Analysis
Descriptive statistics were used to characterize the sample overall and by modality. Paired t tests were used to assess changes on each assessment measure over time and for each device separately. The significance of change was assessed for 8 outcomes for each device. In this context, using a conservative Bonferroni correction, significance was set at P < .006. Because AS-M is designed to address depression, anxiety, and sleep as well as pain, whereas LTO is not, device assignments were based on clinical considerations rather than randomization. Therefore, no comparisons were made between devices, and outcomes were assessed independently for the 2 devices. Analyses were performed using SAS 9.4 (Cary, NC).
Results
Device trials were initiated for 161 veterans (LTO, 70; AS-M, 91). Distribution of devices was unequal because veterans are assigned to 1 device or the other based on clinical presentation. Failure to complete a trial (n = 46; 28.6%) typically was because of travel barriers, lack of interest in continuing, and for 3 veterans, reports of headaches that they attributed to the AS-M treatment. Of the 115 participants who completed valid trials, 88 (76.5%) also completed assessment measures at pre- and postintervention (LTO = 38; AS-M = 50). None of the participants in this study completed trials with both the AS-M and LTO devices.
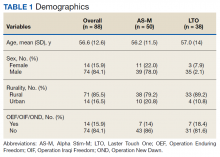
Most participants were male (84.1%) and rural residents (85.5%) (Table 1).
The average age of participants was 56.6 years, and < 20% were Operation Enduring Freedom/Operation Iraqi Freedom/Operation New Dawn-era veterans.Pain Reduction
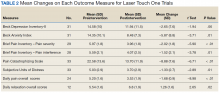
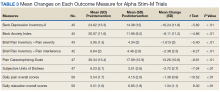
Treatment with AS-M or LTO was associated with statistically significant reductions in pain severity (BPI), pain interference (BPI), daily pain intensity scores (daily pain log), and pain catastrophizing (PCS) (Tables 2 and 3).
Impact on Mood
Use of AS-M was associated with statistically significant improvements in depression (BDI-II), anxiety (BAI), and distress (SUD) scores. In addition, veterans completing AS-M treatment showed a statistically significant improvement in self-reported relaxation scores. Interestingly, use of LTO also resulted in a statistically significant decrease in anxiety (BAI) and a nonstatistically significant decrease in depression (BDI-II).
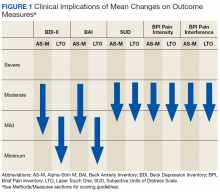
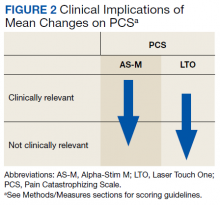
Figure 1 and 2 illustrates the clinical impact of each device in shifting participants from 1 level of symptom severity to another.
Scores on all instruments except the PCS are categorized as minimal, mild, moderate, or severe; PCS scores are dichotomized into clinically relevant and not clinically relevant. Clinically important reductions in pain levels were noted for both AS-M and LTO, with each group of participants improving by 1 category. It also is notable that depression scores (BDI-II) and anxiety scores (BAI) each decreased 1 clinical level with both AS-M and LTO.Discussion
Use of both AS-M and LTO at EOVAHC was associated with reduced pain intensity. The devices also had positive effects beyond pain in areas such as depression, anxiety, and distress. Remission of depression and anxiety symptoms has been associated with significant decline in pain symptoms, suggesting that pain is best treated through multimodal approaches.22