RESULTS
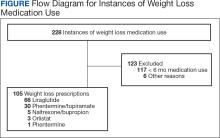
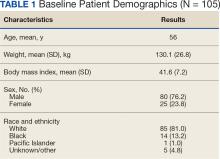
A total of 228 instances of medication use were identified based on prescription fills; 123 did not meet inclusion criteria (117 for < 6 consecutive months of medication use) (Figure). The study included 105 participants with a mean age of 56 years; 80 were male (76.2%), and 85 identified as White race (81.0%). Mean (SD) weight was 130.1 kg (26.8) and BMI was 41.6 (7.2). The most common comorbid disease states among patients included hypertension, dyslipidemia, obstructive sleep apnea, and T2DM (Table 1). The baseline characteristics were comparable to those of Hood and colleagues.16
Most patients at VHI started on liraglutide (63%) or phentermine/topiramate (28%). For primary and secondary outcomes, statistics were calculated to determine whether the results were statistically significant for comparing the liraglutide and phentermine/topiramate subgroups. Sample sizes were too small for statistical analysis for bupropion/naltrexone, phentermine, and orlistat.
Primary Outcomes
The mean (SD) weight of participants dropped 8.1% from 130.1 kg to 119.5 kg over the patient-specific duration of weight management medication therapy for an absolute difference of 10.6 kg (9.7). Duration of individual medication use varied from 6 to 48 months. Weight loss was recorded at 6, 12, 24, 36, and 48 months of weight management therapy. Patient weight was not recorded after the medication was discontinued.
When classified by medication choice, the mean change in weight over the duration of the study was −23.9 kg for 2 patients using orlistat, −10.2 kg for 46 patients using liraglutide, −11.0 kg for 25 patients using phentermine/topiramate, -7.4 kg for 1 patient using phentermine, and -13.0 kg for 4 patients using naltrexone/bupropion. Patients without a weight documented at the end of their therapy or at the conclusion of the data collection period were not included in the total weight loss at the end of therapy. There were 78 documented instances of weight loss at the end of therapy (Table 2).
Body weight loss percentage was recorded at 6, 12, 24, 36, and 48 months of weight management therapy. The mean (SD) body weight loss percentage over the duration of the study was 9.2% (11.2). When classified by medication choice, the mean percentage of body weight loss was 16.8% for 2 patients using orlistat, 9.4% for 46 patients using liraglutide, 8.2% for 25 patients using phentermine/topiramate, 6.0% for 1 patient using phentermine alone, and 10.6% for 4 patients using naltrexone/bupropion (Table 3).
Secondary Outcomes
While none of the secondary outcomes were statistically significant, the results of this study suggest that both medications may contribute to weight loss in many patients included in this study. Almost two-thirds of the included patients analyzed lost ≥ 5% of weight from baseline while taking weight management medication. Sixty-six patients (63%) lost ≥ 5% of body weight at any time during the data collection period. When stratified by liraglutide and phentermine/topiramate, 41 patients (63%) taking liraglutide and 20 patients (67%) taking phentermine/topiramate lost ≥ 5% of weight from baseline. Of the 66 patients who lost ≥ 5% of body weight from baseline, 36 (55%) lost ≥ 10% of body weight from baseline at any time during the data collection period.
The mean (SD) duration for weight management medication use was 23 months (14.9). Phentermine/topiramate was tolerated longer than liraglutide: 22.7 months vs 21.7 months, respectively (Table 4).
The average overall documented medication discontinuation rate was 35.2%. Reasons for discontinuation included 21 patient-elected discontinuations, 8 patients no longer met criteria for use, 4 medications were no longer indicated, and 4 patients experienced AEs. It is unknown whether weight management medication was discontinued or not in 18 patients (17.2%).