About 1 in 10 Americans have diabetes mellitus (DM), of which about 90% to 95% are diagnosed with type 2 DM (T2DM) and veterans are disproportionately affected.1,2 About 25% enrolled in the Veterans Health Administration (VHA) have T2DM, which has been attributed to exposure to herbicides (eg, Agent Orange), decreased physical activity resulting from past physical strain, chronic pain, and other physical limitations resulting from military service.3-5
Pharmacologic management of DM is guided by the effectiveness of lifestyle interventions and comorbid diagnoses. Current DM management guidelines recommend patients with comorbid atherosclerotic cardiovascular disease, chronic kidney disease, or congestive heart failure receive first-line diabetes therapy with a sodium-glucose cotransporter-2 (SGLT-2) inhibitor or glucagon-like peptide-1 receptor (GLP-1) agonist.
Metformin remains a first-line pharmacologic option for the treatment of T2DM with the goal of achieving glycemic management when lifestyle interventions are insufficient.6,7 Newer antihyperglycemic therapies have been studied as adjunct therapy to metformin. However, there is limited literature comparing metformin directly to other medication classes for the treatment of T2DM.8-13 A systematic review of treatment-naive patients found HbA1c reductions were similar whether patients received metformin vs an SGLT-2 inhibitor, GLP-1 agonist, sulfonylurea, or thiazolidinedione monotherapy.10 The analysis found dipeptidyl-peptidase-4 (DPP-4) inhibitors had inferior HbA1c reduction compared to metformin.10 A Japanese systematic review compared metformin to thiazolidinediones, sulfonylureas, glinides, DPP-4 inhibitors, α-glucosidase inhibitors, or SGLT-2 inhibitors for ≥ 12 weeks but found no statistically significant differences in HbA1c reduction.11 The AWARD-3 trial compared once-weekly dulaglutide to metformin in treatment-experienced patients and found greater improvement in HbA1c and achievement of HbA1c goal with dulaglutide.13 While these studies show some comparisons of metformin to alternative pharmacologic therapy, researchers have not looked at what happens to patients’ HbA1c levels when an event, such as a recall, prompts a rapid change to a different antihyperglycemic agent.
On May 28, 2020, the US Food and Drug Administration (FDA) asked 5 pharmaceutical companies to voluntarily recall certain formulations of metformin. This action was taken when FDA testing revealed unacceptably high levels of N-Nitrosodimethylamine, a probable carcinogen.14 This FDA recall of metformin extended-release, referred to as metformin sustained-action (SA) within the VHA electronic medication file but the same type of formulation, prompted clinicians to revisit and revise the pharmacologic regimens of patients taking the drug. Because of the paucity of head-to-head trials comparing metformin with newer alternative antihyperglycemic therapies, the effect of treatment change was unknown. In response, we aimed to establish a data registry within Veterans Integrated Service Network (VISN) 6.
Registry Development
The VISN 6 registry was established to gather long-term, observational, head-to-head data that would allow review of HbA1c levels before and after the recall, as well as HbA1c levels broken down by the agent that patients were switched to after the recall. Another goal was to explore prescribing trends following the recall.
Data Access Request Tracker approval was obtained and a US Department of Veterans Affairs (VA) Information and Computing Infrastructure workspace was developed to host the registry data. The research cohort was established from this data, and the registry framework was finalized using Structured Query Language (SQL). The SQL coding allows for recurring data updates for all individuals within the cohort including date of birth, race, sex, ethnicity, VHA facility visited, weight, body mass index, HbA1c level, creatinine clearance, serum creatinine, antihyperglycemic medication prescriptions, adverse drug reactions, medication adherence (as defined by ≥ 80% refill history), and hospitalizations related to diabetes. For the purposes of this initial analysis, registry data included demographics, diabetes medications, and HbA1c results.
METHODS
This study was a concurrent, observational, multicenter, registry-based study conducted at the Western North Carolina VA Health Care System (WNCVAHCS). The study was approved by the WNCVAHCS institutional review board and research and development committees.
