Case Presentation: A 62-year-old male presented with shortness of breath and a cough productive of green sputum. He had a history of hyperlipidemia, posttraumatic stress disorder, bipolar disorder, obstructive sleep apnea, and a 50 pack-year history of smoking. His medications included prazosin, melatonin, lithium, and gabapentin. He also had a significant exposure history including asbestos and chemical paints following his leave from the military. At the initial evaluation, laboratory work revealed a leukocytosis with white blood cell (WBC) count 20 k/cm3and otherwise normal transaminases, albumin, and electrolytes. A chest X-ray revealed a new left hilar mass.
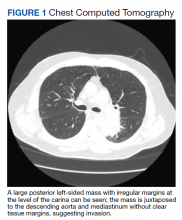
►Manisha Apte, MD, Chief Medical Resident, VA Boston Healthcare System (VABHS) and Boston Medical Center (BMC): To work up his new left hilar mass, a computed tomography (CT) of the chest was ordered (Figure 1), which revealed an apical left lower lobe mass extending into the left hilum encasing part of the ascending aorta. Enlarged mediastinal subcentimeter paratracheal and superior mediastinal lymph nodes also were identified and the pattern raised the concern for lymphangitic carcinomatosis. Dr. Fine, what do you make of the CT findings?
► Alan Fine, MD, Section of Pulmonary and Critical Care, VABHS and Professor of Medicine, Boston University School of Medicine: This mass had irregular edges with septal thickening, which may be why there was a concern for lymphangitic spread. There were no clear tissue planes to see if this process was invading the mediastinum. The mass was irregular, a single lesion, and proximal, making it consistent with a lung cancer. In fact, with his history of smoking, asbestos exposure, the numbers 1 to 10 diagnoses were lung cancer. The lack of demarcation of tissue planes supports this. There are some infections, classically actinomycosis, that do cross and invade anatomical barriers.1 But this looked like a primary lung cancer.
► Dr. Apte: The patient was referred to a pulmonologist where an additional history of night sweats and weight loss were noted. Dr. Fine, we have a patient with a newly identified lung mass, and while we have reason to suspect malignancy as you have already noted, there are many other etiologies to consider, including infections (histoplasmosis, cryptococcosis, bacterial abscess), inflammatory processes (sarcoidosis, rheumatoid nodule) and vasculitis (granulomatosis with polyangiitis). What should be the next step taken to make a diagnosis?
►Dr. Fine: For cancer specifically, we would like to both stage and make a diagnosis with one procedure. That’s part of the utility of a positron emission tomography (PET) scan: We can see lymph node involvement and stage the cancer. We must consider the patient’s comorbidities and the location of the lesion (ie, is it amenable to needle biopsy?). In this case, there are enlarged mediastinal lymph nodes, so one could perform a bronchoscopy with endobronchial ultrasound, which is a relatively noninvasive way to sample the lymph nodes to ideally stage and make a diagnosis as safely as possible. If we are considering infection, needle aspiration is not as sensitive.2
► Dr. Apte: The patient underwent a PET CT, which redemonstrated the lung mass with a loss of aortic fat plane suspicious for aortic involvement as well as lymph nodes in levels 7 and 8 that were concerning for malignancy. Subsequent bronchoscopy with biopsy and endobronchial ultrasound did not show evidence of malignancy; washing and brushing from the mass and lymph node specimens did not identify malignant cells. Benign respiratory mucosa with mild chronic inflammation was noted. Dr. Fine, given the nonspecific findings on the PET scan, negative findings on our bronchoscopy, and a negative biopsy, should we be satisfied that we have ruled out cancer?
► Dr. Fine: No, bronchoscopy has its limitations. It’s highly sensitive to the diagnosis of malignancy if you can see an endobronchial lesion, but we did not see one here. You can only go so far with the scope, and it’s not uncommon for us not to be able to make the diagnosis with bronchoscopy. Malignancy is still the most likely diagnosis, and we need to work this up further. I would perform another biopsy.
►Dr. Apte: Four weeks later, the patient presented with continued shortness of breath, fatigue, and fever. A repeat chest CT showed an opacity suggestive of pneumonia. Given the continued concern for cancer a CT-guided needle biopsy was performed and was once again negative for malignancy. The decision was made to pursue a video-assisted thorascopic surgery (VATS). Following the VATS, the patient developed rigors, fever, and tachycardia with new atrial fibrillation. While being evaluated hypercalcemia was identified, with further workup revealing a low parathyroid hormone (PTH) and low PTH-related peptide. Dr. Fine, the presence of hypercalcemia and a new arrythmia raised the possibility of sarcoidosis. Could this be sarcoidosis?
