of referrals to tobacco cessation clinics within the COPD CARE group. In addition, use of tools that enabled patients to self-manage their care, such as the COPD plan, was greater in the COPD CARE group.
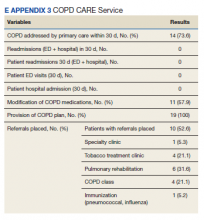
The interventions made in-clinic likely contributed to service results (eAppendix 3).
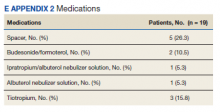
Key interventions included the correction of inhaler technique and prescribing rescue and/or maintenance inhaler therapy as recommended by the GOLD guidelines.In addition, the COPD CARE service provided necessary referrals to pulmonary rehabilitation, nutrition, and tobacco treatment clinics at a higher rate than those patients in the standard of care group. The high percentage of referrals placed to tobacco treatment clinic and pulmonary rehabilitation contributes to improvements in COPD disease control long-term.25
In addition, the high percentage of patients who received care within 30 days of discharge provided additional opportunities to improve disease control and triage high-risk patients (eAppendix 4).The COPD CARE service may best be described as a model for application of the interprofessional team in clinical practice, with the clinical pharmacist uniquely positioned for chronic disease management in the postacute care setting.26 Previously, literature has documented pharmacists as integral members of the team during patient care transitions. Pharmacist completion of medication reconciliation compared with usual care has shown a 28% relative risk (RR) reduction in ED visits and a 67% RR reduction in adverse drug event-related hospital revisits.27 Findings of the COPD CARE service are consistent with the literature and advance the role of pharmacists within the medical home model as prescribers for disease management.27
The interprofessional, team-based design of the COPD CARE service also is supported by recent recommendations from the COPD Foundation, as detailed in the 2nd National COPD Readmission Summit.28 Use of a proactive, team-based care model is emphasized as a central element to coordinating care transitions, with an expectation of 360 degree accountability by all team members for the patients care both during and after hospitalization. The clearly defined roles of each team member within the COPD CARE service, coupled with the expectation that each team member practices with autonomy and accountability, exemplifies the COPD Foundation vision for enhancing COPD care. In addition, the COPD CARE service uses many of the best practices detailed by the COPD Foundation, including the use of spirometry, referrals to pulmonary rehabilitation, and use of motivational interviewing for tobacco treatment clinic referral.
Limitations
This QI initiative has several limitations. By virtue of the study being designed as a practice improvement intervention with rapid implementation, the existing clinic referral structures were used to offer the service to eligible patients. This standard of care included routine telephone contact by a nurse case manager following hospital discharge. Although all patients in the COPD CARE service received the intervention, 5 patients were not seen within the 30-day window, resulting in an implementation rate of 73%. Of the 5 patients that were not seen, 4 were discharged from the ED. Timely follow-up in primary care clinic from the ED required the use of a time-intensive chart review for referral and subsequent delay in intervention delivery.A streamlined clinic referral process from the ED likely would further improve patient scheduling and result in a greater number of patients who would receive the intervention within 30 days of discharge. Despite this limitation, the COPD CARE service was able to see a large percentage of patients within the 30-day time frame postdischarge.