All patients aged ≥ 18 years with T2DM and receiving health care from VISN 6 facilities who had an active metformin SA prescription on, and 1 year prior to, June 1, 2020 (the initial date VHA began implementing the FDA metformin recall) were entered into the registry. Data from 1 year prior were collected to provide a baseline. Veterans were excluded if they received metformin SA for any indication other than T2DM, there was no pre- or postrecall HbA1c measurement, or death. We included 15,594 VISN 6 veterans.
Registry data were analyzed to determine whether a significant change in HbA1c level occurred after the metformin recall and in response to alternative agents being prescribed. Data from veterans who met all inclusion criteria were assessed during the year before and after June 1, 2020. Demographic data were analyzed using frequency and descriptive statistics. The Shapiro Wilkes test was performed, and data were found to be nonparametric; therefore the Wilcoxon signed-rank test was used to evaluate the hypothesis that HbA1c levels were not impacted by the recall.
Our sample size allowed us to create exact matched pairs of 9130 individuals and utilize rank-biserial correlation to establish effect size. Following this initial population-level test, we constructed 2 models. The first, a linear mixed-effects model, focused solely on the interaction effects between the pre- and postrecall periods and various medication classes on HbA1c levels. Second, we constructed a random-effects within-between model (REWB) to evaluate the impact ofmedication classes and demographic variables. Statistical significance was measured at P < .05 with conservative power at .90. The effect size was set to 1.0, reflecting a minimum clinically important difference. Literature establishes 0.5 as a modest level of HbA1c improvement and 1.0 as a clinically significant improvement.
RESULTS
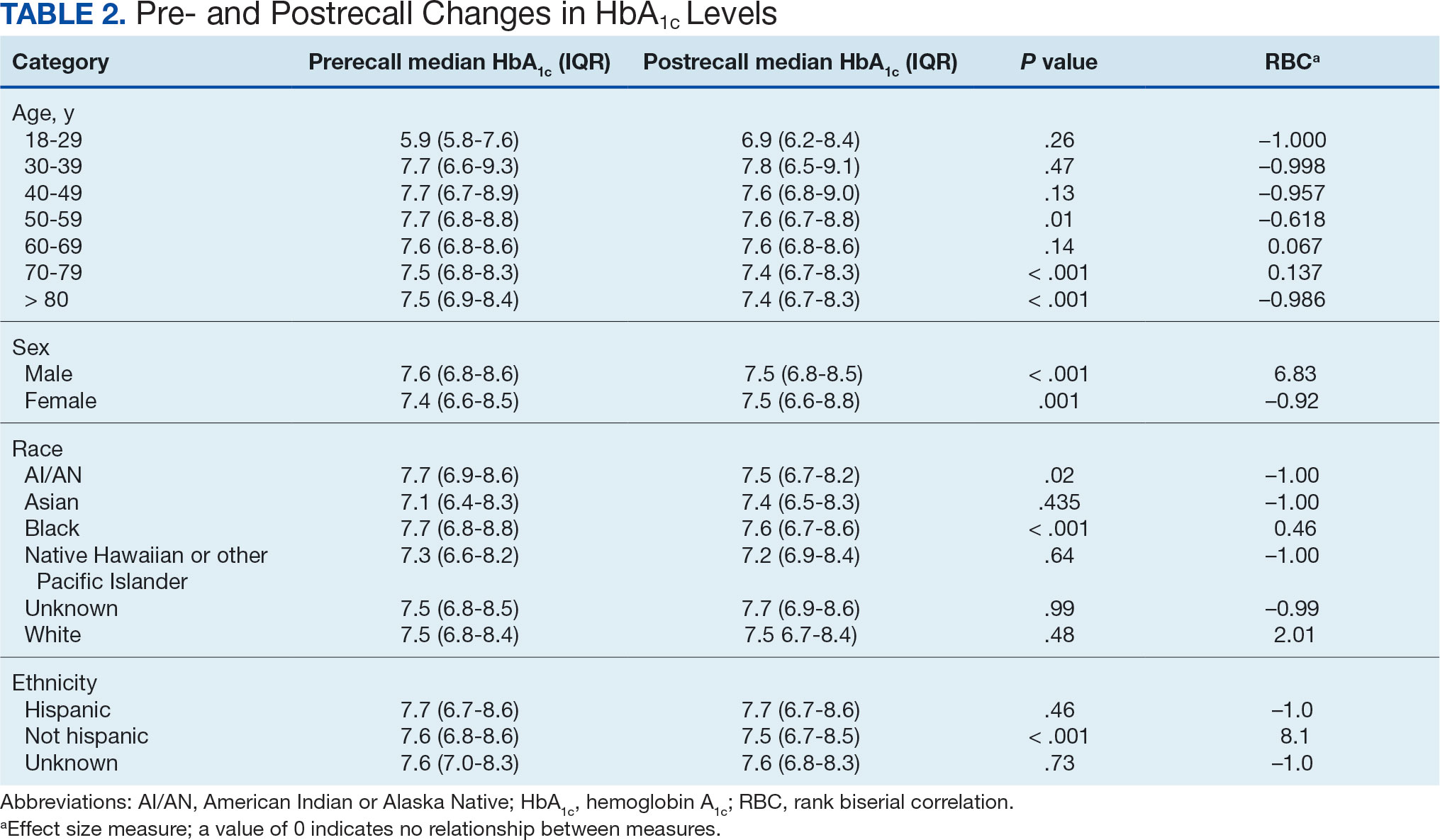