►Dr. Fine: Sarcoidosis is one of the great masqueraders in medicine. There is a type of sarcoidosis called nodular sarcoidosis where you see masslike distribution in the lung, but generally there are multiple masses and so this presentation would be atypical.3 There is also a phenomenon called sarcoidal reactions usually in the presence of cancer. Again, one tends to see multiple tiny lesions in the lung. It is certainly on the differential, but I would consider it to be less likely than cancer. It is also relatively common to develop atrial fibrillation after manipulation from a lung surgery.4 The other possibility I am concerned about is whether the mass is invading the mediastinum and involving the pericardium.
►Dr. Apte: Results from the VATS biopsy once again returned negative for malignancy and instead showed signs of focal micro-abscesses, atypical pneumocytes, and prominent neutrophils. A diagnosis of acute fibrinous organizing pneumonia (AFOP) was offered. Dr. Fine, what is AFOP?
►Dr. Fine: This is the first case of AFOP I had seen and probably the first case many in our department have seen. This is a relatively new entity with limited reported cases in the literature and is a pathological diagnosis originally recorded in autopsies from patients at the National Institutes of Health.5,6 Given the complexity of the lesion, the diagnosis is difficult to make. Most commonly, AFOP is associated with other systemic entities, most commonly hematologic malignancies like lymphomas and leukemias. It has also been associated with vasculitis and certain drugs. The mechanism is poorly understood, and although pneumonia is a part of the term, this just implies there is inflammation of the lung (ie, pneumonitis).
► Dr. Apte: Given the association of AFOP with underlying hematologic malignancies, an emphasis was placed on another finding: the patient’s increasing WBC count. The total WBC count had been 20 k/cm3 at the time of his lung mass discovery but had increased to > 40 k/cm3 with a differential of neutrophils > 80%. Flow cytometry was negative, and his peripheral smear was read as normal. Dr. Gilbert, what might explain this patient’s leukocytosis?
►Gary Gilbert, MD, Section of Hematology and Oncology, VABHS and Associate Professor of Medicine, Harvard Medical School (HMS): This patient had an elevated WBC for 4 months. Initially, the cause was likely lithium as this is known to cause a leukocytosis.7 More recently, the total WBC had increased and there were a couple of other abnormalities: A consistently elevated absolute monocyte count and a markedly elevated mature neutrophil count. These findings are consistent with a leukemoid reaction (ie, a WBC count > 50,000/µL from causes other than leukemia). The question becomes what is this a leukemoid reaction in response to? Once we have excluded a lung malignancy (a well-known common cause of a leukemoid reaction) we must consider a clonal myeloproliferative disorder. This is particularly true because many things that cause a leukemoid reaction (eg, lobar pneumonia) do not cause a persistently elevated neutrophil count. That this patient does have a persistently elevated neutrophil count suggests something abnormal about the neutrophils themselves.
► Dr. Apte: A bone marrow biopsy was performed. Dr. Gilbert, can you comment on this patient’s bone marrow biopsy and whether a myeloproliferative disorder may have played a role in the marked leukocytosis?
► Dr. Gilbert: The bone marrow biopsy was hyperplastic with myeloid predominance and normal maturation in all lineages. A deep sequencing analysis demonstrated the absence of chromosomal abnormalities or genetic mutations that are associated with myeloproliferative disorders. This excludes the possibility of a myeloproliferative disorder.
► Dr. Apte: The patient was started on 60 mg of prednisone daily, which led to marked improvement in his symptoms. He was discharged in stable condition but presented again with abdominal pain. A complete blood count once again showed increased WBC and new thrombocytosis. A CT angiogram (CTA) showed the prior lung mass with new signs of central necrosis. In the abdomen, new splenic and renal infarct were identified, along with signs of multiple arterial thrombi in the abdomen and internal and external iliac vessel wall thickening. These findings were read as concerning for a medium vessel vasculitis. Dr. Kaur, what are some of the imaging findings you would expect to see in vasculitis, and what about this patient’s CT is consistent with a medium vessel vasculitis?
► Maneet Kaur, MD, Section of Rheumatology, VABHS: Vasculitis is inflammation of the vessel wall that can lead to vascular injury and activation of the coagulation cascade. Sometimes these findings can be seen on imaging with evidence of stenosis, microaneurysms, and thrombosis distal to the stenosis. The nomenclature of vasculitis is not simple and has been revised many times. Medium-vessel vasculitis does not just affect the medium vessels (eg, visceral arteries) but can overlap with distal large vessels and smaller cutaneous vessels.