Immune checkpoint inhibitors (CPIs) have revolutionized cancer therapy and improved the prognosis for a variety of advanced solid tumors and Hodgkin lymphoma, but evidence is growing regarding severe endocrine disturbances.1,2 CPIs block inhibitory molecules on activated T cells to increase tumor cell destruction but also can breach normal tolerance, resulting in a spectrum of immune-related adverse events (irAE).1,2 Programmed cell death-1 (PD-1) inhibitors are one type of CPIs. Pembrolizumab is a humanized monoclonal antibody that targets the PD-1 checkpoint pathway and is approved for the treatment of malignant melanoma and non-small cell lung cancer.3,4 When the PD-1 checkpoint pathway is inhibited, T cells targeting cancer are activated, as are autoreactive T cells, such as those regulating pancreatic islet cell survival, which can lead to type 1 diabetes mellitus (T1DM).5
Case Presentation
A 95-year-old male veteran with long-standing, stable prediabetes was treated with pembrolizumab for stage 4 melanoma. Four months after treatment initiation and 3 weeks after completion of his sixth treatment cycle of pembrolizumab (2 mg/kg every 3 weeks), he presented for surveillance positron emission tomography (PET) and was incidentally found to have a serum glucose of 423 mg/dL. Hypothesis-driven history taking revealed polyuria, polydipsia, and a 12-lb weight loss during the previous 3 months. The patient reported no abdominal pain, nausea, or vomiting. He showed no evidence of pancreatic metastases on recent imaging. His family history was notable for a daughter with T1DM diagnosed at a young age.
On examination, the patient’s vital signs were normal aside from a blood pressure of 80/40 mm Hg. His body mass index was 30. He was alert and oriented with comfortable respirations and no Kussmaul breathing. He exhibited dry mucous membranes and poor skin turgor. Laboratory studies revealed 135 mmol/L sodium (reference, 135-145), 4.6 mmol/L potassium (reference, 3.6-5.2), 100 mmol/L chloride (reference, 99-106), bicarbonate of 26.5 mmol/L (reference, 23-29), serum blood urea nitrogen 27 mg/dL (reference, 6-24), 1.06 mg/dL creatinine (reference, 0.74-1.35), and 423 mg/dL glucose (reference, 70-100), with negative urine ketones. Further studies demonstrated 462 µmol/L fructosamine (reference, 190-270), correlating with hemoglobin A1c (HbA1c) close to 11.0% (HbA1c was drawn on admission but cancelled by the laboratory for unknown reasons).6,7 Later, an inappropriately low C-peptide level of 0.56 ng/mL (reference, 0.8-3.85) and a negative antiglutamic acid decarboxylase (GAD) antibody titer resulted. The patient was given IV hydration and admitted to the hospital. With input from endocrinology, the patient was started on 0.3 units per kg of body weight basal-prandial insulin therapy. Pembrolizumab was held. Six weeks after discharge, his HbA1c was 7.2%, and C-peptide improved to 1.95 ng/mL and plasma glucose 116 mg/dL. After shared decision making with his health care team, the patient decided against restarting pembrolizumab. The patient reported that his functional status was preserved, and he preferred to take fewer medications at his advanced age. He died comfortably 6 months after this presentation from complications of metastatic melanoma.
Dicussion
Immunotherapy is now an integral part of cancer treatment and can result in endocrine disturbances.1,2 Life-threatening irAEs are rare and may mimic more common conditions; thus, there is growing recognition of the need to educate health care professionals in appropriate screening and management of these conditions. CPI-induced T1DM is an uncommon but clinically significant event with an incidence of 0.4 to 1.27% and a median onset of 20 weeks after initiation of therapy (range, 1-228 weeks).8-12In case seriesfrom 3 academic centers, 59 to81% of patients with CPI-induced T1DM presented with diabetic ketoacidosis (DKA), and only 40 to 71% of patients were autoantibody positive.13-16 These patients are older than those presenting with classic T1DM, often require intensive care unit admission, and nearly invariably require exogenous insulin injections for metabolic control.13-16
Based on the later age of onset of cancers that may be treated with CPI, patients with CPI-induced T1DM may be misdiagnosed with T2DM or hyperglycemia from other causes, such as medications or acute illness in the outpatient setting, risking suboptimal treatment.
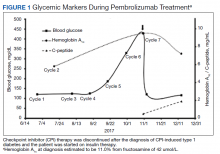
Given the infrequent incidence and lack of controlled trials, screening and treatment recommendations for CPI-induced T1DM are based on principles derived from case series and expert opinion. Development of polyuria, polydipsia, weight loss, nausea, and/or vomiting should prompt investigation for possible development or worsening of hyperglycemia, suggestive of development of T1DM.17 American Society of Clinical Oncology (ASCO) guidelines recommend that serum glucose be assessed at baseline and with each treatment cycle during induction for 12 weeks, then every 3 to 6 weeks thereafter.17 There is no reported association between the number of CPI treatments and the development of DM.8,9 Following our patient’s fifth pembrolizumab cycle, a random glucose reading was noted to be 186 mg/dL (Figure 1). Under the ASCO guidelines, ideally the patient would have received close clinical follow-up given the striking increase in plasma glucose compared with prior baseline lower values and perhaps been further evaluated with an anti-GAD antibody titer to screen for T1DM.17
This patient's case adds to the published reports of CPI-induced T1DM without DKA and represents the oldest patient experiencing this irAE in the literature.13-16 The degree of elevation of his initial fructosamine, which is comparable to an average plasma glucose of approximately 270 mg/dL, belied the rapid rate of rise of his recent plasma glucose. Given the trajectory of glycemic markers and symptoms, one could certainly be concerned about imminent decompensation to DKA. However, fortuitous point-of-care glucose reading prior to surveillance PET resulted in a new critical diagnosis and initiation of treatment.