Preliminary results included 15,594 veterans who received a metformin SA prescription as of June 1, 2020 from VISN 6 facilities; 15,392 veterans had a drug exposure end on June 1, 2020, indicating their standard therapy of metformin SA was discontinued following the FDA recall. Two hundred and two veterans were excluded from the registry because they continued to receive metformin SA from existing stock at a VISN6 facility. After identifying veterans with data for 1 year prior (June 1, 2019) to the index date and 1 year after (June 1, 2021) the study population was adjusted to 9130. The population was predominantly males aged> 60 years. Roughly 55% of the registry identified as White and nearly 40% as Black, and 2% indentified as Hispanic (Table 1).
Wilcoxon Signed-Rank Test
We created exact pairs by iterating the data and finding the closest measurements for each patient before and after the recall. This has the advantage over averaging a patient’s pre- and post-HbA1c levels, as it allows for a rank-biserial correlation. Using the nonparametric Wilcoxon signed-rank test, V was 20,100,707 (P < .001), indicating a significant effect. The –0.29 rank-biserial correlation, which was computed to assess the effect size of the recall, suggests that the median HbA1c level was lower postrecall vs prerecall. The magnitude of the correlation suggests a moderate effect size, and while the recall had a noticeable impact at a population level, it was not extreme (Table 2).
Linear Mixed-Effects Model
The binary variable for medication class exposure suggests the use of a logit link function for binary outcomes within the multilevel modeling framework.15 We employed a linear mixed-effects model to investigate the impact that switching from metformin SA to other T2DM medications had on HbA1c levels. The model was adjusted for patient-specific random effects and included interaction terms between the recall period (before and after) and the usage of different T2DM medications.
