Vaccinations have substantially reduced morbidity and mortality from many infectious diseases. Despite the clear value of vaccinations in public health, efforts to better understand adverse events (AEs) following immunization are important to sustain public trust and vaccine confidence. Noninfectious inflammation of the heart may manifest as myocarditis or pericarditis, or occasionally, with shared signs and symptoms of each, as myopericarditis. This is a rare AE following some immunizations. Vaccine-associated myocarditis, pericarditis, or myopericarditis (VAMP) has been most clearly associated with smallpox vaccines and mRNA COVID-19 vaccines.1-6 Although extremely rare, VAMP also has been associated with other vaccines.7,8 Limited information exists to guide shared clinical decision making on COVID-19 vaccination in persons with a history of VAMP. It is unknown whether individuals with a history of VAMP are at higher risk for developing a recurrence or experiencing a more severe outcome following COVID-19 vaccination.
Methods
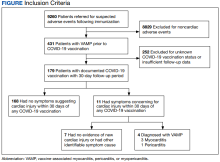
As part of the collaborative public health mission with the Centers for Disease Control and Prevention (CDC) for enhanced vaccine AE surveillance, the Defense Health Agency Immunization Healthcare Division (IHD) maintains a clinical database of service members and beneficiaries referred for suspected AEs following immunizations. A review of all AEs following immunization cases in this database from January 1, 2003, through February 28, 2022, identified individuals meeting the following criteria: (a) VAMP prior to receipt of COVID-19 vaccine; (b) receipt of COVID-19 vaccine in 2021; and (c) medical documentation in available electronic health records sufficient to describe health status at least 30 days following COVID-19 vaccination.9 If medical entries suggested cardiac symptoms following a COVID-19 vaccine, additional information was sought to verify VAMP based on current published criteria.10,11 Both the initial VAMP cases and the suspected COVID-19 VAMP cases were adjudicated by a team of vaccine experts and specialists in immunology, cardiology, and preventive medicine.
This retrospective review was approved and conducted in accordance with the Walter Reed National Military Medical Center Institutional Review Board protocol #20664. All individuals with recurrent VAMP consented to share their health records and clinical details.
Results
Among 9260 cases in the IHD database, 431 met the case definition for VAMP.
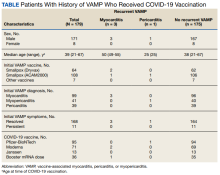
Within this cohort, 179 individuals had records available that confirmed receipt of a COVID-19 vaccine in 2021 and described their health status for at least 30 days after vaccination (Figure). Vaccines associated with the initial VAMP episode included 172 smallpox (64 Dryvax and 108 ACAM2000), 3 influenza, 1 Tdap, 1 anthrax, and 2 multiple vaccines. Subsequent COVID-19 vaccines received included 95 Pfizer-BioNTech, 71 Moderna, and 13 Janssen. Thirty-six patients also received mRNA vaccine boosters.Among the 179 patients included in this analysis, 171 (96%) were male. Their median age was 39 years at the time of COVID-19 vaccination.
The Table describes the cohort’s history of VAMP and subsequent experience with COVID-19 vaccination. Prior VAMP presentations included 99 cases of myocarditis, 39 cases of pericarditis, and 41 cases showing mixed features of myocarditis and pericarditis (myopericarditis).