The US Food and Drug Administration (FDA) approved HGNS in 2014. However, it is not considered a first-line treatment for OSA by the AASM. Original candidate criteria for HGNS included an AHI score of 15 to 65 events/hour, age ≥ 18 years, failed CPAP use, body mass index (BMI) < 32, absence of palatal complete concentric collapse, and central apneas comprising < 25% of total events.16 In June 2023, the FDA expanded approval to increase the upper limit of AHI to 100 events/hour and the BMI to < 40.17
HGNS has been reported to be effective in appropriately selected patients with OSA at tertiary care centers with established multidisciplinary sleep surgical programs. These benefits have not been confirmed in larger, community-based settings, where most of these surgeries occur. In community practice, there is significant confusion among patients and clinicians about the optimal pathway for patient selection and clinical follow-up. Many patients view HGNS as a viable alternative to CPAP, but initially do not understand that it requires surgery. Surgical treatments for OSA, such as HGNS, are appealing because they suggest a 1-time intervention that permanently treats the condition, without need for follow-up or equipment resupply. HGNS might be an appealing treatment option because it is less obtrusive than CPAP and requires fewer resources for set-up and maintenance. Also, it does not cause skin irritation (a possible adverse effect of nightly use of a CPAP mask), allows the individual to sleep in a variety of positions, has less impact on social and sex life, and does not require an electric outlet. In the long term, HGNS might be more cost effective because there is no yearly physician follow-up or equipment resupply and/or maintenance.
The military population has specific demands that impact delivery and effectiveness of health care. Among service members with OSA, CPAP treatment can be challenging because of low adherence, required annual follow-up despite frequent moving cycles that pose a challenge for care continuity, and duty limitations for affected service members (ie, the requirement for a waiver to deploy and potential medical separation if symptoms are not adequately controlled). As the incidence of OSA continues to increase among service members, so does the need for OSA treatment options that are efficacious as CPAP but better tolerated and more suitable for use during military operations. The aim of this review is to assess the effectiveness of HGNS and its potential use by the military OSA patient population.
METHODS
To identify eligible studies, we employed PICOS: Population (patients aged ≥ 18 years with a history of OSA), Intervention (HGNS), Comparator (standard of care PAP therapy), Outcome (AHI or Epworth Sleepiness Scale [ESS], and Study (randomized control trial [RCT] or clinical trial). Studies were excluded if they were not written in English or included pediatric populations. The ESS is a subjective rating scale used to determine and quantify a patient’s level of daytime sleepiness, using a 4-point scale for the likelihood of falling asleep totaled across 8 different situations.18 Daytime sleepiness is considered lower normal(0-5 points), higher normal (6-10 points), mild or moderate excessive (11-15 points), and severe excessive (16-24 points).
Literature Search
We conducted a review of PubMed and Scopus for RCTs and controlled trials published from 2013 to 2023 that included the keywords and phrases: obstructive sleep apnea and either hypoglossal nerve stimulation or upper airway stimulation. The final literature search was performed December 8, 2023.
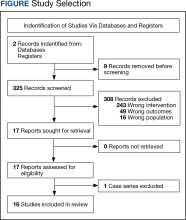
Two authors independently assessed the titles and abstracts of studies identified in the literature search based on the predefined inclusion criteria. If it was not clear whether an article met inclusion criteria based on its title and/or abstract, the 2 review authors assessed the full text of study and resolved any disagreement through consensus. If consensus was not obtained, a third author was consulted. No duplicates were identified. The PRISMA study selection process is presented in the Figure.
Data extraction was performed by 1 independent reviewer. A second author reviewed the extracted data. Any identified discrepancies were resolved through discussion and consensus. If consensus was not obtained, a third author was consulted. Study data included methods (study design and study objective), participants mean age, inclusion criteria, exclusion criteria, interventions and comparators, and primary study outcomes.
The quality of evidence was assessed using a rating of 1 to 5 based on a modified version of the Oxford Centre for Evidence-based Medicine Levels of Evidence and Grades of Recommendation.19 A rating of 1 indicated a properly powered and conducted RCT, 2 demonstrated a well-designed controlled trial without randomization or prospective comparative cohort trial, 3 designated a case-control study or retrospective cohort study, 4 signified a case series with or without intervention or a cross-sectional study, and 5 denoted an opinion of respected authorities or case reports. Two reviewers independently evaluated the quality of evidence. Any identified discrepancies were resolved through discussion and consensus. If consensus was not obtained, a third review author was consulted.