Model Fit and Random Effects
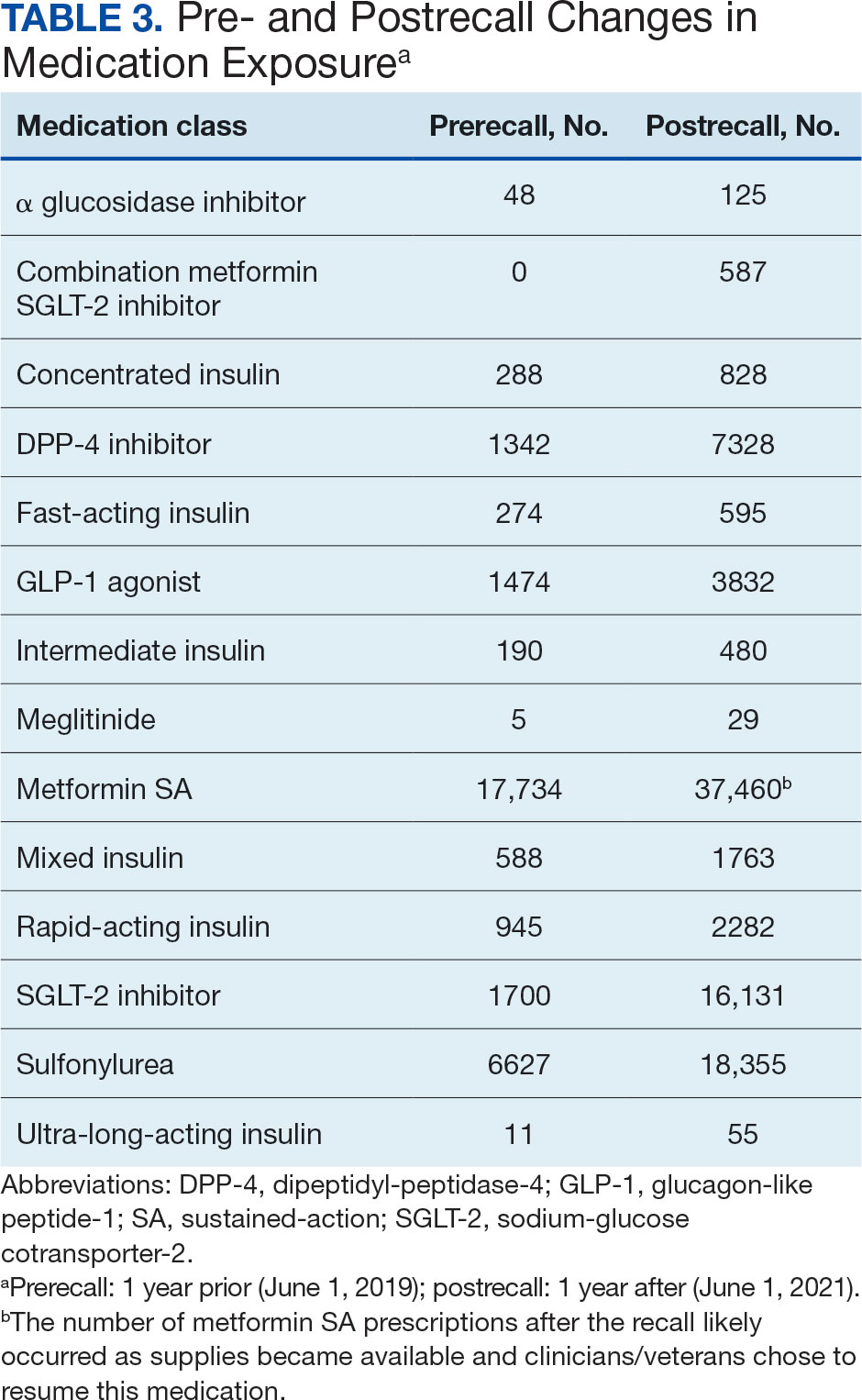
The model demonstrated a residual maximum likelihood criterion of 100,219.7, indicating its fit to the data. Notably, the random effects analysis revealed a substantial variability in baseline HbA1c levels across patients (SD, 0.94), highlighting the importance of individual differences in DM management. Medication classes with zero or near-zero exposure rate were removed. Due to demographic homogeneity, the model did not converge on demographic variables. Veterans were taking a mean of 1.8 T2DM medications and metformin SA was most common (Table 3).
During the postrecall period, metformin SA remained the most frequently prescribed medication class. This may be attributed to the existence of multiple manufacturers of metformin SA, some of which may not have been impacted by the recall. VISN 6 medical centers could have sought metformin SA outside of the usual procurement path following the recall.
Complex Random Effects Model
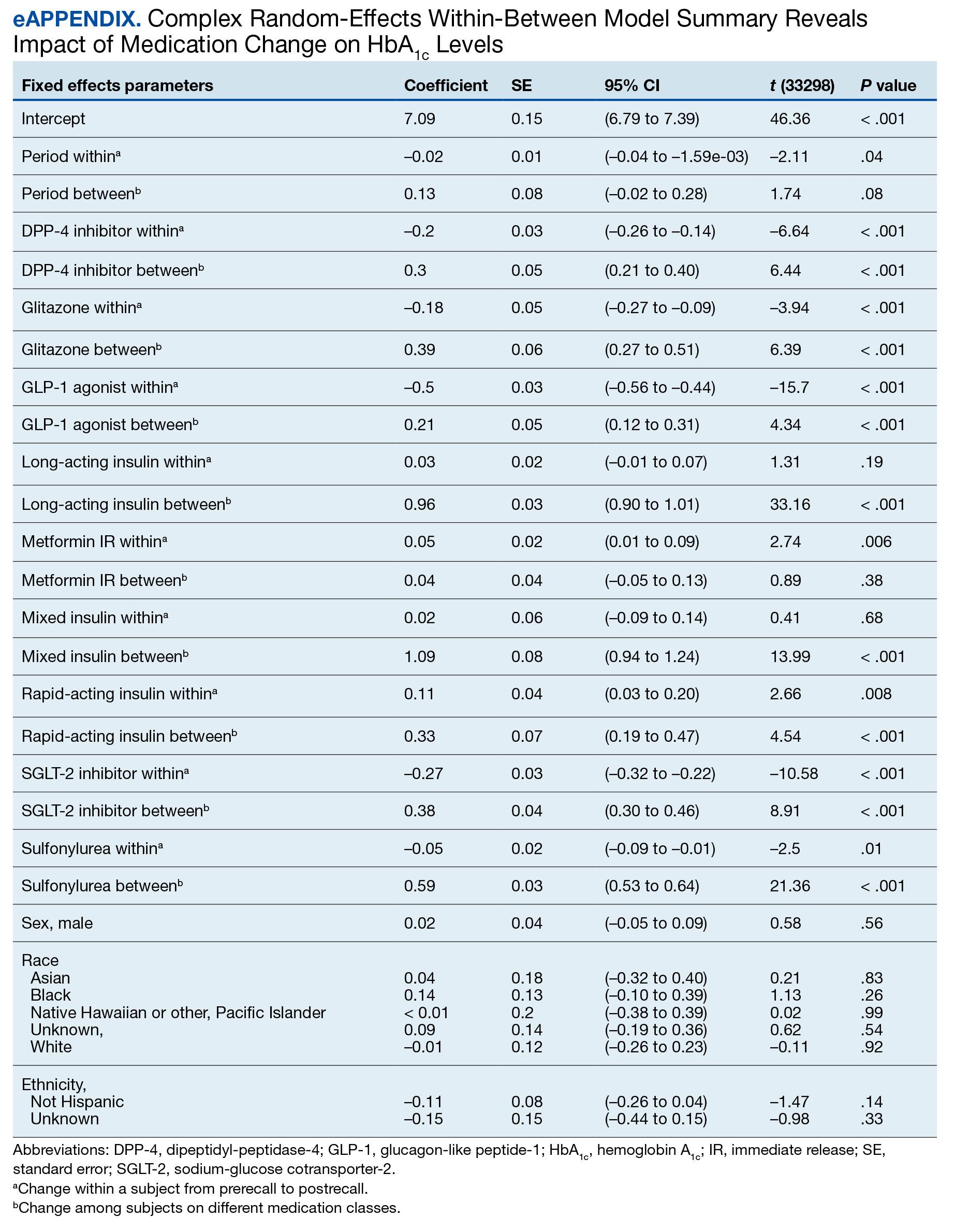
We employed a complex REWB model that evaluated the impact of medication classes on HbA1c levels, accounting for both within and between subject effects of these medications, along with demographic variables (sex, race, and ethnicity) (eAppendix). This model accounts for individual-level changes over time (within-patient effects) and between groups of patients (between-patient effects). This is a more comprehensive model aimed at understanding the broader impact of medications on HbA1c levels across diverse patient groups.
Most demographic categories did not demonstrate significant effects in this model. Black individuals experienced a slight increase in HbA1c levels compared with other racial categories that was not statistically significant. However, this model confirms the findings from the linear mixed-effects model that GLP-1 agonists showed a substantial decrease in HbA1c levels within patients (coefficient –0.5; 95% CI, –0.56 to –0.44; P < .001) and a moderate increase between patients (coefficient, 0.21; 95% CI, 0.12-0.31; P < .001). Additionally, SGLT-2 inhibitors had a notable decrease within patients (coefficient, –0.27; 95% CI, –0.32 to –0.22; P < .001).Another notable finding with our REWB model is insulin usage was associated with high HbA1c levels, but only between subjects. Long-acting insulin (coefficient, 0.96; 95% CI, 0.90-1.01; P <. 001) and mixed insulin (coefficient, 1.09; 95% CI, 0.94-1.24; P < .001) both displayed marked increases between patients, suggesting future analysis may benefit from stratifying across insulin users and nonusers.
Fixed Effect Analysis
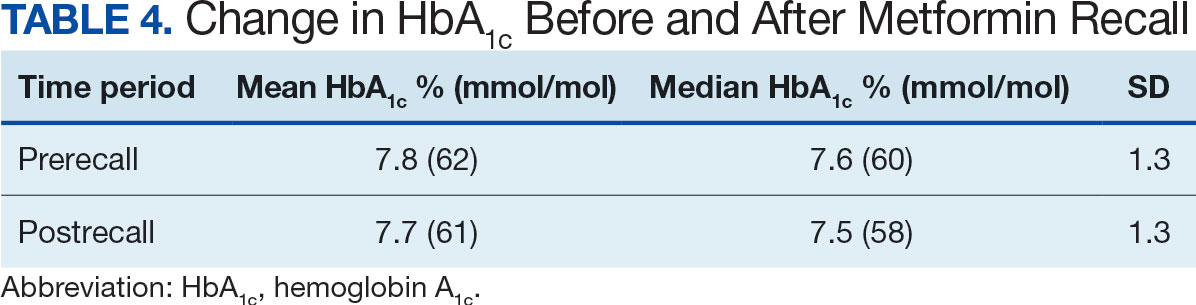
The fixed effects analysis yielded several notable findings. The intercept, representing the mean baseline HbA1c level, was estimated at 7.8% (58 mmol/mol). The coefficient for the period (postrecall) was not statistically significant, indicating no overall change in HbA1c levels from before to after the recall when specific medication classes were not considered (Table 4). Among medication classes examined, several showed significant associations with HbA1c levels. DPP-4 inhibitors and GLP-1 agonists were associated with a decrease in HbA1c levels, with coefficients of −0.08 and −0.24, respectively. Long-acting insulin and metformin immediate-release (IR) were associated with an increase in HbA1c levels, as indicated by their positive coefficients of 0.38 and 0.16, respectively. Mixed insulin formulations and sulfonylureas showed an association with decreased HbA1c levels.
Interaction Effects
The interaction terms between the recall period and the medication classes provided insights into the differential impact of the medication switch postrecall. Notably, the interaction term for long-acting insulin (coefficient, −0.10) was significant, suggesting a differential effect on HbA1c levels postrecall. Other medications, like metformin IR, also exhibited significant interaction effects, indicating changes in the impact on HbA1c levels in the postrecall period. The binary variable for medication class exposure suggests the use of a logit link function for binary outcomes within the multilevel modeling framework.15 We did not address the potential for cross cluster heterogeneity due to different medication classes.
DISCUSSION
This study is an ongoing, concurrent, observational, multicenter, registry-based study consisting of VISN 6 veterans who have T2DM and were prescribed metformin SA on June 1, 2020. This initial aim was to evaluate change in HbA1c levels following the FDA metformin recall. While there was substantial variability in baseline HbA1c levels across the patients, the mean baseline HbA1c level at 7.5% (58 mmol/mol). Patients taking GLP-1 agonists showed substantial decrease in HbA1c levels (coefficient; –0.5; 95% CI, –0.56 to –0.44; P <. 001). Patients taking SGLT-2 inhibitors had a notable decrease in HbA1c (coefficient, –0.27; 95% CI, –0.32 to –0.22; P < .001). Despite this, the coefficient for the postrecall period was not statistically significant, indicating no overall change in HbA1c levels from pre- to postrecall when specific medication classes were not considered.
Further analysis included assessment of prescribing trends postrecall. There was an increase in SGLT-2 inhibitor, GLP-1 agonist, and DPP-4 inhibitor prescribing. Considering the growing evidence of the cardiovascular and renal benefits of these medication classes, specifically the GLP-1 agonists and SGLT-2 inhibitors, this trend would be expected.
Limitations
This study cohort did not capture veterans with T2DM who transferred their health care to VISN 6 after June 1, 2020, and continued to receive metformin SA from the prior facility. Inclusion of these veterans would have increased the registry population. Additionally, the cohort did not identify veterans who continued to receive metformin SA through a source other than the VA. Without that information, the registry cohort may include veterans thought to have either transitioned to a different therapy or to no other T2DM therapy after the recall.
Given that DM can progress over time, it is possible the transition to a new medication after the recall was the result of suboptimal management, or in response to an adverse effect from a previous medication, and not solely due to the metformin SA recall. In addition, there are several factors that could impact HbA1c level over time that were not accounted for in this study, such as medication adherence and lifestyle modifications.
The notable level of metformin SA prescriptions, despite the recall, may be attributed to several factors. First, not all patients stopped metformin completely. Review of the prescription data indicated that some veterans were provided with limited refills at select VA medical centers that had supplies (medication lots not recalled). Access to a safe supply of metformin SA after the recall may have varied among VISN 6 facilities. It is also possible that as new supplies of metformin SA became available, veterans restarted metformin SA. This may have been resumed while continuing a new medication prescribed at the beginning of the recall. As the year progressed after the recall, an increase in metformin SA prescriptions likely occurred as supplies became available and clinicians/veterans chose to resume this medication therapy.
Conclusions
Results of this initial registry study found no difference in HbA1c levels across the study population after the metformin SA recall. However, there was clinical difference in the HbA1c within veterans prescribed SGLT-2 inhibitors and GLP-1 agonists. As expected, prescribing trends showed an increase in these agents after the recall. With the known benefits of these medications beyond glucose lowering, it is anticipated the cohort of veterans prescribed these medications will continue to grow.
The VISN 6 research registry allowed this study to gain an important snapshot in time following the metformin SA recall, and will serve as an important resource for future DM research endeavors. It will allow for ongoing evaluation of the impact of the transition to alternative T2DM medications after the metformin SA recall. Future exploration will include evaluation of adverse drug reactions, DM-related hospitalizations, emergency department visits related to T2DM, changes in renal function, and cardiovascular events among all diabetes medication classes.
Acknowledgments
The study team thanks the Veterans Affairs Informatics and Computing Infrastructure for their help and expertise throughout this project. The authors acknowledge the contributions of Philip Nelson, PharmD, and Brian Peek, PharmD.